siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer
Abstract
:1. Introduction
2. Small Interfering RNA (siRNA) for Lung Cancer Therapy
2.1. Background
2.2. Mechanism of Action
2.3. Challenges
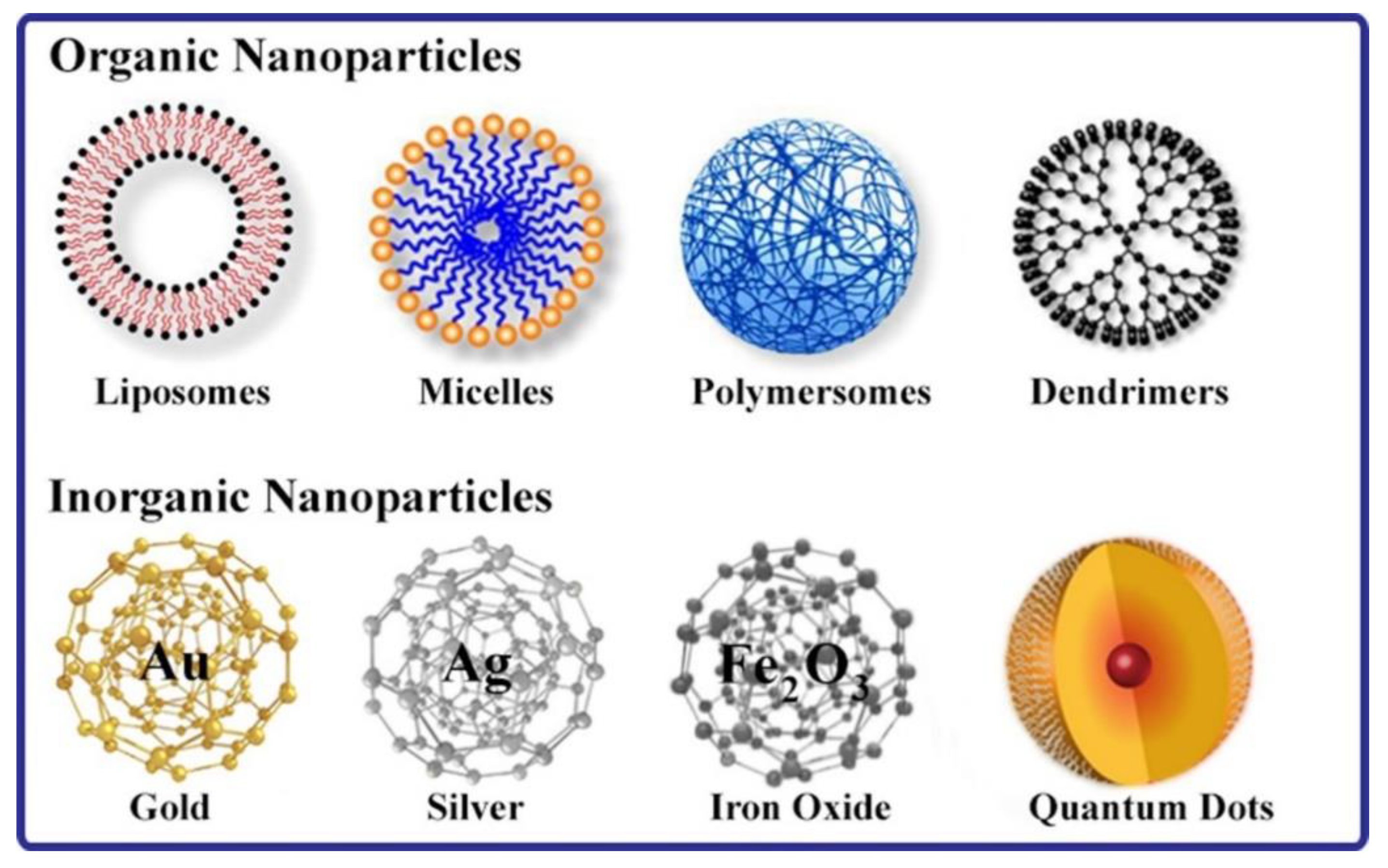
3. Nanoparticles for the Delivery of Therapeutic Drugs and Compounds
3.1. Background
3.2. Lipid-Based Nanoparticles
3.3. Polymer Nanoparticles
3.4. Dendrimers
3.5. Inorganic Metal-Based Nanoparticles
4. siRNA Conjugated Nanoparticles for Lung Cancer Therapy
4.1. Background
4.2. Effects of Nanoparticle Modification on Delivery of siRNA
4.3. Types of Nanoparticles Used for siRNA Delivery to the Lungs
4.3.1. Organic Nanoparticles
Lipid-Based Nanocarriers
Polymer-Based Nanocarriers
4.3.2. Inorganic Nanocarriers
Gold Nanocarriers
Iron Oxide Nanocarriers
4.4. Delivery Mechanisms of siRNA Loaded Nanoparticles for Lung Cancer Treatment
4.4.1. Intratracheal Delivery
4.4.2. Intranasal Delivery
4.4.3. Intravenous Delivery
4.5. Barriers of siRNA-Loaded Nanoparticles Delivery to the Lungs
5. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 30 July 2019).
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, C. Radioactivity of tobacco leaves and radiation dose induced from smoking. Int. J. Environ. Res. Public Health 2009, 6, 558–567. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- De Wever, W.; Coolen, J.; Verschakelen, J.A. Imaging techniques in lung cancer. Breathe 2011, 7, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Scarlata, S.; Fuso, L.; Lucantoni, G.; Varone, F.; Magnini, D.; Antonelli Incalzi, R.; Galluccio, G. The technique of endoscopic airway tumor treatment. J. Thorac. Dis. 2017, 9, 2619–2639. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Guo, W.; Chen, W.; Yu, W.; Huang, W.; Deng, W. Small interfering RNA-based molecular therapy of cancers. Chin. J. Cancer 2013, 32, 488–493. [Google Scholar] [CrossRef]
- Fujita, Y.; Kuwano, K.; Ochiya, T. Development of small RNA delivery systems for lung cancer therapy. Int. J. Mol. Sci. 2015, 16, 5254–5270. [Google Scholar] [CrossRef] [Green Version]
- Vicentini, F.T.; Borgheti-Cardoso, L.N.; Depieri, L.V.; de Macedo Mano, D.; Abelha, T.F.; Petrilli, R.; Bentley, M.V. Delivery systems and local administration routes for therapeutic siRNA. Pharm. Res. 2013, 30, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Narenji, M.; Talaee, M.R.; Moghimi, H.R. Investigating the effects of size, charge, viscosity and bilayer flexibility on liposomal delivery under convective flow. Int. J. Pharm. 2016, 513, 88–96. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, C.H.; Huang, T.H.; Fang, J.Y. Lipid-Based Nanoparticles as a Potential Delivery Approach in the Treatment of Rheumatoid Arthritis. Nanomaterials (Basel) 2018, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Judson, I.; Radford, J.A.; Harris, M.; Blay, J.Y.; van Hoesel, Q.; le Cesne, A.; van Oosterom, A.T.; Clemons, M.J.; Kamby, C.; Hermans, C.; et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2001, 37, 870–877. [Google Scholar] [CrossRef]
- Stathopoulos, G.P.; Antoniou, D.; Dimitroulis, J.; Stathopoulos, J.; Marosis, K.; Michalopoulou, P. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2011, 68, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Jung, J.; Park, S.J.; Chung, H.K.; Kang, H.W.; Lee, S.W.; Seo, M.H.; Park, H.J.; Song, S.Y.; Jeong, S.Y.; Choi, E.K. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e77–e83. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.E.; Muflih Al Harthi, S.; Ebrahimi Shahmabadi, H.; Akbarzadeh, A. Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved Properties as an Anticancer Agent. Int. J. Mol. Sci. 2019, 20, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J.; Tian, H.; Chen, X. Pulmonary delivery by exploiting doxorubicin and cisplatin co-loaded nanoparticles for metastatic lung cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2019, 295, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Su, W.Y.; Yen, K.C.; Yang, K.C.; Lin, F.H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Wang, T.W.; Dong, G.C.; Yueh-Hsiu Wu, S.; Young, T.H.; Shieh, M.J.; Lou, P.J.; Lin, F.H. Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 2007, 28, 3996–4005. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, Z.; Li, M.; Lv, S.; Sun, H.; Deng, M.; Liu, H.; Chen, X. Polypeptide-based combination of paclitaxel and cisplatin for enhanced chemotherapy efficacy and reduced side-effects. Acta Biomater. 2014, 10, 1392–1402. [Google Scholar] [CrossRef]
- Morgan, M.T.; Carnahan, M.A.; Immoos, C.E.; Ribeiro, A.A.; Finkelstein, S.; Lee, S.J.; Grinstaff, M.W. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003, 125, 15485–15489. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.S.; Lou, P.J.; Peng, C.L.; Pai, C.L.; Yen, W.N.; Huang, M.Y.; Young, T.H.; Shieh, M.J. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release Off. J. Control. Release Soc. 2007, 122, 39–46. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Zheng, Y.; Guo, R.; Wang, S.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Shi, X. Doxorubicin-Conjugated PAMAM Dendrimers for pH-Responsive Drug Release and Folic Acid-Targeted Cancer Therapy. Pharmaceutics 2018, 10, 162. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Wang, G.; Alves, C.S.; Tomas, H.; Xiong, Z.; Shen, M.; Rodrigues, J.; Shi, X. Multifunctional Dendrimer-Entrapped Gold Nanoparticles Conjugated with Doxorubicin for pH-Responsive Drug Delivery and Targeted Computed Tomography Imaging. Langmuir ACS J. Surf. Colloids 2018, 34, 12428–12435. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Merkel, O.M.; Reineke, J.J.; da Rocha, S.R. Effect of the Route of Administration and PEGylation of Poly(amidoamine) Dendrimers on Their Systemic and Lung Cellular Biodistribution. Mol. Pharm. 2016, 13, 1866–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barash, O.; Peled, N.; Tisch, U.; Bunn, P.A., Jr.; Hirsch, F.R.; Haick, H. Classification of lung cancer histology by gold nanoparticle sensors. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Chen, D.H.; Wang, C.R.; Shiau, A.L.; Wu, C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Chattopadhyay, A.; Ghosh, S.S. Implications of silver nanoparticle induced cell apoptosis for in vitro gene therapy. Nanotechnology 2008, 19, 075104. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Sefah, K.; Bamrungsap, S.; Chang, H.T.; Tan, W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir ACS J. Surf. Colloids 2008, 24, 11860–11865. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and t2* magnetic resonance imaging. Investig. Radiol. 2011, 46, 132–140. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Behlke, M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef] [Green Version]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of siRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Koli, U.; Krishnan, R.A.; Pofali, P.; Jain, R.; Dandekar, P. siRNA-Based Therapies for Pulmonary Diseases. J. Biomed. Nanotechnol. 2014, 10, 1953–1997. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano- and microparticles for drug delivery. Glob. Cardiol. Sci. Pract. 2015, 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Paranjpe, M.; Muller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Dabbagh, A.; Abu Kasim, N.H.; Yeong, C.H.; Wong, T.W.; Abdul Rahman, N. Critical Parameters for Particle-Based Pulmonary Delivery of Chemotherapeutics. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Liu, W.; Gu, H.; Wang, D.; Wang, Y. The Transport and Deposition of Nanoparticles in Respiratory System by Inhalation. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release Off. J. Control. Release Soc. 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunov, O.; Syrovets, T.; Loos, C.; Nienhaus, G.U.; Mailänder, V.; Landfester, K.; Rouis, M.; Simmet, T. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano 2011, 5, 9648–9657. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, J.; Bao, G.; Zhang, S. Variable nanoparticle-cell adhesion strength regulates cellular uptake. Phys. Rev. Lett. 2010, 105, 138101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, X.; Gu, N. Computational Investigation of Interaction between Nanoparticles and Membranes—Hydrophobic Hydrophilic Effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. Adv. Exp. Med. Biol. 2014, 811, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Sørensen, D.R. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem. Biophys. Res. Commun. 2003, 312, 1220–1225. [Google Scholar] [CrossRef]
- Beyerle, A.; Braun, A.; Merkel, O.; Koch, F.; Kissel, T.; Stoeger, T. Comparative in vivo study of poly (ethylene imine)/siRNA complexes for pulmonary delivery in mice. J. Control. Release Off. J. Control. Release Soc. 2011, 151, 51–56. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Hafdi, N.; Behr, J.P.; Erbacher, P.; Peng, L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006, 22, 2362–2364. [Google Scholar] [CrossRef]
- Tan, S.J.; Kiatwuthinon, P.; Roh, Y.H.; Kahn, J.S.; Luo, D. Engineering Nanocarriers for siRNA Delivery. Small 2011, 7, 841–856. [Google Scholar] [CrossRef]
- Rosi, N.L.; Giljohann, D.A.; Thaxton, C.S.; Lytton-Jean, A.K.; Han, M.S.; Mirkin, C.A. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 2006, 312, 1027–1030. [Google Scholar] [CrossRef]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investig. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Preat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release Off. J. Control. Release Soc. 2014, 194, 82–91. [Google Scholar] [CrossRef]
- Al Faraj, A.; Shaik, A.S.; Shaik, A.P.; Al Sayed, B. Enhanced magnetic delivery of superparamagnetic iron oxide nanoparticles to the lung monitored using noninvasive MR. J. Nanopart. Res. 2014, 16, 2667. [Google Scholar] [CrossRef]
- Al Faraj, A.; Shaik, A.S.; Halwani, R.; Alfuraih, A. Magnetic Targeting and Delivery of Drug-Loaded SWCNTs Theranostic Nanoprobes to Lung Metastasis in Breast Cancer Animal Model: Noninvasive Monitoring Using Magnetic Resonance Imaging. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2016, 18, 315–324. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Zwier, R.; Junginger, H.E.; Borchard, G. Non-invasive pulmonary aerosol delivery in mice by the endotracheal route. Eur. J. Pharm. Biopharm. 2005, 61, 214–218. [Google Scholar] [CrossRef]
- Conde, J.; Tian, F.; Hernandez, Y.; Bao, C.; Cui, D.; Janssen, K.P.; Ibarra, M.R.; Baptista, P.V.; Stoeger, T.; de la Fuente, J.M. In vivo tumor targeting via nanoparticle-mediated therapeutic siRNA coupled to inflammatory response in lung cancer mouse models. Biomaterials 2013, 34, 7744–7753. [Google Scholar] [CrossRef]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Bonnet, M.E.; Gossart, J.B.; Benoit, E.; Messmer, M.; Zounib, O.; Moreau, V.; Behr, J.P.; Lenne-Samuel, N.; Kedinger, V.; Meulle, A.; et al. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J. Control. Release Off. J. Control. Release Soc. 2013, 170, 183–190. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Panyam, J. Polymer nanoparticles: Weighing up gene delivery. Nat. Nanotechnol. 2013, 8, 805–806. [Google Scholar] [CrossRef]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itani, R.; Al Faraj, A. siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. Int. J. Mol. Sci. 2019, 20, 6088. https://doi.org/10.3390/ijms20236088
Itani R, Al Faraj A. siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. International Journal of Molecular Sciences. 2019; 20(23):6088. https://doi.org/10.3390/ijms20236088
Chicago/Turabian StyleItani, Rasha, and Achraf Al Faraj. 2019. "siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer" International Journal of Molecular Sciences 20, no. 23: 6088. https://doi.org/10.3390/ijms20236088
APA StyleItani, R., & Al Faraj, A. (2019). siRNA Conjugated Nanoparticles—A Next Generation Strategy to Treat Lung Cancer. International Journal of Molecular Sciences, 20(23), 6088. https://doi.org/10.3390/ijms20236088




