Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4
Abstract
:1. Introduction
2. Results
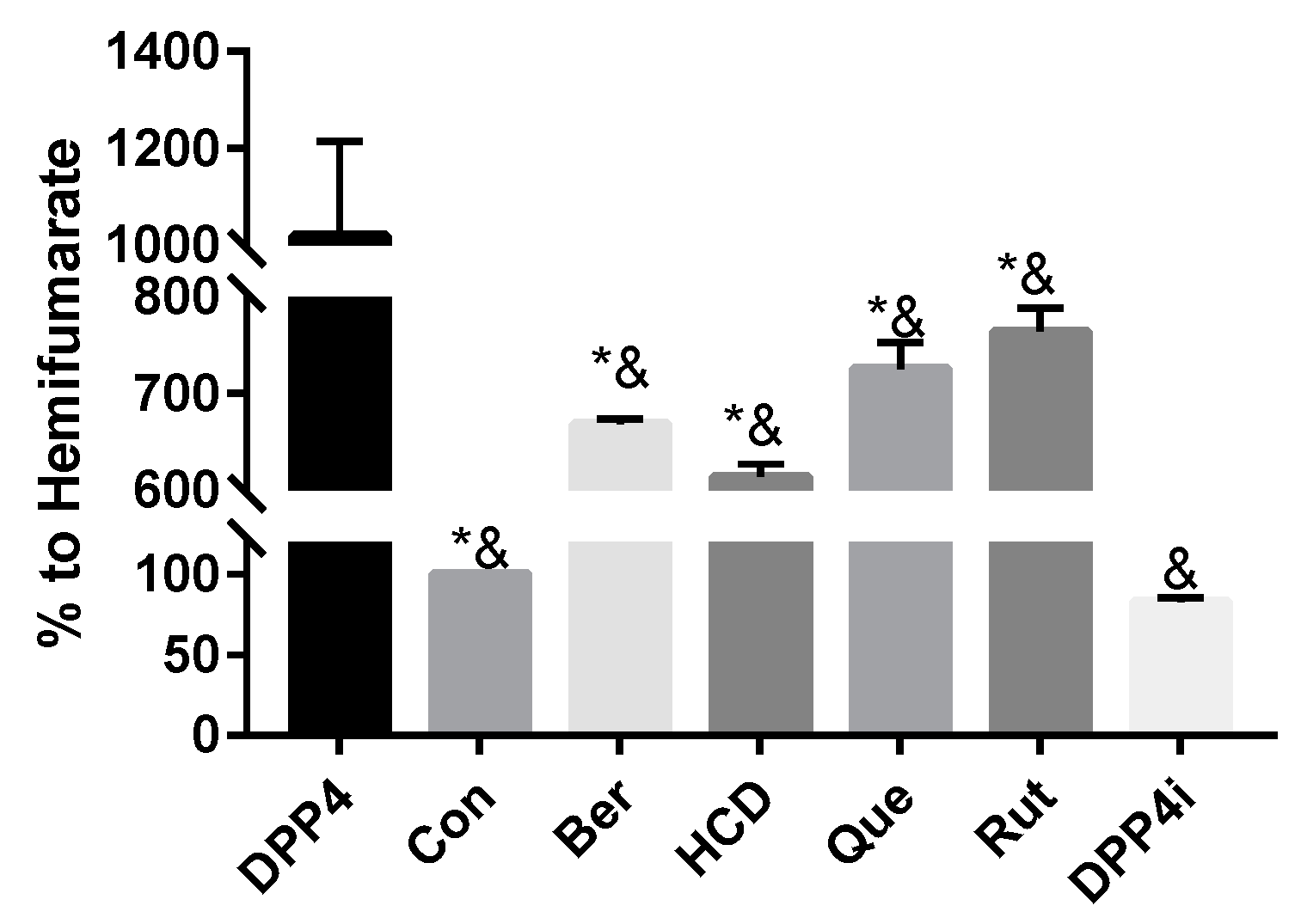
2.1. In Vitro Inhibition of DPP-4 Activity by Natural Compounds
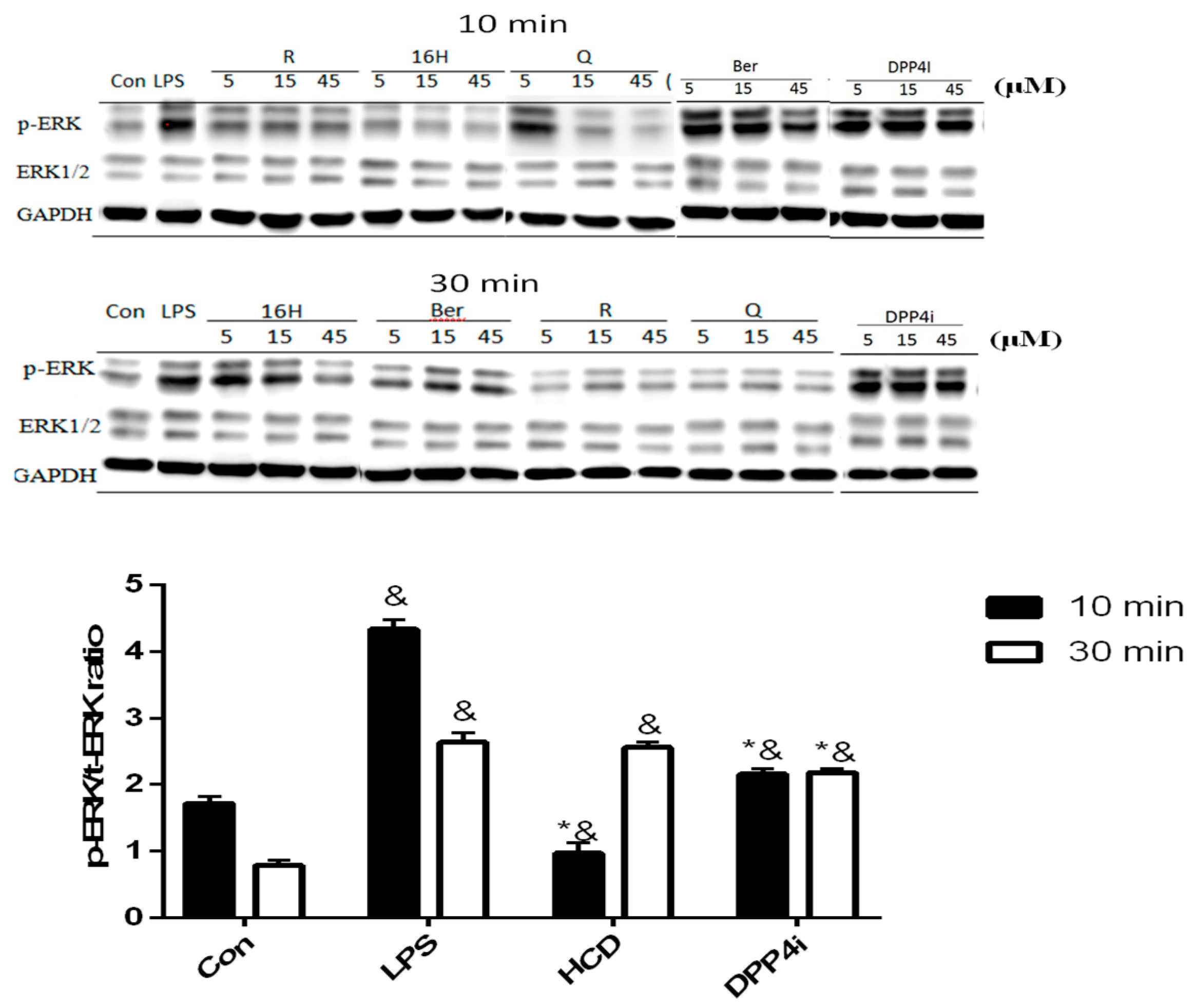
2.2. Natural Compounds against DPP-4 Expression and Downstream Signaling Pathway
2.3. Single-Dose Hypoglycemic Effect of Natural Compounds
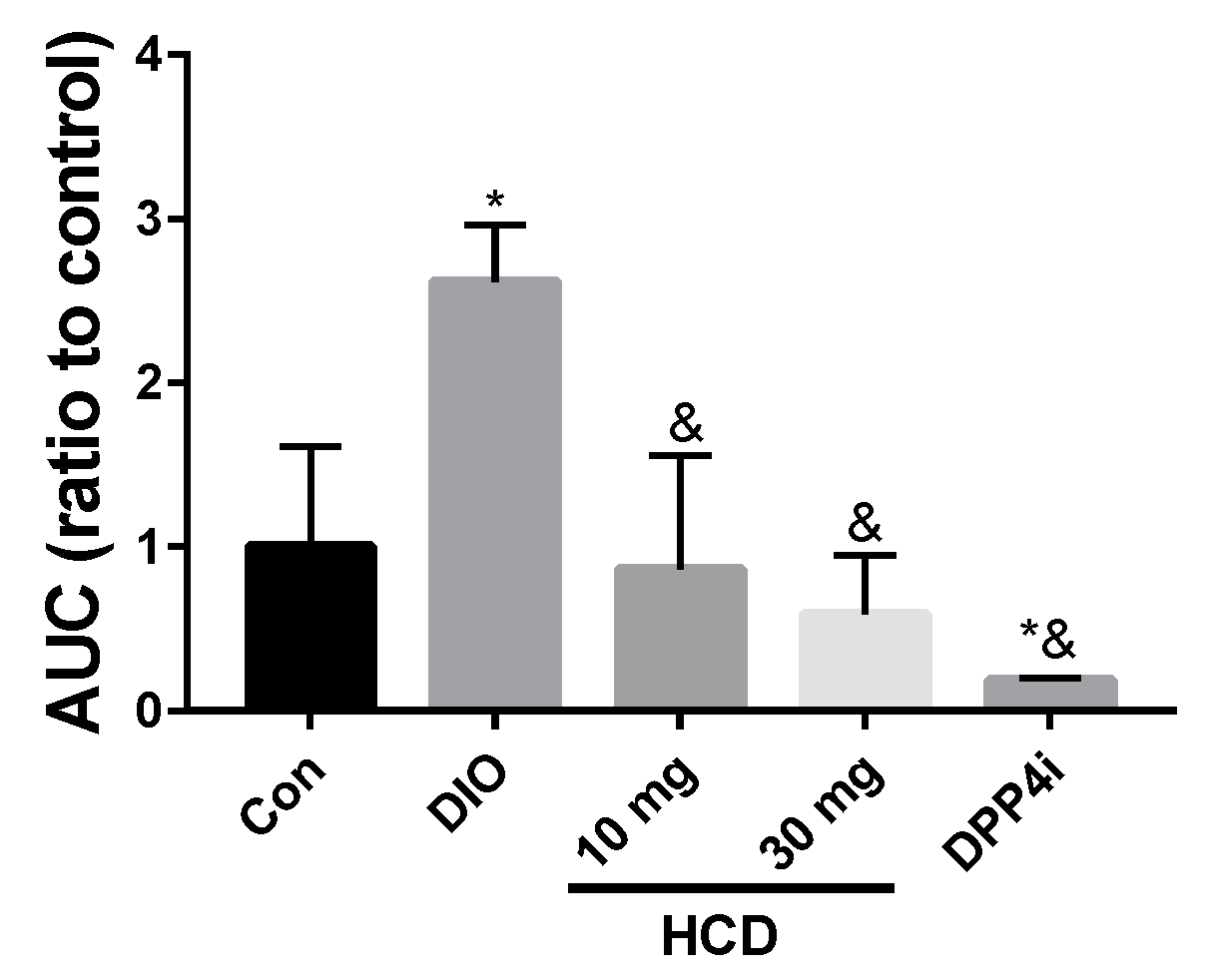
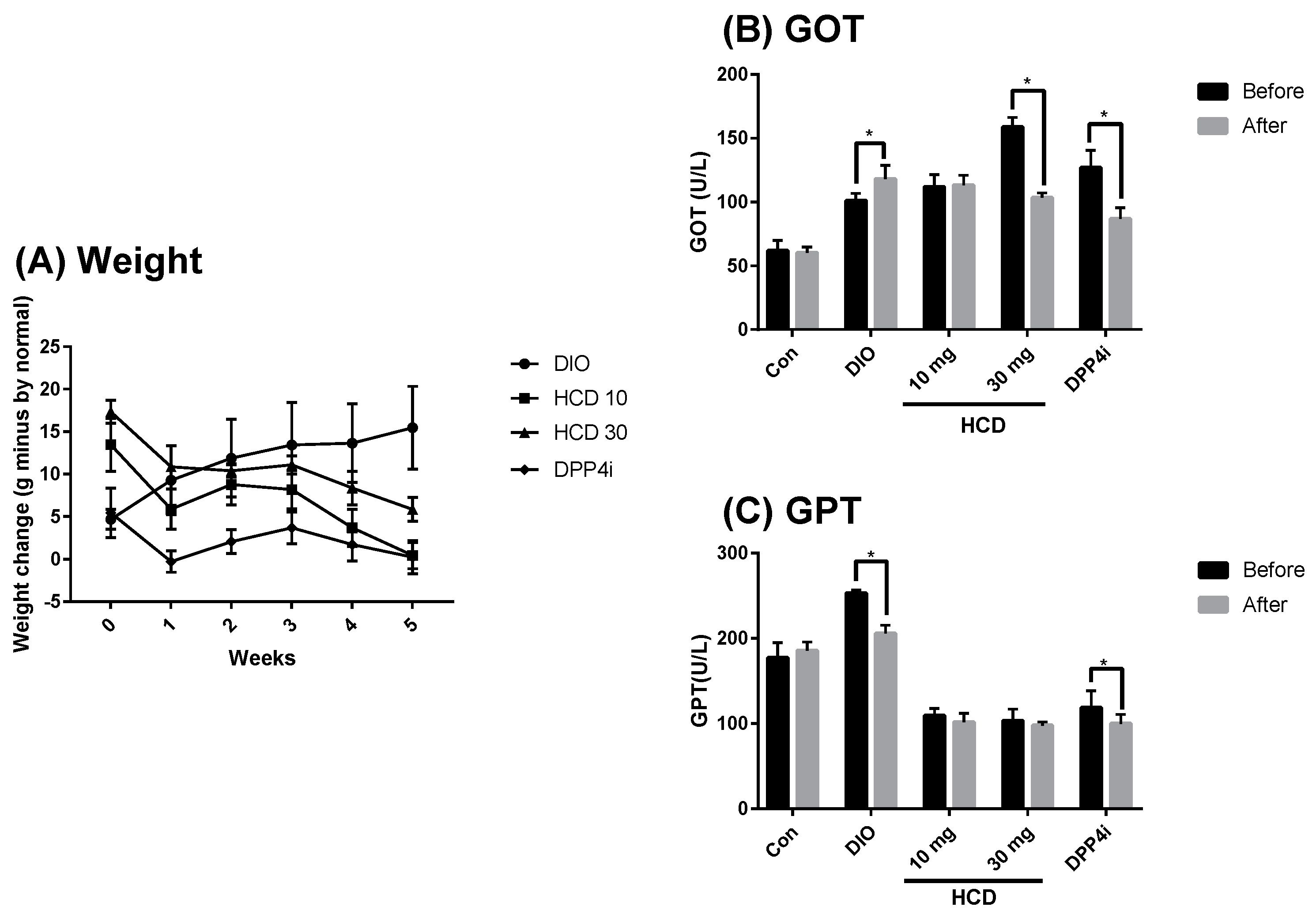
2.4. Long-Term Blood Sugar Administration of Natural Compounds
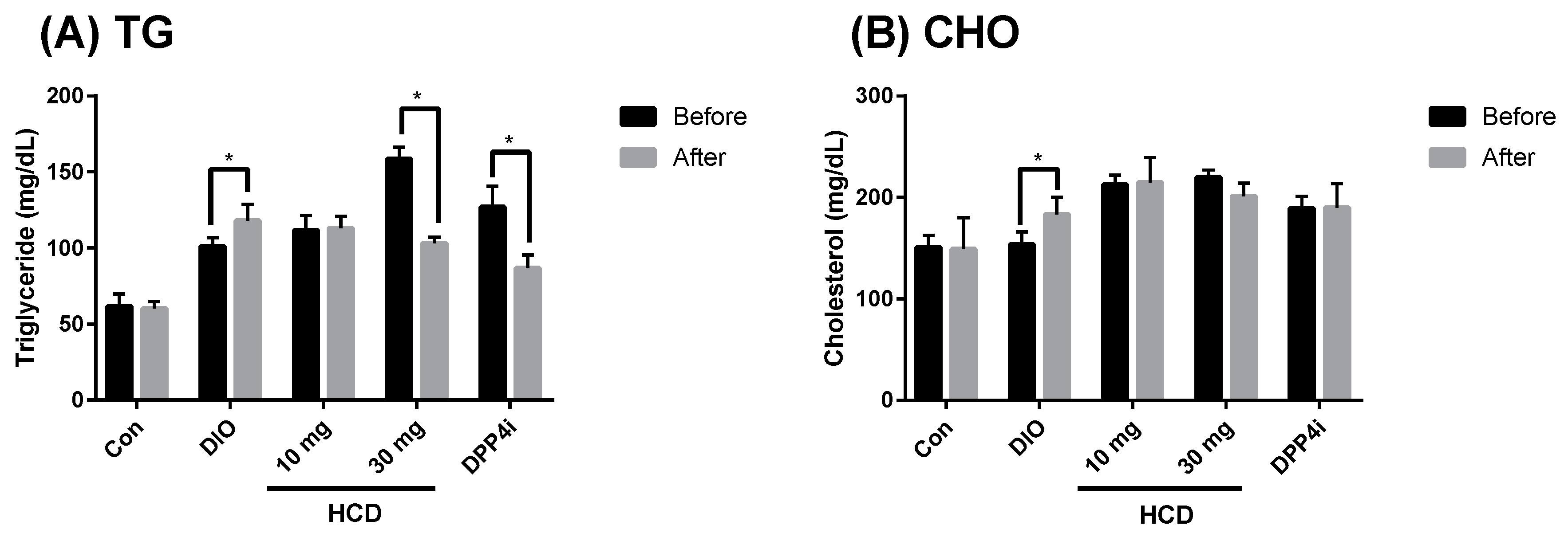
2.5. Dyslipidemia Reducing Effect in Long-Term Administration
2.6. Toxicity in Long-Term Administration
3. Discussion
4. Materials and Methods
4.1. Natural Compounds and Reagents
4.2. Western Blotting
4.3. In Vitro DPP-4 Inhibitory Assay
4.3.1. Cell Culture
4.3.2. Enzymatic Inhibition Assay
4.3.3. Cellular DPP-4 Inhibition Assay
4.4. In Vivo Animal Test
4.4.1. Animals and Obese Induction
4.4.2. Oral Glucose Tolerance Test (OGTT) and Insulin Intolerance Test
4.4.3. Single-Dose Hypoglycemic Efficacy
4.4.4. Long Term Administration
4.4.5. Blood Biochemical Detection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area under curve |
| CHO | Total cholesterol |
| DIO | Diet-induced obese |
| DPP-4 | Dipeptidyl peptidase 4 |
| ECL | Enhanced chemiluminescence |
| ERK | Extracellular signal–regulated kinases |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GLP-1 | Glucagon like peptide 1 |
| GOT | Glutamic oxaloacetic transaminase |
| GPT | Glutamic-pyruvic transaminase |
| HbA1c | Glycated hemoglobin |
| HCD | 16-hydroxycleroda-3,13-dien-15,16-olide |
| HRP | Horseradish peroxidase |
| LPS | Lipopolysaccharide |
| OGTT | Oral glucose tolerance test |
| PKA | Protein kinase A |
| PVDF | Polyvinylidene difluoride |
| TG | Triglyceride |
| TII DM | Type 2 diabetes mellitus |
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. Idf diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- DALYs, G.B.D.; Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar]
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S73–S85. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int. J. Clin. Pract. 2006, 60, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Lambeir, A.M.; Durinx, C.; Scharpe, S.; De Meester, I. Dipeptidyl-peptidase iv from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.M.; Slaitini, L.; Gysbers, V.; Riekhoff, A.G.; Kahne, T.; Knott, H.M.; De Meester, I.; Abbott, C.A.; McCaughan, G.W.; Gorrell, M.D. Soluble CD26/dipeptidyl peptidase IV enhances human lymphocyte proliferation in vitro independent of dipeptidyl peptidase enzyme activity and adenosine deaminase binding. Scand. J. Immunol. 2011, 73, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Casrouge, A.; Sauer, A.V.; Barreira da Silva, R.; Tejera-Alhambra, M.; Sanchez-Ramon, S.; ICAReB; Cancrini, C.; Ingersoll, M.A.; Aiuti, A.; Albert, M.L. Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4. Clin. Exp. Immunol. 2018, 194, 166–179. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Rohrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Al Tulaihi, B.; Alhabib, S. Uncertainties around incretin-based therapies: A literature review. Saudi Pharm. J. 2017, 25, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.R.; Fu, Y.S.; Tsai, M.J.; Cheng, H.; Weng, C.F. Natural compounds from herbs that can potentially execute as autophagy inducers for cancer therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Rognan, D. The impact of in silico screening in the discovery of novel and safer drug candidates. Pharmacol. Ther. 2017, 175, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of dipeptidyl peptidase IV. Sci. Rep. 2018. under press. [Google Scholar]
- Darmoul, D.; Lacasa, M.; Baricault, L.; Marguet, D.; Sapin, C.; Trotot, P.; Barbat, A.; Trugnan, G. Dipeptidyl peptidase IV (CD 26) gene expression in enterocyte-like colon cancer cell lines HT-29 and Caco-2. Cloning of the complete human coding sequence and changes of dipeptidyl peptidase IV mRNA levels during cell differentiation. J. Biol. Chem. 1992, 267, 4824–4833. [Google Scholar] [PubMed]
- Hasan, A.A.; Hocher, B. Role of soluble and membrane-bound dipeptidyl peptidase-4 in diabetic nephropathy. J. Mol. Endocrinol. 2017, 59, R1–R10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wronkowitz, N.; Gorgens, S.W.; Romacho, T.; Villalobos, L.A.; Sanchez-Ferrer, C.F.; Peiro, C.; Sell, H.; Eckel, J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim. Biophys. Acta 2014, 1842, 1613–1621. [Google Scholar] [CrossRef] [Green Version]
- Omar, B.A.; Liehua, L.; Yamada, Y.; Seino, Y.; Marchetti, P.; Ahren, B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia 2014, 57, 1876–1883. [Google Scholar] [CrossRef] [Green Version]
- Dalle, S.; Burcelin, R.; Gourdy, P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic beta-cell impairments in type 2 diabetes. Cell. Signal. 2013, 25, 570–579. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef]
- Khatoon, A.; Rashid, I.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Pathak, N.; Mir, S.S.; Ahmad, K.; Hussain, T.; Srivastava, P. Adncd: A compendious database on anti-diabetic natural compounds focusing on mechanism of action. 3 Biotech 2018, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Wang, H.J.; Chiang, B.H. Anti-diabetic effect of a traditional Chinese medicine formula. Food Funct. 2012, 3, 1161–1169. [Google Scholar] [CrossRef]
- Kalhotra, P.; Chittepu, V.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Structure(-)activity relationship and molecular docking of natural product library reveal chrysin as a novel dipeptidyl peptidase-4 (DPP-4) inhibitor: An integrated in silico and in vitro study. Molecules 2018, 23, 1368. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Song, L.; Zhang, J.F.; Shen, Z.F.; Liu, Q.; Liu, S.N.; Zheng, W.S.; Yao, C.S. Cyanogenetic glycosides and simple glycosides from the linseed meal. Fitoterapia 2015, 106, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Le, D.D.; Nguyen, P.H.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Shin, H.M.; Rhee, H.I.; Woo, M.H. PTB1B, α-glucosidase, and DPP-IV inhibitory effects for chromene derivatives from the leaves of Smilax china L. Chem. Biol. Interact. 2016, 253, 27–37. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Soybean- and lupin-derived peptides inhibit DPP-IV activity on in situ human intestinal Caco-2 cells and ex vivo human serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chen, C.R.; Wu, W.H.; Wen, C.L.; Chang, C.I.; Hou, W.C. Anti-alpha-glucosidase and anti-dipeptidyl peptidase-IV activities of extracts and purified compounds from Vitis thunbergii var. Taiwaniana. J. Agric. Food Chem. 2015, 63, 6393–6401. [Google Scholar] [CrossRef]
- Saleem, S.; Jafri, L.; ul Haq, I.; Chang, L.C.; Calderwood, D.; Green, B.D.; Mirza, B. Plants Fagonia cretica L. and Hedera nepalensis K. Koch contain natural compounds with potent dipeptidyl peptidase-4 (DPP-4) inhibitory activity. J. Ethnopharmacol. 2014, 156, 26–32. [Google Scholar] [CrossRef]
- Suman, R.K.; Mohanty, I.R.; Maheshwari, U.; Borde, M.K.; Deshmukh, Y.A. Natural dipeptidyl peptidase-iv inhibitor mangiferin mitigates diabetes- and metabolic syndrome-induced changes in experimental rats. Diabetes Metab. Syndr. Obes. 2016, 9, 261–272. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Fan, H.; Wu, P.; Zhang, F.; Zhang, C.; Liu, W.; Li, M. Screening of a natural compound library identifies emodin, a natural compound from Rheum palmatum linn. that inhibits DPP4. Peer J. 2017, 5, e3283. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Bhandari, K.; Singla, R.K.; Katakam, P.; Samanta, T.; Kushwaha, D.K.; Gundamaraju, R.; Mitra, A. Chemometrics optimized extraction procedures, phytosynergistic blending and in vitro screening of natural enzyme inhibitors amongst leaves of tulsi, banyan and jamun. Pharmacogn. Mag. 2015, 11, S522–S532. [Google Scholar] [PubMed]
- Dey, B.; Mitra, A.; Katakam, P.; Singla, R.K. Exploration of natural enzyme inhibitors with hypoglycemic potentials amongst Eucalyptus spp. by in vitro assays. World J. Diabetes 2014, 5, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sila, A.; Alvarez, O.M.; Haddar, A.; Frikha, F.; Dhulster, P.; Nedjar-Arroume, N.; Bougatef, A. Purification, identification and structural modelling of DPP-IV inhibiting peptides from barbel protein hydrolysate. J. Chromatogr. B 2016, 1008, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katkar, K.V.; Suthar, A.C.; Chauhan, V.S. The chemistry, pharmacologic, and therapeutic applications of Polyalthia longifolia. Pharmacogn. Rev. 2010, 4, 62–68. [Google Scholar] [PubMed]
- Wu, T.H.; Cheng, Y.Y.; Chen, C.J.; Ng, L.T.; Chou, L.C.; Huang, L.J.; Chen, Y.H.; Kuo, S.C.; El-Shazly, M.; Wu, Y.C.; et al. Three new clerodane diterpenes from Polyalthia longifolia var. Pendula. Molecules 2014, 19, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.T.; Hsu, Y.Y.; Chang, F.R.; Wu, Y.C.; Lo, Y.C. 6-hydroxycleroda-3,13-dien-15,16-olide protects neuronal cells from lipopolysaccharide-induced neurotoxicity through the inhibition of microglia-mediated inflammation. Planta Med. 2010, 76, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, C.C.; Chang, F.R.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-hydroxycleroda-3,13-dien-15,16-olide regulates the expression of histone-modifying enzymes PRC2 complex and induces apoptosis in CML K562 cells. Life Sci. 2011, 89, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, W.C.; Huang, B.M.; Chia, Y.C.; Chen, Y.C.; Chen, Y.C. 16-hydroxycleroda-3, 13-dien-15, 16-olide inhibits the proliferation and induces mitochondrial-dependent apoptosis through Akt, mTOR, and MEK-ERK pathways in human renal carcinoma cells. Phytomedicine 2017, 36, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.F.; Lin, S.R.; Tseng, F.J.; Huang, Y.C.; Tsai, M.J.; Fu, Y.S.; Weng, C.F. The autophagic inhibition oral squamous cell carcinoma cancer growth of 16-hydroxy-cleroda-3,13-dien-15,16-olide. Oncotarget 2017, 8, 78379–78396. [Google Scholar] [PubMed]
- Thiyagarajan, V.; Sivalingam, K.S.; Viswanadha, V.P.; Weng, C.F. 16-hydroxy-cleroda-3,13-dien-16,15-olide induced glioma cell autophagy via ROS generation and activation of p38 MAPK and ERK-1/2. Environ. Toxicol. Pharmacol. 2016, 45, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, B.K.; Wang, P.C.; Weng, C.F. 16-hydroxycleroda-3,13-dien-15,16-olide and n-methyl-actinodaphne potentiate tamoxifen-induced cell death in breast cancer. Molecules 2018, 23, 1966. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, B.M.; Lee, W.C.; Chen, Y.C. 16-hydroxycleroda-3,13-dien-15,16-olide induces anoikis in human renal cell carcinoma cells: Involvement of focal adhesion disassembly and signaling. Onco Targets Ther. 2018, 11, 7679–7690. [Google Scholar] [CrossRef] [PubMed]
- Riyaphan, J.; Jhong, C.H.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Sung, P.J.; Leong, M.K.; Weng, C.F. Hypoglycemic efficacy of docking selected natural compounds against alpha-glucosidase and alpha-amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef] [PubMed]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.K.; Lin, S.X.; Tsai, M.J.; Leong, M.K.; Lin, S.R.; Kankala, R.K.; Lee, C.H.; Weng, C.F. Encapsulation of 16-hydroxycleroda-3,13-dien-16,15-olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycemia in diabetic mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Lin, S.X.; Lee, C.H.; Weng, C.F. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surf. B 2016, 141, 120–131. [Google Scholar] [CrossRef]
- Meng, F.C.; Wu, Z.F.; Yin, Z.Q.; Lin, L.G.; Wang, R.; Zhang, Q.W. Coptidis rhizoma and its main bioactive components: Recent advances in chemical investigation, quality evaluation and pharmacological activity. Chin. Med. 2018, 13, 13. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 377–387. [Google Scholar] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-K.; Lin, S.-R.; Riyaphan, J.; Fu, Y.-S.; Weng, C.-F. Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4. Int. J. Mol. Sci. 2019, 20, 530. https://doi.org/10.3390/ijms20030530
Huang P-K, Lin S-R, Riyaphan J, Fu Y-S, Weng C-F. Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4. International Journal of Molecular Sciences. 2019; 20(3):530. https://doi.org/10.3390/ijms20030530
Chicago/Turabian StyleHuang, Po-Kai, Shian-Ren Lin, Jirawat Riyaphan, Yaw-Syan Fu, and Ching-Feng Weng. 2019. "Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4" International Journal of Molecular Sciences 20, no. 3: 530. https://doi.org/10.3390/ijms20030530
APA StyleHuang, P.-K., Lin, S.-R., Riyaphan, J., Fu, Y.-S., & Weng, C.-F. (2019). Polyalthia Clerodane Diterpene Potentiates Hypoglycemia via Inhibition of Dipeptidyl Peptidase 4. International Journal of Molecular Sciences, 20(3), 530. https://doi.org/10.3390/ijms20030530





