5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells
Abstract
1. Introduction
2. Results
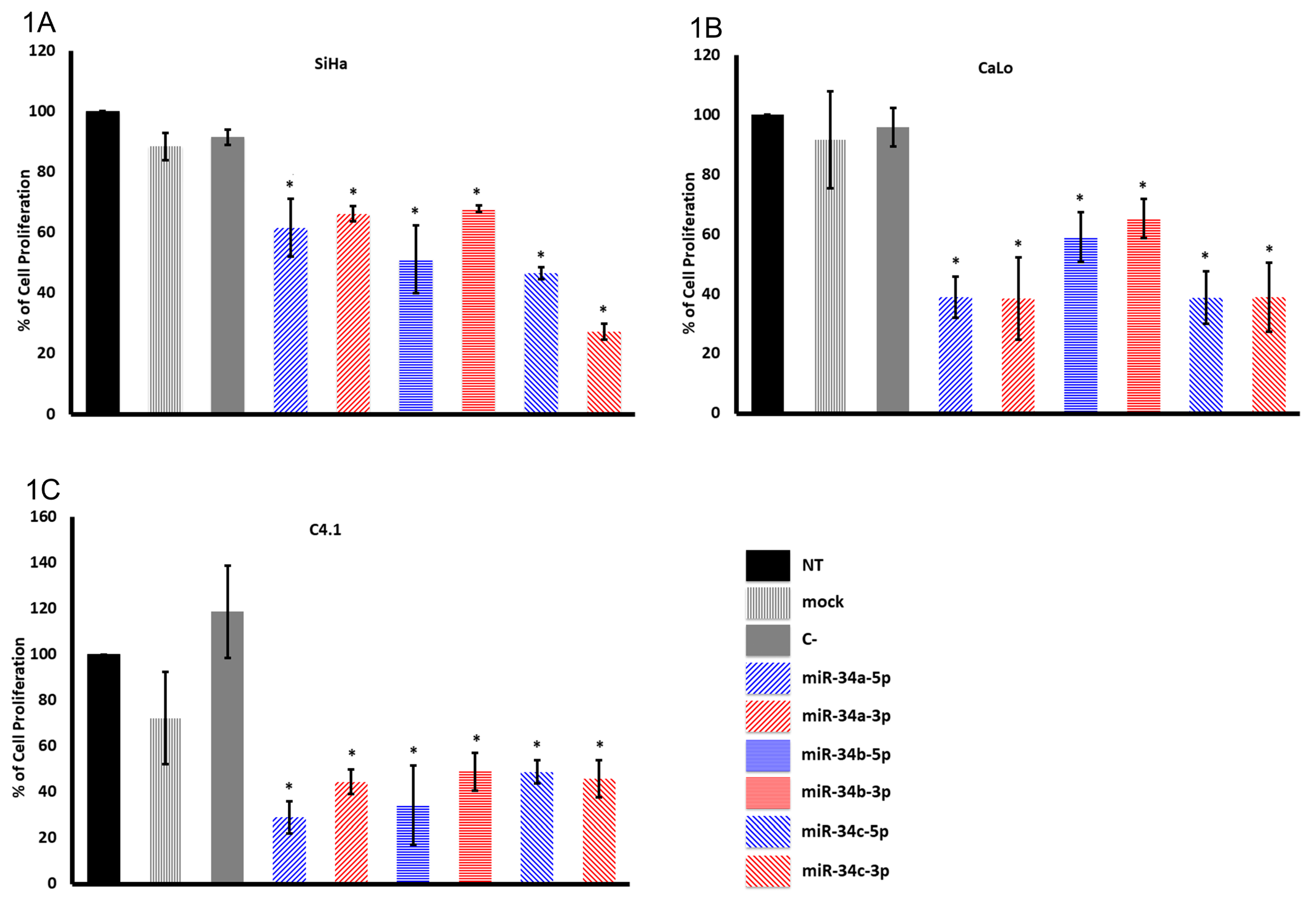
2.1. The miR-34 Family Members Inhibit Cervical Cancer Cell Proliferation
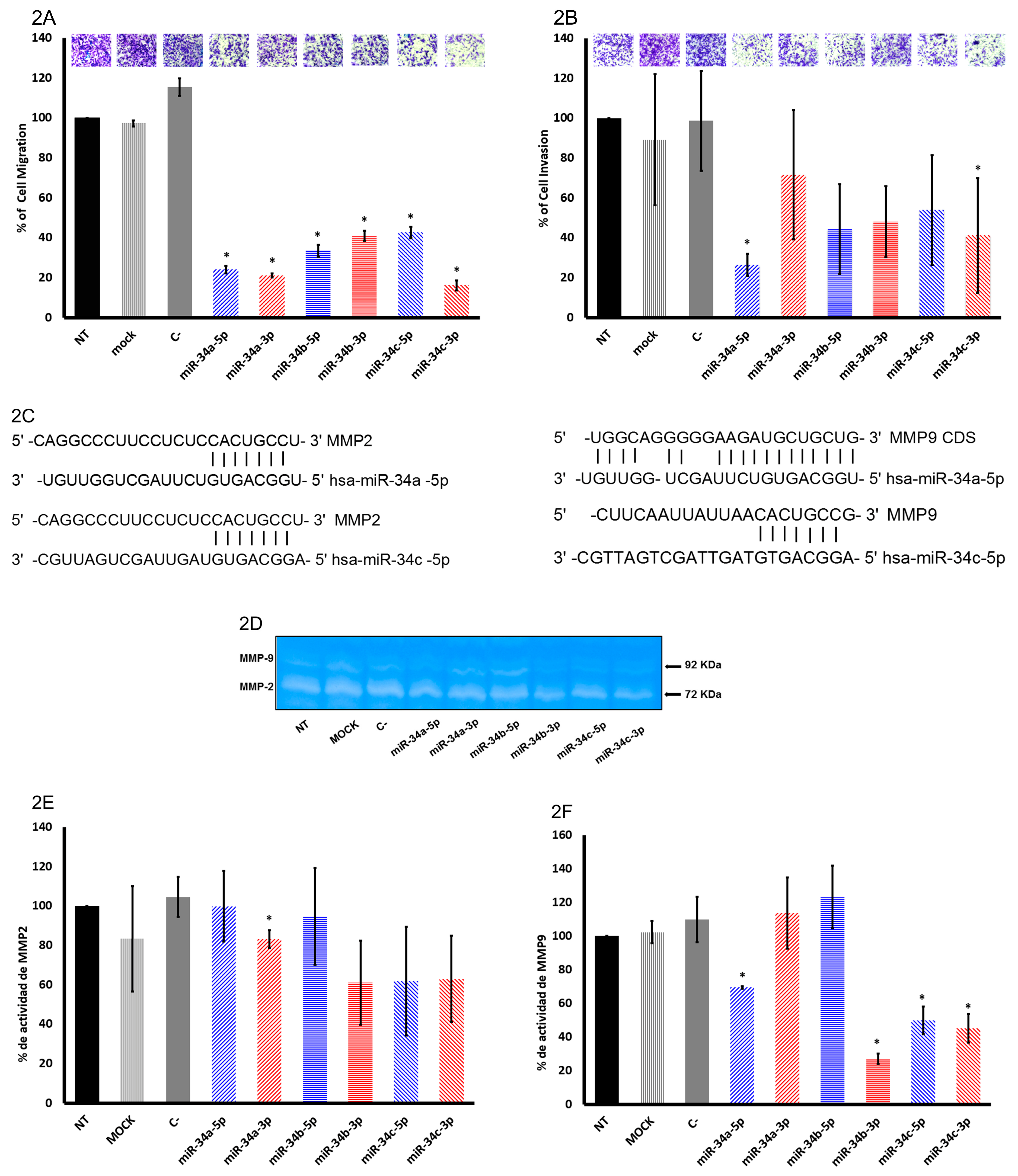
2.2. The miR-34 Family Members Inhibit Migration and Invasion in SiHa Cells
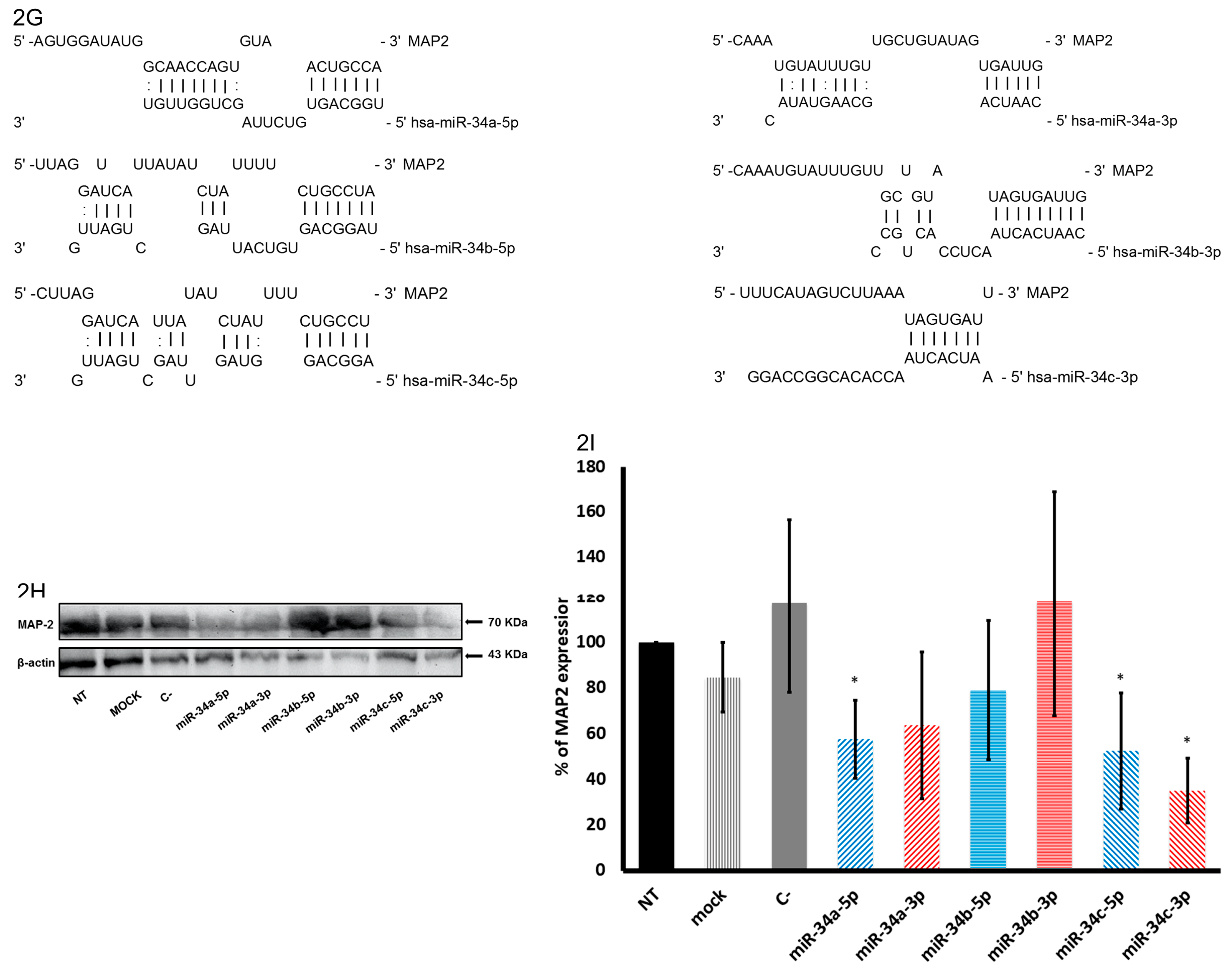
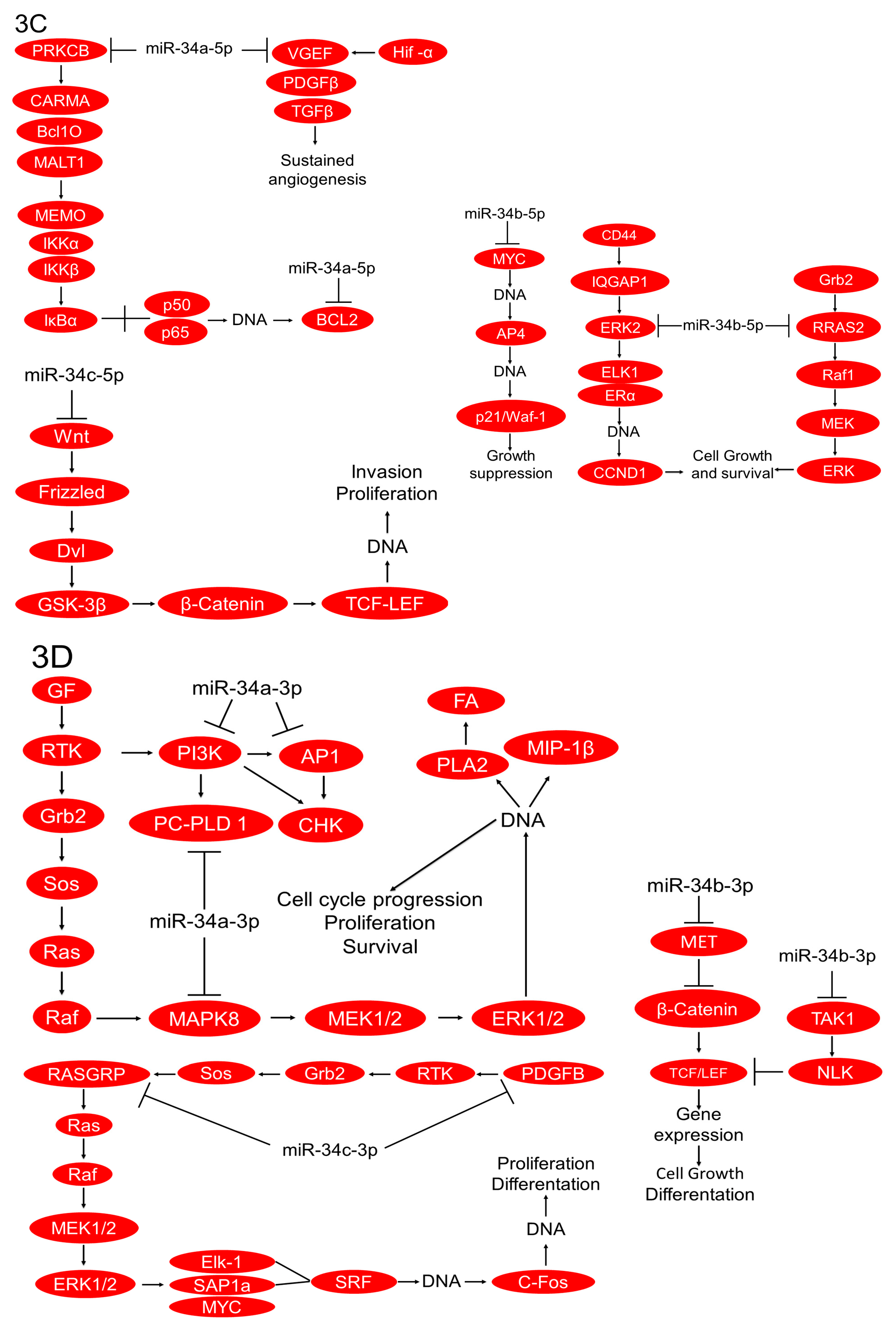
2.3. In Silico Analysis of Targets Regulated by miR-34 Family Members
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transient miRNA Transfection
4.3. Cell Proliferation Analysis
4.4. Cell Migration and Invasion Measured by Transwell Assays
4.5. Zymography
4.6. Immunoblotting
4.7. Messenger RNA Target Prediction Analysis in RNA22-HAS, DIANA-microT, and miRDB Databases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, C.D.; Cheng, S.; Simpson, S.; Hamacher, L.; Chow, L.T.; Broker, T.R.; DiPaolo, J.A. Recombinant retroviruses encoding human papillomavirus type 18 E6 and E7 genes stimulate proliferation and delay differentiation of human keratinocytes early after infection. Oncogene 1992, 7, 619–626. [Google Scholar] [PubMed]
- Zur Hausen, H. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 1999, 9, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Servin-Gonzalez, L.S.; Granados-Lopez, A.J.; Lopez, J.A. Families of microRNAs Expressed in Clusters Regulate Cell Signaling in Cervical Cancer. Int. J. Mol. Sci. 2015, 16, 12773–12790. [Google Scholar] [CrossRef] [PubMed]
- Granados Lopez, A.J.; Lopez, J.A. Multistep model of cervical cancer: Participation of miRNAs and coding genes. Int. J. Mol. Sci. 2014, 15, 15700–15733. [Google Scholar] [CrossRef]
- Tehler, D.; Hoyland-Kroghsbo, N.M.; Lund, A.H. The miR-10 microRNA precursor family. RNA Biol. 2011, 8, 728–734. [Google Scholar] [CrossRef]
- Wang, W.X.; Danaher, R.J.; Miller, C.S.; Berger, J.R.; Nubia, V.G.; Wilfred, B.S.; Neltner, J.H.; Norris, C.M.; Nelson, P.T. Expression of miR-15/107 family microRNAs in human tissues and cultured rat brain cells. Genom. Proteom. Bioinform. 2014, 12, 19–30. [Google Scholar] [CrossRef]
- Yu, J.; Wang, F.; Yang, G.H.; Wang, F.L.; Ma, Y.N.; Du, Z.W.; Zhang, J.W. Human microRNA clusters: Genomic organization and expression profile in leukemia cell lines. Biochem. Biophys. Res. Commun. 2006, 349, 59–68. [Google Scholar] [CrossRef]
- Chhabra, R.; Dubey, R.; Saini, N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24-2 cluster and its implication in human diseases. Mol. Cancer 2010, 9, 232. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Rådmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415. [Google Scholar] [CrossRef] [PubMed]
- Denli, A.M.; Tops, B.B.J.; Plasterk, R.H.A.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Meijer, H.A.; Smith, E.M.; Bushell, M. Regulation of miRNA strand selection: Follow the leader? Biochem. Soc. Trans. 2014, 42, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Davis-Dusenbery, B.N.; Hata, A. Mechanisms of control of microRNA biogenesis. J. Biochem. 2010, 148, 381–392. [Google Scholar] [CrossRef]
- Suzuki, H.I.; Miyazono, K. Emerging complexity of microRNA generation cascades. J. Biochem. 2011, 149, 15–25. [Google Scholar] [CrossRef]
- Lopez, J.A.; Alvarez-Salas, L.M. Differential effects of miR-34c-3p and miR-34c-5p on SiHa cells proliferation apoptosis, migration and invasion. Biochem. Biophys. Res. Commun. 2011, 409, 513–519. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.K.; McCoy, J.P.; Banerjee, N.S.; Rader, J.S.; Broker, T.R.; Meyers, C.; Chow, L.T.; Zheng, Z.M. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 2009, 15, 637–647. [Google Scholar] [CrossRef]
- Kasinski, A.L.; Slack, F.J. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012, 72, 5576–5587. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Song, Y.C.; Cao, P.L.; Zhang, H.; Guo, Q.; Yan, R.; Diao, D.M.; Cheng, Y.; Dang, C.X. Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med. Oncol. 2014, 31, 894. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Chen, X.; Zhu, D.; Luo, Z.; Yang, M. Low Expression of Circulating MicroRNA-34c is Associated with Poor Prognosis in Triple-Negative Breast Cancer. Yonsei Med. J. 2017, 58, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Li, Y.; Wang, F.; Li, Y.; Xu, J.; Shen, Y.; Ye, F.; Wang, X.; Cheng, X.; Chen, Y.; et al. MicroRNA detection in cervical exfoliated cells as a triage for human papillomavirus-positive women. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Werness, B.; Levine, A.; Howley, P. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990, 248, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, N.L.; Sedman, S.A.; Schiller, J.T. Human papillomavirus type 16 E6 increases the degradation rate of p53 in human keratinocytes. J. Virol. 1992, 66, 6237–6241. [Google Scholar] [PubMed]
- Dyson, N.; Howley, P.; Munger, K.; Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989, 243, 934–937. [Google Scholar] [CrossRef]
- Münger, K.; Basile, J.R.; Duensing, S.; Eichten, A.; Gonzalez, S.L.; Grace, M.; Zacny, V.L. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 2001, 20, 7888. [Google Scholar] [CrossRef]
- Duensing, S.; Duensing, A.; Flores, E.R.; Do, A.; Lambert, P.F.; Münger, K. Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J. Virol. 2001, 75, 7712–7716. [Google Scholar] [CrossRef]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; Desano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef]
- Corney, D.C.; Flesken-Nikitin, A.; Godwin, A.K.; Wang, W.; Nikitin, A.Y. MicroRNA-34b and MicroRNA-34c Are Targets of p53 and Cooperate in Control of Cell Proliferation and Adhesion-Independent Growth. Cancer Res. 2007, 67, 8433–8438. [Google Scholar] [CrossRef] [PubMed]
- Bommer, G.T.; Gerin, I.; Feng, Y.; Kaczorowski, A.J.; Kuick, R.; Love, R.E.; Zhai, Y.; Giordano, T.J.; Qin, Z.S.; Moore, B.B.; et al. p53-Mediated Activation of miRNA34 Candidate Tumor-Suppressor Genes. Curr. Biol. 2007, 17, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, K.; Spillare, E.A.; Fujita, K.; Horikawa, I.; Yamashita, T.; Appella, E.; Nagashima, M.; Takenoshita, S.; Yokota, J.; Harris, C.C. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008, 68, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Cannell, I.G.; Kong, Y.W.; Johnston, S.J.; Chen, M.L.; Collins, H.M.; Dobbyn, H.C.; Elia, A.; Kress, T.R.; Dickens, M.; Clemens, M.J.; et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc. Natl. Acad. Sci. USA 2010, 107, 5375–5380. [Google Scholar] [CrossRef]
- Mudduluru, G.; Ceppi, P.; Kumarswamy, R.; Scagliotti, G.V.; Papotti, M.; Allgayer, H. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 2011, 30, 2888–2899. [Google Scholar] [CrossRef]
- Tanaka, N.; Toyooka, S.; Soh, J.; Kubo, T.; Yamamoto, H.; Maki, Y.; Muraoka, T.; Shien, K.; Furukawa, M.; Ueno, T.; et al. Frequent methylation and oncogenic role of microRNA-34b/c in small-cell lung cancer. Lung Cancer 2012, 76, 32–38. [Google Scholar] [CrossRef]
- Hagman, Z.; Larne, O.; Edsjö, A.; Bjartell, A.; Ehrnström, R.A.; Ulmert, D.; Lilja, H.; Ceder, Y. miR-34c is downregulated in prostate cancer and exerts tumor suppressive functions. Int. J. Cancer 2010, 127, 2768–2776. [Google Scholar] [CrossRef]
- Cai, K.-M.; Bao, X.-L.; Kong, X.-H.; Jinag, W.; Mao, M.-R.; Chu, J.-S.; Huang, Y.-J.; Zhao, X.-J. Hsa-miR-34c suppresses growth and invasion of human laryngeal carcinoma cells via targeting c-Met. Int. J. Mol. Med. 2010, 25, 565–571. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Izant, J.G.; McIntosh, J.R. Microtubule-associated proteins: A monoclonal antibody to MAP2 binds to differentiated neurons. Proc. Natl. Acad. Sci. USA 1980, 77, 4741–4745. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G.; Briones, E.; Koszka, C.; Artlieb, U.; Krepler, R. Widespread occurrence of polypeptides related to neurotubule-associated proteins (MAP-1 and MAP-2) in non-neuronal cells and tissues. EMBO J. 1984, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Chen, Y.T.; Tseng, M.Y.; Hung, C.C.; Chiang, W.F.; Chen, H.R.; Shieh, T.Y.; Chen, C.H.; Jou, Y.S.; Chen, J.Y. Involvement of microtubule-associated protein 2 (MAP2) in oral cancer cell motility: A novel biological function of MAP2 in non-neuronal cells. Biochem. Biophys. Res. Commun. 2008, 366, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, V.; Törnquist, K. MMP2 and MMP9 participate in S1P-induced invasion of follicular ML-1 thyroid cancer cells. Mol. Cell. Endocrinol. 2015, 404, 113–122. [Google Scholar] [CrossRef]
- Yang, L.; Song, X.; Zhu, J.; Li, M.; Ji, Y.; Wu, F.; Chen, Y.; Cui, X.; Hu, J.; Wang, L.; et al. Tumor suppressor microRNA-34a inhibits cell migration and invasion by targeting MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int. J. Oncol. 2017, 51, 378–388. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kay, N.; Yang, J.M.; Lin, C.T.; Chang, H.L.; Wu, Y.C.; Fu, C.F.; Chang, Y.; Lo, S.; Hou, M.F.; et al. Total synthetic protoapigenone WYC02 inhibits cervical cancer cell proliferation and tumour growth through PIK3 signalling pathway. Basic Clin. Pharmacol. Toxicol. 2013, 113, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Delcuratolo, M.; Fertey, J.; Schneider, M.; Schuetz, J.; Leiprecht, N.; Hudjetz, B.; Brodbeck, S.; Corall, S.; Dreer, M.; Schwab, R.M.; et al. Papillomavirus-Associated Tumor Formation Critically Depends on c-Fos Expression Induced by Viral Protein E2 and Bromodomain Protein Brd4. PLoS Pathog. 2016, 12, e1005366. [Google Scholar] [CrossRef]
- Guan, S.; Lu, J.; Zhao, Y.; Woodfield, S.E.; Zhang, H.; Xu, X.; Yu, Y.; Zhao, J.; Bieerkehazhi, S.; Liang, H.; et al. TAK1 inhibitor 5Z-7-oxozeaenol sensitizes cervical cancer to doxorubicin-induced apoptosis. Oncotarget 2017, 8, 33666–33675. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, Y.; Chao, X.; Shi, S.; Liang, M.; Qiao, Y.; Wang, B.; Wang, P.; Zhu, Z. HOTAIR contributes to cell proliferation and metastasis of cervical cancer via targetting miR-23b/MAPK1 axis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, M.; Xia, X.; Huang, Y.; Zhang, Q.; Wang, X. Jumonji domain-containing protein 1A promotes cell growth and progression via transactivation of c-Myc expression and predicts a poor prognosis in cervical cancer. Oncotarget 2016, 7, 85151–85162. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, Y.; Lu, S.; Xiang, Y.Y. Downregulation of long noncoding RNA DGCR5 contributes to the proliferation, migration, and invasion of cervical cancer by activating Wnt signaling pathway. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rabachini, T.; Boccardo, E.; Andrade, R.; Perez, K.R.; Nonogaki, S.; Cuccovia, I.M.; Villa, L.L. HPV-16 E7 expression up-regulates phospholipase D activity and promotes rapamycin resistance in a pRB-dependent manner. BMC Cancer 2018, 18, 485. [Google Scholar] [CrossRef] [PubMed]
- Refaat, T.; Donnelly, E.D.; Sachdev, S.; Parimi, V.; El Achy, S.; Dalal, P.; Farouk, M.; Berg, N.; Helenowski, I.; Gross, J.P.; et al. c-Met Overexpression in Cervical Cancer, a Prognostic Factor and a Potential Molecular Therapeutic Target. Am. J. Clin. Oncol. 2017, 40, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Q.; Zuo, Y.; Liu, L.; Liu, S.; Chen, L.; Wang, K.; Lei, Y.; Zhao, X.; Li, Y. Isoprenaline/beta2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer 2017, 17, 875. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Erlandsson, S.; Herrero-Vidal, P.; Fernandez-Alfara, M.; Hernandez-Garcia, S.; Gonzalo-Flores, S.; Mudarra-Rubio, A.; Fresno, M.; Cubelos, B. R-RAS2 overexpression in tumors of the human central nervous system. Mol. Cancer 2013, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Doucette, T.A.; Kong, L.Y.; Yang, Y.; Ferguson, S.D.; Yang, J.; Wei, J.; Qiao, W.; Fuller, G.N.; Bhat, K.P.; Aldape, K.; et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro-oncology 2012, 14, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Ksionda, O.; Limnander, A.; Roose, J.P. RasGRP Ras guanine nucleotide exchange factors in cancer. Front. Biol. 2013, 8, 508–532. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.A.; Pitarresi, J.R.; Sharma, N.; Palettas, M.; Cuitino, M.C.; Sizemore, S.T.; Yu, L.; Sanderlin, A.; Rosol, T.J.; Mehta, K.D.; et al. Protein kinase C Beta in the tumor microenvironment promotes mammary tumorigenesis. Front. Biol. 2014, 4, 87. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Cannell, I.; Bushell, M. Regulation of Myc by miR-34c: A mechanism to prevent genomic instability? Cell Cycle 2010, 9, 2798–2802. [Google Scholar] [CrossRef]
- Jiang, J.; Gusev, Y.; Aderca, I.; Mettler, T.A.; Nagorney, D.M.; Brackett, D.J.; Roberts, L.R.; Schmittgen, T.D. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin. Cancer Res. 2008, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.T. MicroRNAs: Critical Regulators of Development, Cellular Physiology and Malignancy. Cell Cycle 2005, 4, 1179–1184. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, Q.; Zhang, S.; Zhang, Q.; Chang, J.; Qiu, X.; Wang, E. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer 2010, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Dehmelt, L.; Halpain, S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Bhat, K.M.; Maddodi, N.; Shashikant, C.; Setaluri, V. Transcriptional regulation of human MAP2 gene in melanoma: Role of neuronal bHLH factors and Notch1 signaling. Nucleic Acids Res. 2006, 34, 3819–3832. [Google Scholar] [CrossRef]
- Liu, Y.; Sturgis, C.D.; Grzybicki, D.M.; Jasnosz, K.M.; Olson, P.R.; Tong, M.; Dabbs, D.D.; Raab, S.S.; Silverman, J.F. Microtubule-associated protein-2: A new sensitive and specific marker for pulmonary carcinoid tumor and small cell carcinoma. Mod. Pathol. 2001, 14, 880–885. [Google Scholar] [CrossRef]
- Leclerc, N.; Baas, P.W.; Garner, C.C.; Kosik, K.S. Juvenile and mature MAP2 isoforms induce distinct patterns of process outgrowth. Mol. Biol. Cell 1996, 7, 443–455. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Zhang, H.; Artiles, K.L.; Fire, A.Z. Functional relevance of “seed” and “non-seed” sequences in microRNA-mediated promotion of C. elegans developmental progression. RNA 2015, 21, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, Y.; Qi, P.; Chen, Y.; Xu, P.; Yang, X.; Jin, X.; Tian, X. MicroRNA-183 functions as the tumor suppressor via inhibiting cellular invasion and metastasis by targeting MMP-9 in cervical cancer. Gynecol. Oncol. 2016, 141, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Cui, H.; Xu, X.; Lin, Z.; Zhang, X.; Kang, L.; Han, B.; Meng, J.; Yan, Z.; Yan, X.; et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget 2015, 6, 25266–25280. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tao, M.; Jiang, M. MicroRNA-454-3p inhibits cervical cancer cell invasion and migration by targeting c-Met. Exp. Ther. Med. 2018, 15, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhai, Y.X.; Liu, H.Q.; Shi, Y.A.; Li, X.B. MicroRNA-491-5p suppresses cervical cancer cell growth by targeting hTERT. Oncol. Rep. 2015, 34, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Toth, M.; Chvyrkova, I.; Bernardo, M.M.; Hernandez-Barrantes, S.; Fridman, R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: Role of TIMP-2 and plasma membranes. Biochem. Biophys. Res. Commun. 2003, 308, 386–395. [Google Scholar] [CrossRef]
- Yao, J.; Xiong, S.; Klos, K.; Nguyen, N.; Grijalva, R.; Li, P.; Yu, D. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene 2001, 20, 8066–8074. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef]
- Turpeenniemi-Hujanen, T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie 2005, 87, 287–297. [Google Scholar] [CrossRef]
- Granados-Lopez, A.J.; Ruiz-Carrillo, J.L.; Servin-Gonzalez, L.S.; Martinez-Rodriguez, J.L.; Reyes-Estrada, C.A.; Gutierrez-Hernandez, R.; Lopez, J.A. Use of Mature miRNA Strand Selection in miRNAs Families in Cervical Cancer Development. Int. J. Mol. Sci. 2017, 18, 407. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Kedde, M.; Strasser, M.J.; Boldajipour, B.; Vrielink, J.A.F.O.; Slanchev, K.; le Sage, C.; Nagel, R.; Voorhoeve, P.M.; van Duijse, J.; Ørom, U.A.; et al. RNA-Binding Protein Dnd1 Inhibits MicroRNA Access to Target mRNA. Cell 2007, 131, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.C.; Weiler, J.; Großhans, H. Regulating the regulators: Mechanisms controlling the maturation of microRNAs. Trends Biotechnol. 2009, 27, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bail, S.; Swerdel, M.; Liu, H.; Jiao, X.; Goff, L.A.; Hart, R.P.; Kiledjian, M. Differential regulation of microRNA stability. RNA 2010, 16, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
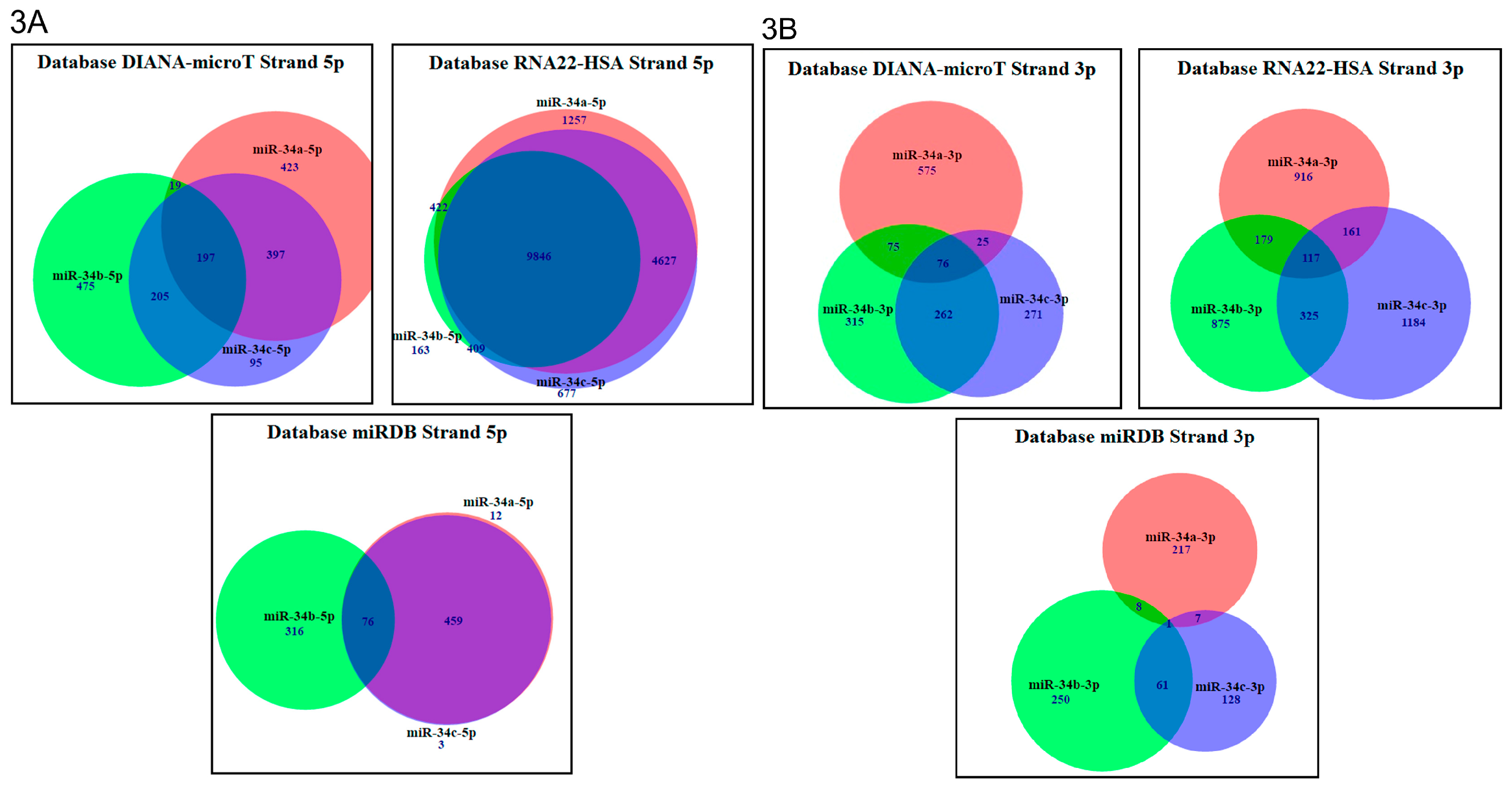
| Data Base | Total Genes Regulated by 5p Strands | Total Genes Regulated by 3p Strands | Genes Regulated by 5p Strands | Genes Regulated by 3p Strands | % of Genes Regulated by 5p Strands | % of Genes Regulated by 3p Strands |
|---|---|---|---|---|---|---|
| miRDB | 866 | 672 | miR-34a (12) miR-34b (316) miR-34c (3) | miR-34a (217) miR-34b (250) miR-34c (128) | miR-34a (1.38%) miR-34b (36.48%) miR-34c (0.34%) | miR-34a (32.29%) miR-34b (37.2%) miR-34c (19.04%) |
| RNA22-HSA | 17,401 | 3752 | miR-34a (1257) miR-34b (163) miR-34c (677) | miR-34a (916) miR-34b (875) miR-34c (1184) | miR-34a (7.22%) miR-34b (0.93%) miR-34c (3.89%) | miR-34a (24.41%) miR-34b (23.32%) miR-34c (31.55%) |
| DIANA-microT | 1811 | 1599 | miR-34a (423) miR-34b (475) miR-34c (95) | miR-34a (575) miR-34b (315) miR-34c (271) | miR-34a (23.35%) miR-34b (26.22%) miR-34c (5.24%) | miR-34a (35.95%) miR-34b (19.69%) miR-34c (16.94%) |
| miRNA | Single Pathways by Strand |
|---|---|
| hsa-miR-34a-5p | DIANA microT |
| -NF-κB signaling pathway -Longevity regulating pathway (multiple species) -Focal adhesion -Amphetamine addiction -Rap1 signaling pathway -Adrenergic signaling in cardiomyocytes -Pathways in cancer | |
| miRDB | |
| -Galactose metabolism -One-carbon pool by folate -Glycosaminoglycan biosynthesis-keratan sulfate -Butirosin and neomycin biosynthesis -Type II diabetes mellitus -Carbohydrate digestion and absorption -Glycosphingolipid biosynthesis, lacto and neolacto series -Amino sugar and nucleotide sugar metabolism -Other types of O-glycan biosynthesis | |
| RNA22-HSA | |
| -Chemical carcinogenesis -Steroid hormone biosynthesis -Metabolism of xenobiotics by cytochrome P450 -Parkinson’s disease | |
| hsa-miR-34a-3p | DIANA microT |
| -GnRH signaling pathway -Choline metabolism in cancer -Tryptophan metabolism -Dopaminergic synapse -Gastric acid secretion -Salmonella infection -Histidine metabolism -Chagas disease (American trypanosomiasis) | |
| miRDB | |
| -Pathways in cancer -Fatty-acid degradation -Histidine metabolism -Adherens junction -Tryptophan metabolism -Axon guidance -Tuberculosis -Apoptosis | |
| RNA22-HSA | |
| Pathways in cancer -GABAergic synapse -Circadian entrainment -Retrograde endocannabinoid signaling -PI3K/Akt signaling pathway -Morphine addiction -Dopaminergic synapse -Glutamatergic synapse -ErbB signaling pathway -Chemokine signaling pathway | |
| hsa-miR-34b-5p | DIANA microT |
| -Shigellosis -Aldosterone-regulated sodium reabsorption -Regulation of actin cytoskeleton -Proteoglycans in cancer -Pathogenic Escherichia coli infection -ErbB signaling pathway -Hepatitis B -Thyroid cancer | |
| miRDB | |
| -Ubiquitin-mediated proteolysis -Thyroid hormone signaling pathway -ErbB signaling pathway -Circadian rhythm -MicroRNAs in cancer -T-cell receptor signaling pathway -Prolactin signaling pathway -Insulin signaling pathway | |
| RNA22-HSA | |
| -Renin/angiotensin system -Lysosome -Nicotinate and nicotinamide metabolism -mRNA surveillance pathway -Influenza A -RNA transport -Fat digestion and absorption -Pyrimidine metabolism -Purine metabolism | |
| hsa-miR-34b-3p | DIANA microT |
| -Glycosaminoglycan degradation -mRNA surveillance pathway -Adherens junction -Glutathione metabolism -AMPK signaling pathway -SNARE interactions in vesicular transport -Transcriptional misregulation in cancer -Cysteine and methionine metabolism | |
| miRDB | |
| -Cysteine and methionine metabolism -Sphingolipid metabolism -Phospholipase D signaling pathway -Choline metabolism in cancer -Renin secretion -Synaptic vesicle cycle -Oocyte meiosis -Dorso–ventral axis formation | |
| RNA22-HSA | |
| -Type II diabetes mellitus -Phosphatidylinositol signaling system -Oxytocin signaling pathway -Protein digestion and absorption -AMPK signaling pathway -Butanoate metabolism -cAMP signaling pathway -Adrenergic signaling in cardiomyocytes -cGMP/PKG signaling pathway -Gastric acid secretion | |
| hsa-miR-34c-5p | DIANA microT |
| -Synaptic vesicle cycle -Vitamin B6 metabolism -Lysosome -Oxidative phosphorylation -Natural killer cell-mediated cytotoxicity -Glycosylphosphatidylinositol (GPI)-anchor biosynthesis -Circadian rhythm -Cocaine addiction | |
| miRDB | |
| -Chemokine signaling pathway -Cytokine–cytokine receptor interaction | |
| RNA22-HSA | |
| -Propanoate metabolism -Terpenoid backbone biosynthesis -Complement and coagulation cascades -Taste transduction -PPAR signaling Pentose phosphate pathway -Basal cell carcinoma -Asthma -Staphylococcus aureus infection | |
| hsa-miR-34c-3p | DIANA microT |
| -Biosynthesis of unsaturated fatty acids -Measles -Glutamatergic synapse -MAPK signaling pathway -T-cell receptor signaling pathway -Ubiquitin-mediated proteolysis -Renin secretion -Renal cell carcinoma -Central carbon metabolism in cancer -Inositol phosphate metabolism | |
| miRDB | |
| -Biosynthesis of unsaturated fatty acids -Maturity onset diabetes of the young -Pentose and glucuronate interconversions -Fatty-acid elongation -Endocrine and other factor-regulated calcium reabsorption -Fatty-acid metabolism -Regulation of actin cytoskeleton -Leukocyte transendothelial migration | |
| RNA22-HSA | |
| -Thyroid hormone signaling pathway -Retinol metabolism -Drug metabolism, cytochrome P450 -Signaling pathways regulating pluripotency of stem cells -Aldosterone-regulated sodium reabsorption |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova-Rivas, S.; Fraire-Soto, I.; Mercado-Casas Torres, A.; Servín-González, L.S.; Granados-López, A.J.; López-Hernández, Y.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Castañeda-Delgado, J.E.; Ramírez-Hernández, L.; et al. 5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 545. https://doi.org/10.3390/ijms20030545
Córdova-Rivas S, Fraire-Soto I, Mercado-Casas Torres A, Servín-González LS, Granados-López AJ, López-Hernández Y, Reyes-Estrada CA, Gutiérrez-Hernández R, Castañeda-Delgado JE, Ramírez-Hernández L, et al. 5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells. International Journal of Molecular Sciences. 2019; 20(3):545. https://doi.org/10.3390/ijms20030545
Chicago/Turabian StyleCórdova-Rivas, Sergio, Ixamail Fraire-Soto, Andrea Mercado-Casas Torres, Luis Steven Servín-González, Angelica Judith Granados-López, Yamilé López-Hernández, Claudia Araceli Reyes-Estrada, Rosalinda Gutiérrez-Hernández, Julio Enrique Castañeda-Delgado, Leticia Ramírez-Hernández, and et al. 2019. "5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells" International Journal of Molecular Sciences 20, no. 3: 545. https://doi.org/10.3390/ijms20030545
APA StyleCórdova-Rivas, S., Fraire-Soto, I., Mercado-Casas Torres, A., Servín-González, L. S., Granados-López, A. J., López-Hernández, Y., Reyes-Estrada, C. A., Gutiérrez-Hernández, R., Castañeda-Delgado, J. E., Ramírez-Hernández, L., Varela-Silva, J. A., & López, J. A. (2019). 5p and 3p Strands of miR-34 Family Members Have Differential Effects in Cell Proliferation, Migration, and Invasion in Cervical Cancer Cells. International Journal of Molecular Sciences, 20(3), 545. https://doi.org/10.3390/ijms20030545





