First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Low-Dose Radiation Does Not Induce Cell Damage
2.1.1. X-Ray Irradiation Effects the Long-Term Survival of pADSCs Discontinuosly
2.1.2. No Cytotoxic Effects after IR with a Maximal Dose of 2 Gy
2.1.3. LD-IR Neither Induces Residual nor Persistent DNA Double Strand Breaks in pADSCs
2.2. Functional Changes
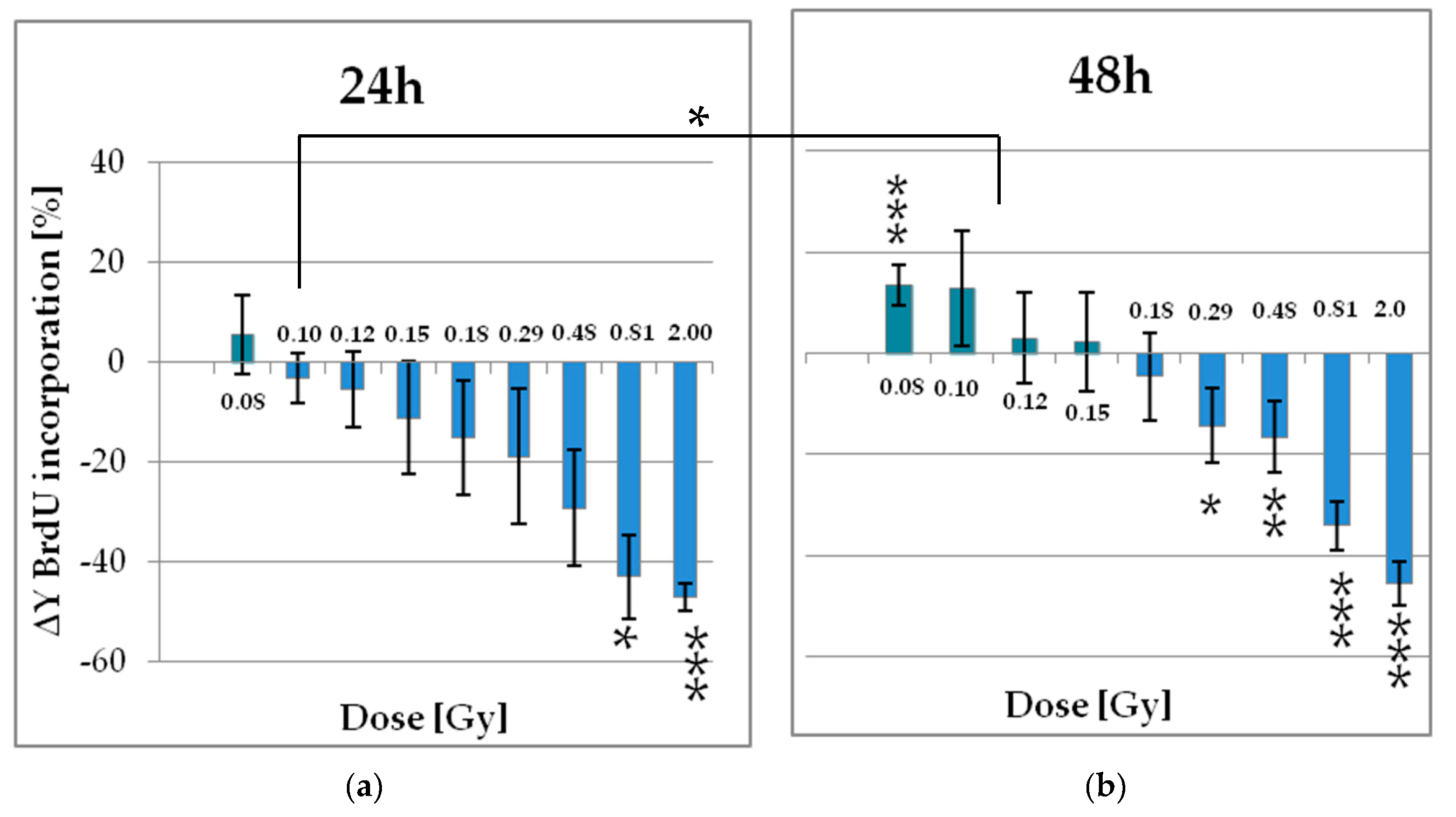
2.2.1. Low Doses of X-Ray Enhances the Proliferation of pADSCs
2.2.2. MMP-2 Secretion of pADSCs is Enhanced after Low Dose X-Ray Therapy with 0.5 Gy
3. Discussion
4. Materials and Methods
4.1. Cell Culture
Isolation of ADSCs
4.2. Irradiation Procedure
4.3. Effects at the Cellular Level
4.3.1. Colony-Forming Units Assay
4.3.2. LDH Cytotoxicity Assay
4.3.3. Cell Proliferation Assay
4.4. DNA Damaging Effects—Measurement of DNA Doublestrand Breaks (γH2AX Assay)
4.5. MMP-2 Enzyme-Linked Immunosorbent Assays (ELISA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose-derived stem cells |
| Akt | Protein kinase B |
| BMSCs | bone-marrow stem cells |
| BrdU | bromodeoxyuridine |
| CAL | cell-assisted lipotransfer |
| DMEM/F12 | Dulbecco′s modified Eagle medium and Kaighn′s modification of Ham′s F12 |
| DMSO | dimethyl sulfoxide |
| DSBs | doublestrand breaks |
| EDTA | Ethylenediaminetetraacetic |
| ELISA | Enzyme-linked Immunosorbent Assay |
| FBS | fetal bovine serum |
| IR | Irradiation |
| LDH | lactate dehydrogenase |
| LD | low dose |
| LD-IR | Low-dose irradiation |
| LD-RT | low-dose radiation therapy |
| MMP 2 | metalloproteinase-2 |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide-Hydrogen |
| pADSCs | pooled adipose-derived stem cells |
| PBS | phosphate-buffered saline |
| P/S | penicillin/streptomycin |
| Raf | rapidly accelerated fibrosarcoma |
| SD | standard deviation |
| VLD | very low-dose |
References
- Richter, E.; Feyerabend, T.; Richter, E.; Feyerabend, T. Strahlentherapie gutartiger Erkrankungen. In Grundlagen der Strahlentherapie; Springer: Berlin/Heidelberg, Germany, 2012; pp. 396–400. [Google Scholar] [CrossRef]
- Niewald, M.; Holtmann, H.; Prokein, B.; Hautmann, M.G.; Rösler, H.P.; Graeber, S.; Dzierma, Y.; Ruebe, C.; Fleckenstein, J. Randomized multicenter follow-up trial on the effect of radiotherapy on painful heel spur (plantar fasciitis) comparing two fractionation schedules with uniform total dose: First results after three months’ follow-up. Radiat. Oncol. 2015, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Uysal, B.; Beyzadeoglu, M.; Sager, O.; Demıral, S.; Gamsız, H.; Dıncoglan, F.; Akın, M.; Dırıcan, B. Role of radiotherapy in the management of heel spur. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Seegenschmiedt, M.H.; Micke, O.; Willich, N. Radiation therapy for nonmalignant diseases in Germany: Current concepts and future perspectives. Strahlenther. Onkol. 2004, 180, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yang, G.; Pan, Z.; Zhao, Y.; Liang, X.; Li, W.; Cai, L. Hormetic response to low-dose radiation: Focus on the immune system and its clinical implications. Int. J. Mol. Sci. 2017, 18, 280. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, J.; Jahns, M.; Hindemith, G. Effects of low dose radiation therapy on adjuvant induced arthritis in rats. Int. J. Radiat. Biol. 2002, 76, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Lödermann, B.; Frey, B.; Kern, P.M.; Fietkau, R.; Rödel, F.; Gaipl, U.S.; Herrmann, M.; Meister, S. Activation-induced cell death and total Akt content of granulocytes show a biphasic course after low-dose radiation. Autoimmunity 2009, 42, 340–342. [Google Scholar]
- Kern, P.; Keilholz, L.; Forster, C.; Seegenschmiedt, M.H.; Sauer, R.; Herrmann, M. In vitro apoptosis in peripheral blood mononuclear cells induced by low- dose radiotherapy displays a discontinuous dose-dependence. Int. J. Radiat. Biol. 1999, 75, 995–1003. [Google Scholar]
- Hildebrandt, G.; Maggiorella, L.; Rödel, F.; Rödel, V.; Willis, D.; Trott, K.R. Mononuclear cell adhesion and cell adhesion molecule liberation after X-irradiation of activated endothelial cells in vitro. Int. J. Radiat. Biol. 2002, 78, 315–325. [Google Scholar] [CrossRef]
- Kern, P.M.; Keilholz, L.; Forster, C.; Hallmann, R.; Herrmann, M.; Seegenschmiedt, M.H. Low-dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiother. Oncol. 2000, 54, 273–282. [Google Scholar] [CrossRef]
- Ott, O.J.; Jeremias, C.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. Radiotherapy for calcaneodynia: Results of a single center prospective randomized dose optimization trial. Strahlenther. Onkol. 2013, 189, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Jeremias, C.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. Radiotherapy for benign calcaneodynia. Long-term results of the Erlangen Dose Optimization (EDO) trial. Strahlenther. Onkol. 2014, 190, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sautter-Bihl, M.-L.; Liebermeister, E.; Scheurig, H.; Heinze, H.-G. Analgetische Bestrahlung degenerativentzündlicher Skeletterkrankungen: Nutzen und Risiko. DMW-Dtsch. Med. Wochenschr. 2008, 118, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xu, Y.; Li, W.; Jiang, H.; Wang, G.; Cai, L.; Ma, K. Low-Dose Radiation Does Not Induce Proliferation in Tumor Cells In Vitro and In Vivo. Radiat. Res. 2008, 170, 477–487. [Google Scholar] [CrossRef]
- Liang, X.; Gu, J.; Yu, D.; Wang, G.; Zhou, L.; Zhang, X.; Zhao, Y.; Chen, X.; Zheng, S.; Liu, Q.; et al. Low-dose radiation induces cell proliferation in human embryonic lung fibroblasts but not in lung cancer cells: Importance of ERK1/2 and AKT signaling pathways. Dose-Response 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Truong, K.; Bradley, S.; Baginski, B.; Wilson, J.R.; Medlin, D.; Zheng, L.; Wilson, R.K.; Rusin, M.; Takacs, E.; Dean, D. The effect of well-characterized, very low-dose X-ray radiation on fibroblasts. PLoS ONE 2018, 13, e0190330. [Google Scholar] [CrossRef]
- Liang, X.; So, Y.H.; Cui, J.; Ma, K.; Xu, X.; Zhao, Y.; Cai, L.; Lu, W. The Low-dose Ionizing Radiation Stimulates Cell Proliferation via Activation of the MAPK/ERK Pathway in Rat Cultured Mesenchymal Stem Cells. J. Radiat. Res. 2011, 52, 380–386. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Chen, C.; Cong, X.; Li, Z.; Zhao, S.; Ren, M. Low-dose radiation modulates human mesenchymal stem cell proliferation through regulating CDK and Rb. Am. J. Transl. Res. 2017, 9, 1914–1921. [Google Scholar]
- Miana, V.V.; Prieto González, E.A. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef]
- Ismail, Y.; Amer, M.; Sherif, S.N.; El-Badri, N.; El-Badawy, A.; Abdelbaset, R.; Abo-Elela, M.; Ghallab, Y.H.; Abdelhamid, H. Adipose Stem Cells Display Higher Regenerative Capacities and More Adaptable Electro-Kinetic Properties Compared to Bone Marrow-Derived Mesenchymal Stromal Cells. Sci. Rep. 2016, 6, 37801. [Google Scholar]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International So. Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or Not the Same? Comparison of Adipose Tissue-Derived Versus Bone Marrow-Derived Mesenchymal Stem and Stromal Cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Morizono, K.; Elbarbary, A.; Alfonso, Z.; Zuk, P.A.; Zhu, M.; Dragoo, J.L.; Ashjian, P.; Thomas, B.; Benhaim, P.; et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003, 174, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Tremolada, C.; Palmieri, G.; Ricordi, C. Adipocyte transplantation and stem cells: Plastic surgery meets regenerative medicine. Cell Transpl. 2010, 19, 1217–1223. [Google Scholar] [CrossRef]
- Fournier, P.F. Fat grafting: My technique. Dermatol. Surg. 2000, 26, 1117–1128. [Google Scholar] [CrossRef]
- Parrish, J.N.; Metzinger, S.E. Review article: Autogenous fat grafting and breast augmentation: A review of the literature. Aesthetic Surg. J. 2010, 30, 549–556. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef]
- Piccinno, M.S.; Veronesi, E.; Loschi, P.; Pignatti, M.; Murgia, A.; Grisendi, G.; Castelli, I.; Bernabei, D.; Candini, O.; Conte, P.; et al. Adipose stromal/stem cells assist fat transplantation reducing necrosis and increasing graft performance. Apoptosis 2013, 18, 1274–1289. [Google Scholar] [CrossRef]
- Kreuzer, M.; Auvinen, A.; Cardis, E.; Durante, M.; Harms-Ringdahl, M.; Jourdain, J.R.; Madas, B.G.; Ottolenghi, A.; Pazzaglia, S.; Prise, K.M.; et al. Multidisciplinary European Low Dose Initiative (MELODI): Strategic research agenda for low dose radiation risk research. Radiat. Environ. Biophys. 2018, 57, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Baaße, A.; Machoy, F.; Juerß, D.; Baake, J.; Stang, F.; Reimer, T.; Krapohl, B.; Hildebrandt, G. Radiation Sensitivity of Adipose-Derived Stem Cells Isolated from Breast Tissue. Int. J. Mol. Sci. 2018, 19, 1988. [Google Scholar] [CrossRef] [PubMed]
- Baaße, A.; Juerß, D.; Reape, E.; Manda, K.; Hildebrandt, G. Promoting effects of adipose-derived stem cells on breast cancer cells are reversed by radiation therapy. Cytotechnology 2018, 70, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Rühle, A.; Xia, O.; Perez, R.L.; Trinh, T.; Richter, W.; Sarnowska, A.; Wuchter, P.; Debus, J.; Saffrich, R.; Huber, P.E.; et al. The Radiation Resistance of Human Multipotent Mesenchymal Stromal Cells Is Independent of Their Tissue of Origin. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1259–1269. [Google Scholar] [CrossRef]
- Shreder, K.; Rapp, F.; Tsoukala, I.; Rzeznik, V.; Wabitsch, M.; Fischer-Posovszky, P.; Fournier, C. Impact of X-ray Exposure on the Proliferation and Differentiation of Human Pre-Adipocytes. Int. J. Mol. Sci. 2018, 19, 2717. [Google Scholar] [CrossRef] [Green Version]
- Rodel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of Inflammatory Immune Reactions by Low-Dose Ionizing Radiation: Molecular Mechanisms and Clinical Application. Curr. Med. Chem. 2012, 19, 1741–1750. [Google Scholar] [CrossRef]
- Marples, B.M.; Spencer, C. Low-Dose Hyper-Radiosensitivity: Past, Present, and Future. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1310–1318. [Google Scholar] [CrossRef]
- Joiner, M.C.; Lambin, P.; Malaise, E.P.; Robson, T.; Arrand, J.E.; Skov, K.A.; Marples, B. Hypersensitivity to very-low single radiation doses: Its relationship to the adaptive response and induced radioresistance. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 1996, 358, 171–183. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, J.K.; Nam, S.Y.; Yang, K.H.; Jeong, M.; Kim, H.S.; Kim, C.S.; Jin, Y.W.; Kim, J. Low-dose radiation stimulates the proliferation of normal human lung fibroblasts via a transient activation of Raf and Akt. Mol. Cells 2007, 24, 424–430. [Google Scholar]
- Guo, W.-Y.; Wang, G.-J.; Wang, P.; Chen, Q.; Tan, Y.; Cai, L. Acceleration of Diabetic Wound Healing by Low-Dose Radiation is Associated with Peripheral Mobilization of Bone Marrow Stem Cells. Radiat. Res. 2010, 174, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K. Human Adipose Tissue Is a Source of Multipotent. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micke, O.; Seegenschmiedt, M.H. Radiotherapy in painful heel spurs (plantar fasciitis)—Results of a national patterns of care study. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 828–843. [Google Scholar] [CrossRef]
- Kriz, J.; Seegenschmiedt, H.M.; Bartels, A.; Micke, O.; Muecke, R. Updated strategies in the treatment of benign diseases—A patterns of care study of the german cooperative group on benign diseases. Adv. Radiat. Oncol. 2018, 3, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Zwicker, F.; Kirchner, C.; Huber, P.E.; Debus, J.; Zwicker, H.; Klepper, R. Breast cancer occurrence after low dose radiotherapy of non-malignant disorders of the shoulder. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Feinendegen, L.E.; Pollycove, M.; Neumann, R.D. Whole-body responses to low-level radiation exposure: New concepts in mammalian radiobiology. Exp. Hematol. 2007, 35, 37–46. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–171. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lo, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef] [Green Version]




| Shielding by | Block | Brass Wedge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shielding [%] | 100 | 96.0 | 95.2 | 94.1 | 92.7 | 91.0 | 85.5 | 76.2 | 59.5 | 0.0 |
| Incoming radiation [Gy] | 0 | 0.080 | 0.096 | 0.118 | 0.146 | 0.180 | 0.290 | 0.476 | 0.810 | 2.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, A.; Kriesen, S.; Hildebrandt, G.; Manda, K. First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2019, 20, 6075. https://doi.org/10.3390/ijms20236075
Schröder A, Kriesen S, Hildebrandt G, Manda K. First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2019; 20(23):6075. https://doi.org/10.3390/ijms20236075
Chicago/Turabian StyleSchröder, Annemarie, Stephan Kriesen, Guido Hildebrandt, and Katrin Manda. 2019. "First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells" International Journal of Molecular Sciences 20, no. 23: 6075. https://doi.org/10.3390/ijms20236075
APA StyleSchröder, A., Kriesen, S., Hildebrandt, G., & Manda, K. (2019). First Insights into the Effect of Low-Dose X-Ray Irradiation in Adipose-Derived Stem Cells. International Journal of Molecular Sciences, 20(23), 6075. https://doi.org/10.3390/ijms20236075





