Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity of L. difformis Extracts in B16F10 Cells
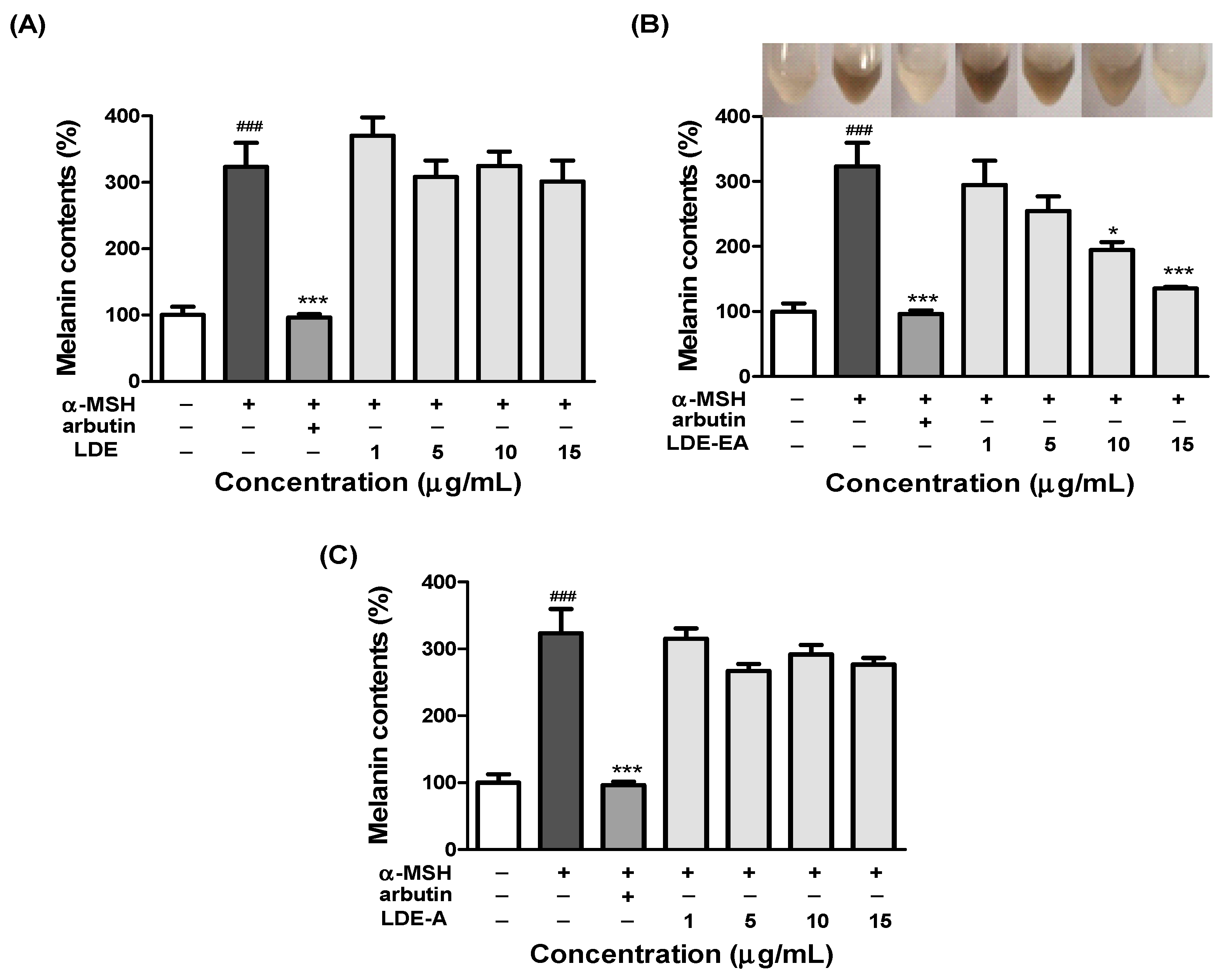
2.2. Effects of L. difformis Extracts on the Melanin Synthesis
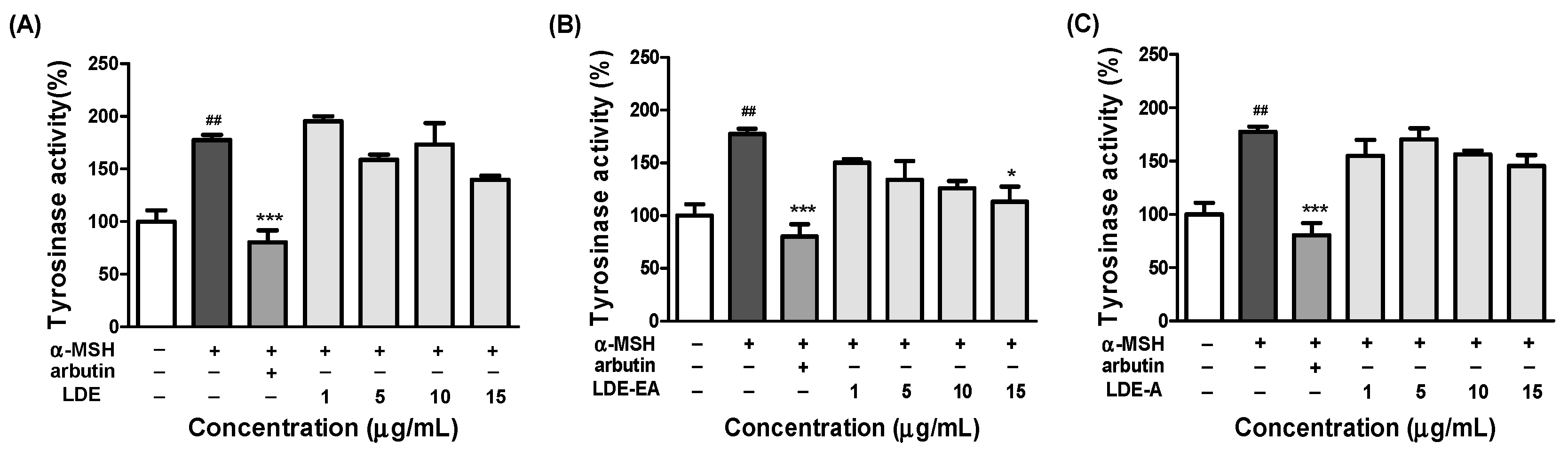
2.3. Effects of the L. difformis Extracts on Tyrosinase Activity
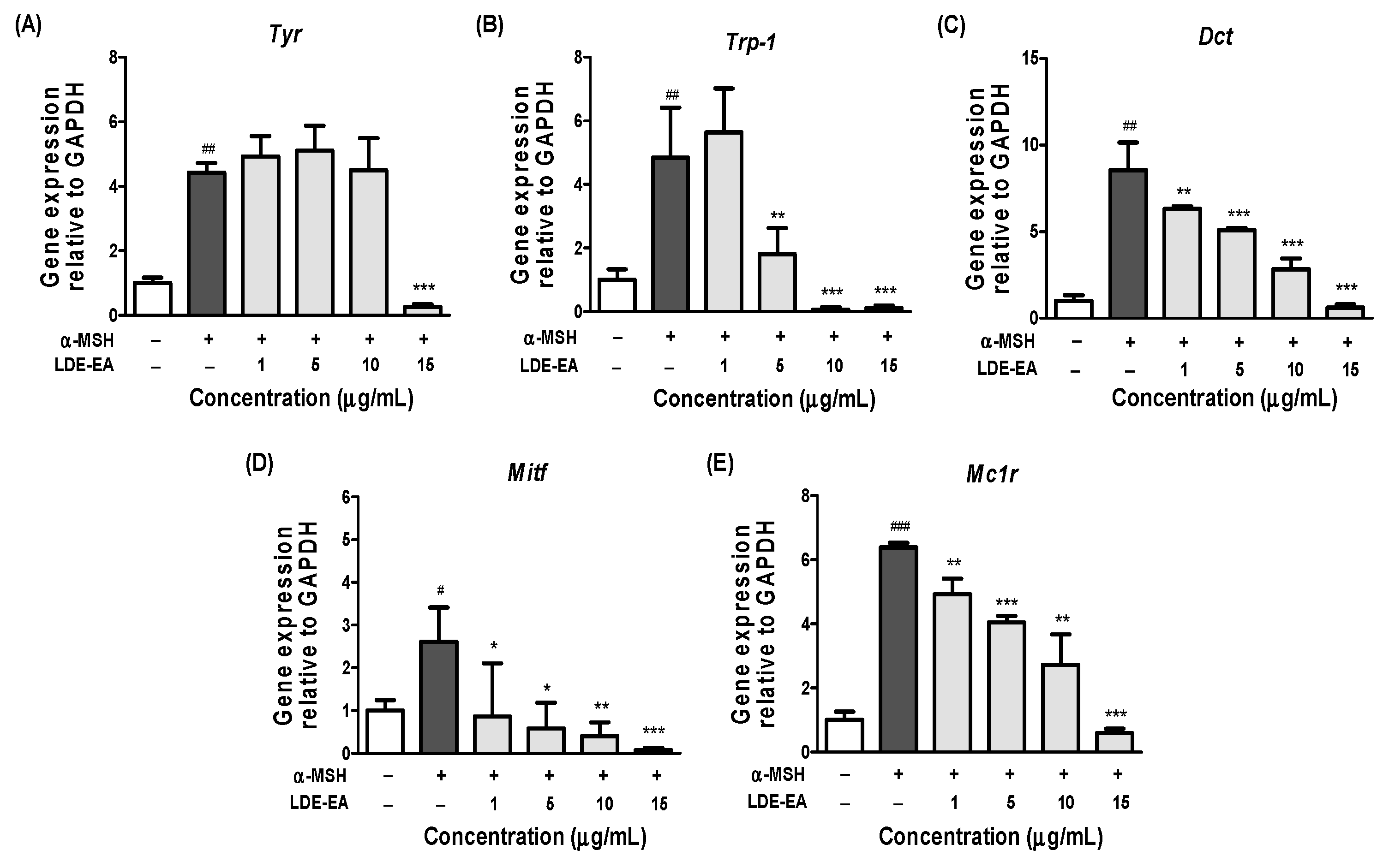
2.4. Effects of LDE-EA on Melanogenesis-Related Gene Expression
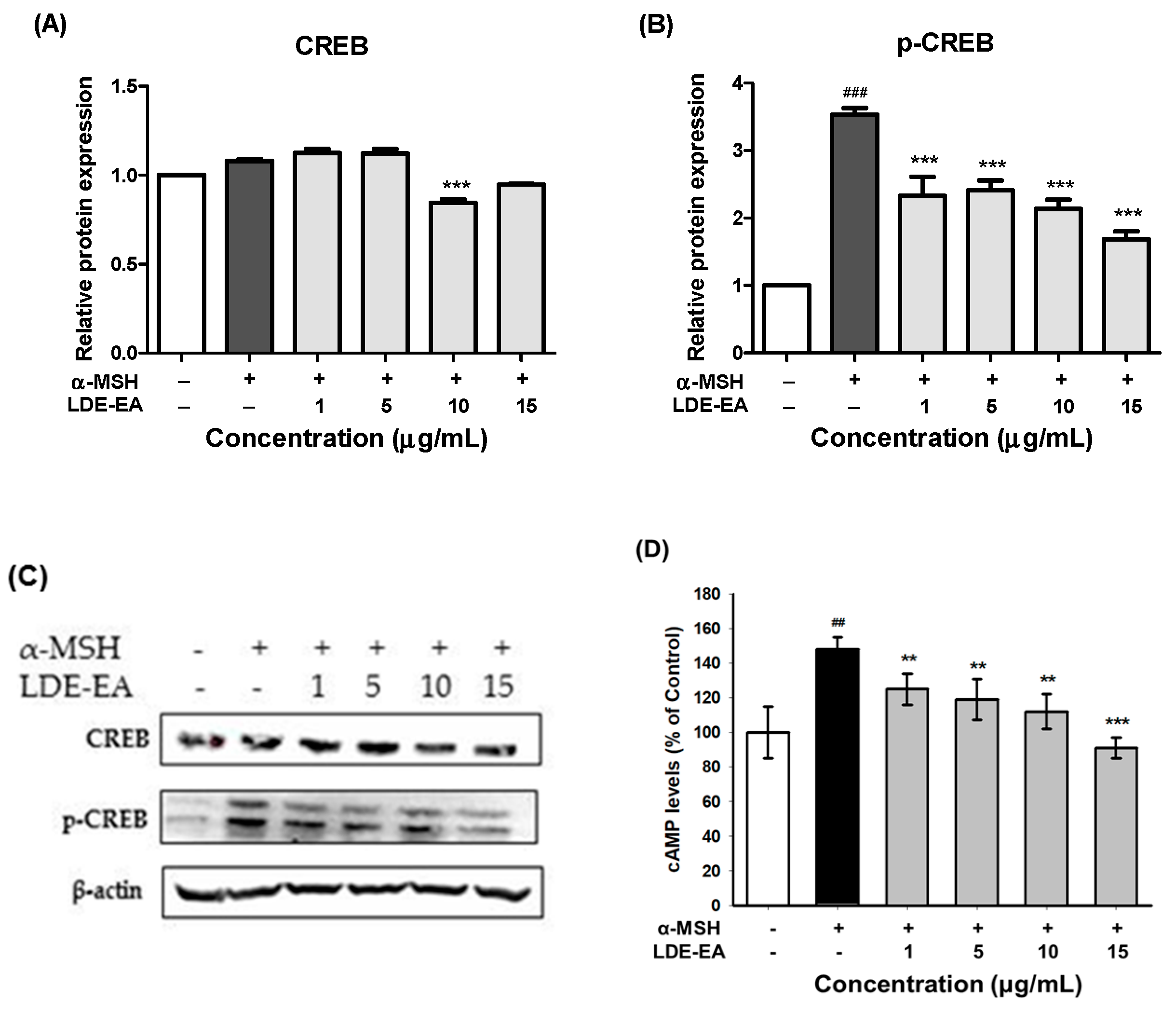
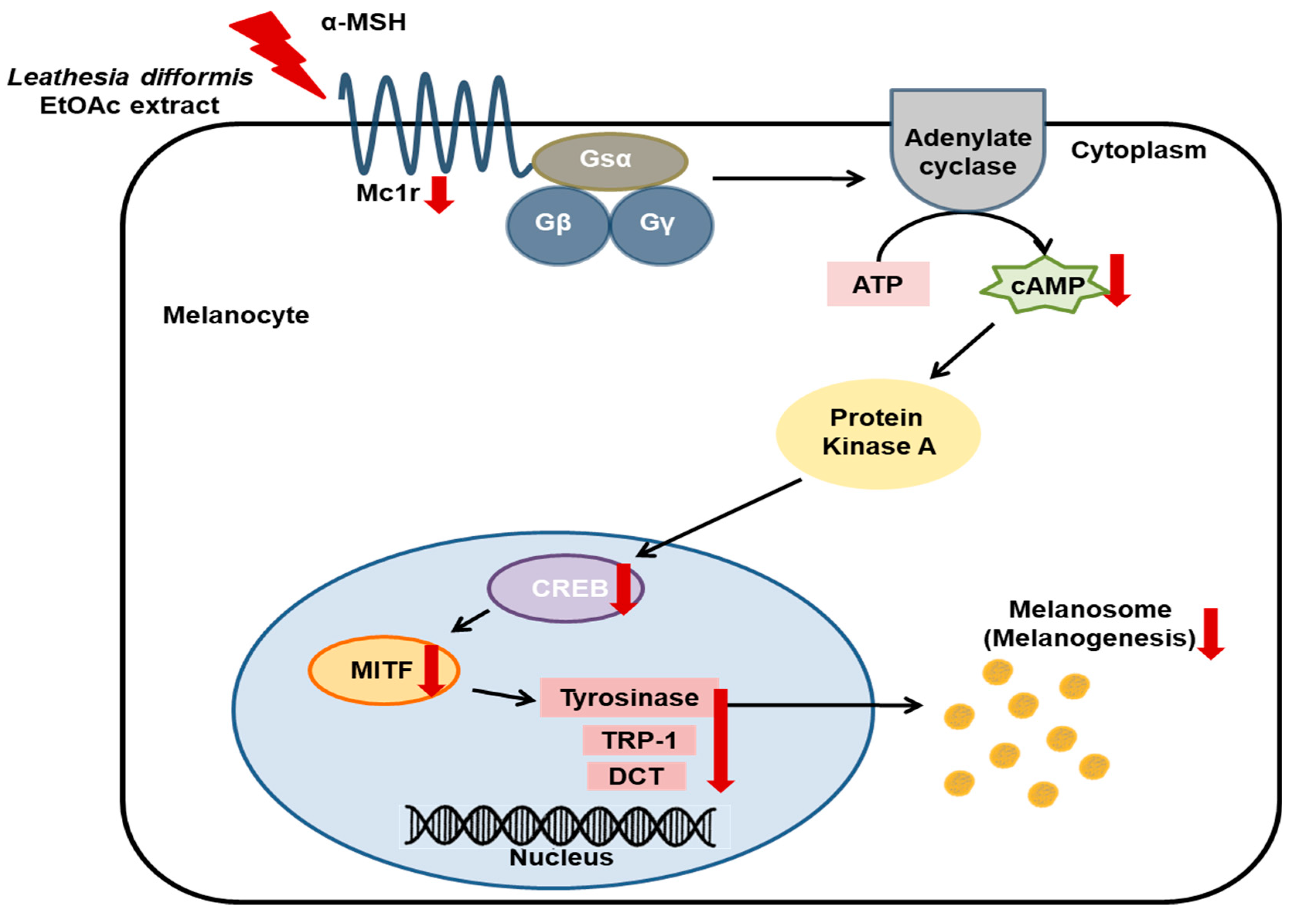
2.5. Effects of LDE-EA on the Melanogenesis-Related Signaling Pathway
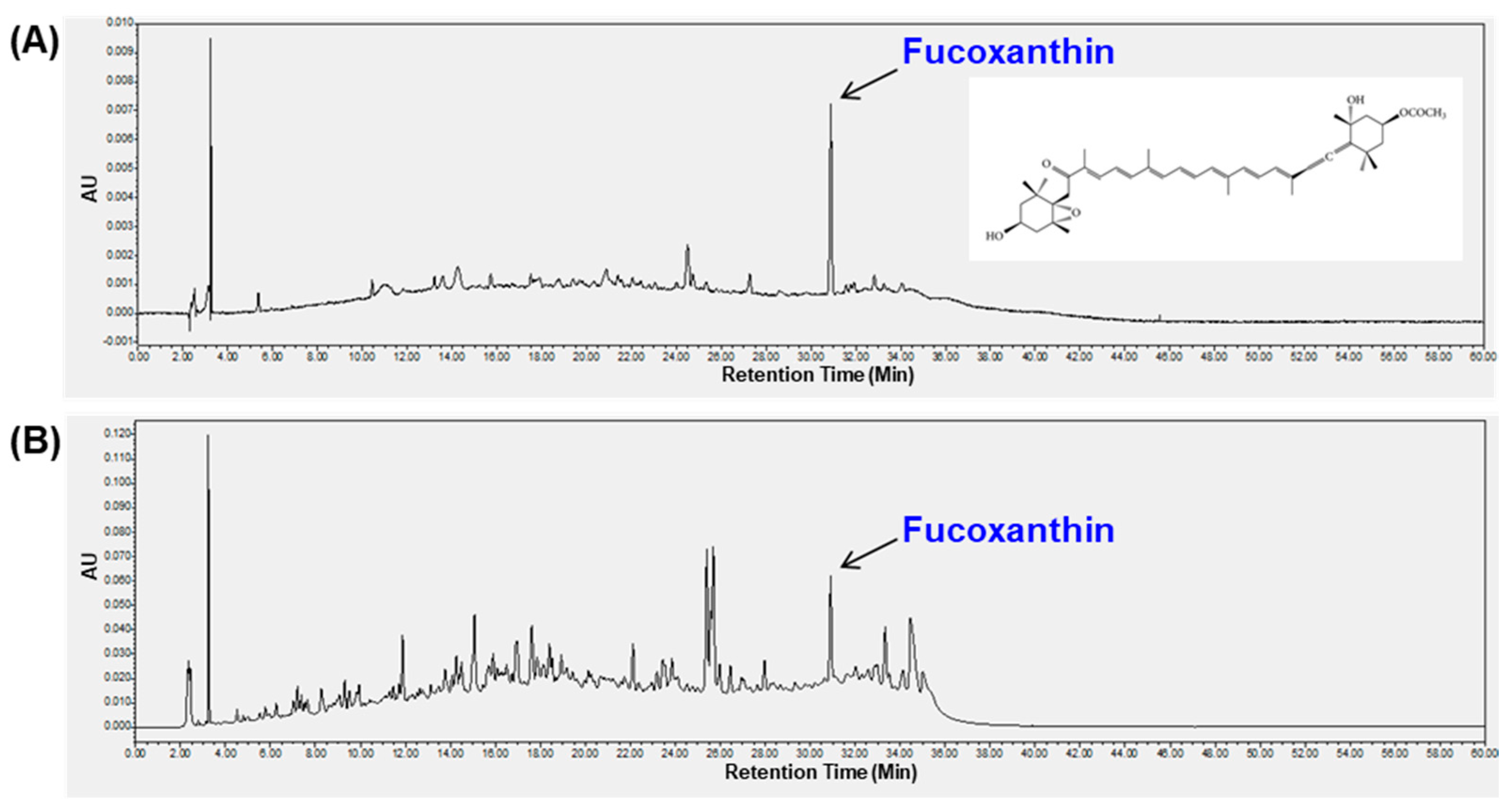
2.6. Analysis of Fucoxanthin in LDE-EA by HPLC
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of L. difformis Extract
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Measurement of Melanin Contents
4.6. Cellular Tyrosinase Activity
4.7. RNA Isolation and Real—Time PCR
4.8. Western Blot Analysis
4.9. cAMP Measurement Assay
4.10. HPLC Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| L. difformis | Leathesia difformis (L.) Areschoug |
| α-MSH | α-melanocyte stimulating hormone |
| LDE | extract of L. difformis using 80% ethanol |
| LDE-EA | ethyl acetate fraction of LDE |
| LDE-A | water fraction of LDE |
| Mitf | microphthalmia-associated transcription factor |
| Tyr | tyrosinase |
| Trp-1 | tyrosinase-related protein-1 |
| Dct | dopachrome tautomerase |
| Mc1r | melanocortin 1 receptor |
| Gapdh | glyceraldehyde 3-phosphate dehydrogenase |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| CREB | cAMP-response element binding |
| p-CREB | phosphorylated cAMP-response element binding |
| DOPA | 3,4-dihydroxy phenylalanine |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra -zolium bromide |
| EtOH | ethanol |
| EtOAc | ethyl acetate |
| DMSO | dimethyl sulfoxide |
| PBS | phosphate-buffer saline |
| BCA | bicinchoninic acid |
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.G.; Nair, N.; Begum, G.; Joshi, N.B.; Sinkar, V.P.; Vora, S. Melanocyte–keratinocyte interaction induces calcium signalling and melanin transfer to keratinocytes. Pigm. Cell Res. 2007, 20, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Adv. Dermatol. Allergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kosmadaki, M.; Yaar, M.; Gilchrest, B. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-W.; Han, J.-M.; Yoon, H.-J.; Ko, W.-S. Inhibitory effects of methanol extract of kaempferia galanga on melanogenesis in b16/f10 melanoma cells. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2013, 26, 1–18. [Google Scholar] [CrossRef]
- Lee, Y.; Ku, B.; Kim, D.; Choi, E.-M. Umbelliferone stimulated melanogenesis and increased glutathione level in b16f10 cells. Toxicol. Environ. Health Sci. 2017, 9, 152–160. [Google Scholar] [CrossRef]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. Anais Brasileiros de Dermatologia 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Kim, H.-N.; Kim, Y.-R.; Kim, B.-W.; Choi, Y.-H.; Choi, B.-T. Studies of inhibitory mechanism on melanogenesis by partially purified asiasari radix in α-msh stimulated b16f10 melanoma cells. J. Life Sci. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Le Pape, E.; Wakamatsu, K.; Ito, S.; Wolber, R.; Hearing, V.J. Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigm. Cell Melanoma Res. 2008, 21, 477–486. [Google Scholar] [CrossRef]
- Oh, S.W.; Park, S.-H.; Lee, H.S.; Kang, M.; Lee, S.E.; Yoo, J.A.; Cho, J.Y.; Lee, J. Melanogenic mechanism of ethanolic extract of dalbergia odorifera. Mol. Cell. Toxicol. 2017, 13, 453–459. [Google Scholar] [CrossRef]
- Park, H.-Y.; Wu, C.; Yonemoto, L.; Murphy-Smith, M.; Wu, H.; Stachur, C.M.; Gilchrest, B.A. Mitf mediates camp-induced protein kinase c-β expression in human melanocytes. Biochem. J. 2006, 395, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Sung, B.-G.; Lee, J.-C.; Lee, B.-K.; Woo, W.-H.; Lim, K.-S. Inhibitory effect of belamcandae rhizoma on the melanogenesis in msh-stimulated b16f10 cells. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2011, 24, 25–35. [Google Scholar]
- Gillbro, J.; Olsson, M. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Imokawa, G.; Bennett, D.C.; Hearing, V.J. Tyrosinase stabilization by tyrp1 (the brown locus protein). J. Biol. Chem. 1998, 273, 31801–31805. [Google Scholar] [CrossRef] [PubMed]
- Orlow, S.J.; Zhou, B.-K.; Drucker, M.; Pifko-Hirst, S.; Chakraborty, A.K.; Pawelek, J.M. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: Evidence for a melanogenic complex. J. Investig. Dermatol. 1994, 103, 196–201. [Google Scholar] [CrossRef]
- Curto, E.V.; Kwong, C.; Hermersdörfer, H.; Glatt, H.; Santis, C.; Virador, V.; Hearing Jr, V.J.; Dooley, T.P. Inhibitors of mammalian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999, 57, 663–672. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–368. [Google Scholar]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigm. Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. Hydroquinone-induced genotoxicity and oxidative DNA damage in hepg2 cells. Chem. Biol. Interact. 2008, 173, 1–8. [Google Scholar] [CrossRef]
- Tse, T.W. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop? J. Dermatol. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Kim, K.N.; Lee, K.W.; Song, C.B.; Jeon, Y.J. Cytotoxic Activities of Green and Brown Seaweeds Collected from Jeju Island against Four Tumor Cell Lines. J. Food Sci. Nutr. 2006, 11, 17–24. [Google Scholar] [CrossRef]
- Feldman, S.; Reynaldi, S.; Stortz, C.; Cerezo, A.; Damonte, E. Antiviral properties of fucoidan fractions from leathesia difformis. Phytomedicine 1999, 6, 335–340. [Google Scholar] [CrossRef]
- Lim, J.-H.; Jung, K.-S.; Lee, J.-S.; Jung, E.-S.; Kim, D.-K.; Kim, Y.-S.; Kim, Y.-W.; Park, D.-H. The study on antimicrobial and antifungal activity of the wild seaweeds of jeju island. J. Soc. Cosmet. Sci. Korea 2008, 34, 201–207. [Google Scholar]
- Muhammad, S.A.; Muhammad, J.; Muhammad, S.; Muhammad, K.P.; Shaista, H.; Viqar, U.A. Metabolites of marine algae collected from karachi-coasts of arabian sea. Nat. Prod. Sci. 2000, 6, 61–65. [Google Scholar]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.-J.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Antioxidant effects of phlorotannins isolated from ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Park, P.-J.; Park, E.-J.; Cho, S.K.; Kim, S.-K.; Jeon, Y.-J. Antioxidative effect of proteolytic hydrolysates from ecklonia cava on radical scavenging using esr and H2O2-induced DNA damage. Food Sci. Biotechnol. 2005, 14, 614–620. [Google Scholar]
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase a2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66. [Google Scholar] [CrossRef]
- Whitaker, D.M.; Carlson, G.P. Anti-inflammation mechanism of extract from eisenia bicyclis (kjellman) setchell. J. Pharm. Sci. 1975, 64, 1258–1259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jimenez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; Garcia-Borron, J.; Hearing, V. Tyrosinase related protein 1 (trp1) functions as a dhica oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Funasaka, Y.; Slominski, A.; Ermak, G.; Hwang, J.; Pawelek, J.M.; Ichihashi, M. Production and release of proopiomelanocortin (pomc) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet b. BBA Mol. Cell Res. 1996, 1313, 130–138. [Google Scholar] [CrossRef]
- Saha, B.; Singh, S.K.; Sarkar, C.; Bera, R.; Ratha, J.; Tobin, D.J.; Bhadra, R. Activation of the mitf promoter by lipid-stimulated activation of p38-stress signalling to creb. Pigm. Cell Melanoma Res. 2006, 19, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Ballotti, R. Cyclic amp a key messenger in the regulation of skin pigmentation. Pigm. Cell Melanoma Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. Mitf: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Loir, B.; Pérez, S.C.; Ghanem, G.; Lozano, J.; Garcia-Borron, J.; Jimenez-Cervantes, C. Expression of the mc1 receptor gene in normal and malignant human melanocytes. A semiquantitative rt-pcr study. Cell. Mol. Biol. (Noisy-Le-Grandfrance) 1999, 45, 1083–1092. [Google Scholar]
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-S.; Park, H.-Y.; Nam, K.-H. Whitening effects of 4-hydroxyphenethyl alcohol isolated from water boiled with Hizikia fusiformis. Food Sci. Biotechnol. 2014, 23, 555–560. [Google Scholar] [CrossRef]
- Joung, E.-J.; Gwon, W.-G.; Shin, T.; Jung, B.-M.; Choi, J.; Kim, H.-R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Azam, M.S.; Joung, E.-J.; Choi, J.; Kim, H.-R. Ethanolic extract from Sargassum serratifolium attenuates hyperpigmentation through CREB/ERK signaling pathways in α-MSH-stimulated B16F10 melanoma cells. J. Appl. Phycol. 2017, 29, 2089–2096. [Google Scholar] [CrossRef]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Xu, X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.H.; Qian, Z.J.; Choi, I.W.; Jung, W.K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Jeong, H.O.; Chung, H.Y.; Woo, H.C.; Choi, J.S. Promising antidiabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclisand Undaria pinnatifida. Fish. Sci. 2012, 78, 1321–1329. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Yoon, H.D.; Kim, J.I.; Choi, J.S.; Byun, D.S.; Kim, H.R. Dioxinodehydroeckol inhibits melanin synthesis through PI3K/Akt signalling pathway in alpha-melanocyte-stimulating hormone-treated B16F10 cells. Exp. Dermatol. 2012, 21, 471–473. [Google Scholar] [CrossRef]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Ahn, G.N.; Roh, S.W.; Lee, W.; Kim, D.K.; Jeon, Y.J. Whitening Effect of Octaphlorethol A Isolated from Ishige foliacea in an In Vivo Zebrafish Model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK Activation by Fucoidan Leads to Inhibition of Melanogenesis in Mel-Ab Cells. Korean J. Physiol. Pharmacol. 2015, 19, 29–34. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Kim, M.-J.; Moon, J.-Y.; Kang, H.-J.; Kim, G.-O.; Lee, N.H.; Hyun, C.-G. Effect of palmitoleic acid on melanogenic protein expression in murine b16 melanoma. J. Oleo Sci. 2010, 59, 315–319. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, D.S.; Yoon, H.-S.; Lee, W.J.; Lee, N.H.; Hyun, C.-G. Melanogenesis inhibitory activity of korean undaria pinnatifida in mouse b16 melanoma cells. Interdiscip. Toxicol. 2014, 7, 89–92. [Google Scholar] [CrossRef]

| Target Gene | Sequence | |
|---|---|---|
| Tyr | Forward | AAGAATGCTGCCCACCATGG |
| Reverse | CACGGTCATCCACCCCTTTG | |
| Trp-1 | Forward | CAGTGCAGCGTCTTCCTGAG |
| Reverse | TTCCCGTGGGAGCACTGTAA | |
| Dct | Forward | GATGGCGTGCTGAACAAGGA |
| Reverse | ATAAGGGCCACTCCAGGGTC | |
| Mitf | Forward | ATCCCATCCACCGGTCTCTG |
| Reverse | CCGTCCGTGAGATCCAGAGT | |
| Mc1r | Forward | TCATCGTCCTCTGCCCTCAG |
| Reverse | GCAGCACCTCCTTGAGTGTC | |
| Gapdh | Forward | TTGGCATTGTGGAAGGGCTC |
| Reverse | ACCAGTGGATGCAGGGATGA | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, G.-Y.; Ha, Y.; Park, A.-H.; Kwon, O.W.; Kim, Y.-J. Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 536. https://doi.org/10.3390/ijms20030536
Seo G-Y, Ha Y, Park A-H, Kwon OW, Kim Y-J. Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. International Journal of Molecular Sciences. 2019; 20(3):536. https://doi.org/10.3390/ijms20030536
Chicago/Turabian StyleSeo, Ga-Young, Yuna Ha, Ah-Hyun Park, Oh Wook Kwon, and Youn-Jung Kim. 2019. "Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway" International Journal of Molecular Sciences 20, no. 3: 536. https://doi.org/10.3390/ijms20030536
APA StyleSeo, G.-Y., Ha, Y., Park, A.-H., Kwon, O. W., & Kim, Y.-J. (2019). Leathesia difformis Extract Inhibits α-MSH-Induced Melanogenesis in B16F10 Cells via Down-Regulation of CREB Signaling Pathway. International Journal of Molecular Sciences, 20(3), 536. https://doi.org/10.3390/ijms20030536





