Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice
Abstract
:1. Introduction
2. Results
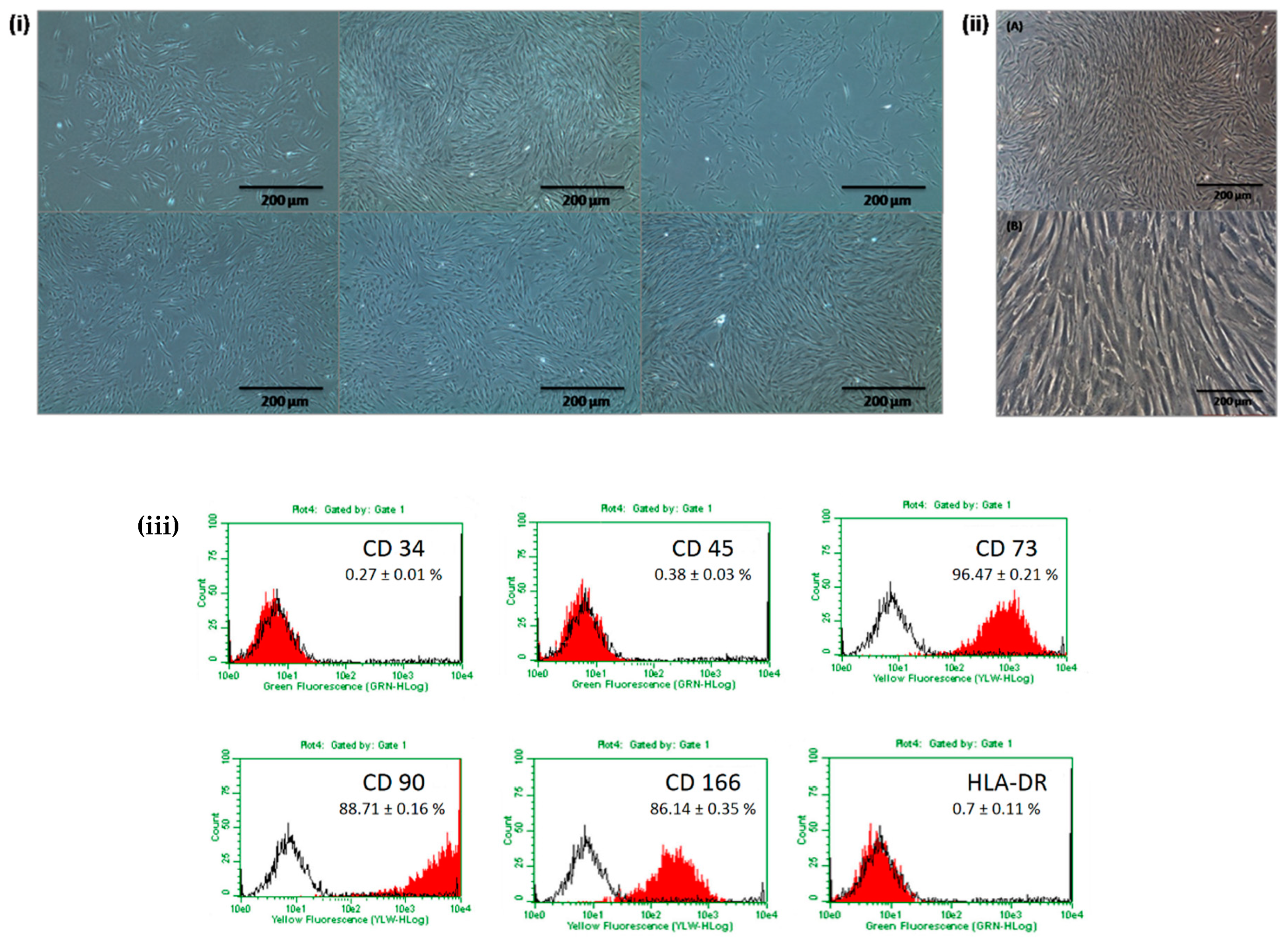
2.1. Basic Characterization of Isolated DPSCs
2.2. Differentiation of DPSCs into Dopaminergic Neuron-Like Cells
2.3. Recovery of Neurological Behaviour in Parkinsonian Mice Following Intranasal Application of DPSCs
2.4. Intranasal DPSC Application Rescued Dopaminergic Neurons from MPTP Toxicity
2.5. Fluorescent Imaging of DPSCs In Vivo
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of DPSCs
4.2. Flow Cytometric Analysis
4.3. Induction of Dopaminergic Neuronal Differentiation
4.4. Real-Time Polymerase Chain Reaction
4.5. Animals
4.6. Establishment of PD Model
4.7. Intranasal Application of DPSCs
4.8. Fluorescent Imaging of DPSCs in Vivo
4.9. Behaviour Testing
4.10. Perfusion and Fixation of Brains
4.11. Tyrosine Hydroxylase (TH) Immunostaining
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Perry, E.K.; Mckeith, I.; Thompson, P.; Marshall, E.; Kerwin, J.; Jabeen, S.; Edwardson, J.A.; Ince, P.; Blessed, G.; Irving, D.; et al. Topography, Extent, and Clinical Relevance of Neurochemical Deficits in Dementia of Lewy Body Type, Parkinson’s Disease, and Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 1991, 640, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.Y.; Soker, S.; Jang, Y.-J.; Kwon, T.G.; Yoo, E.S. Differentiation of Human Dental Pulp Stem Cells into Dopaminergic Neuron-like Cells in Vitro. J. Korean Med. Sci. 2016, 31, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hely, M.A.; Fung, V.S.C.; Morris, J.G.L. Treatment of Parkinson’s disease. J. Clin. Neurosci. 2000, 7, 484–494. [Google Scholar] [CrossRef]
- Apel, C.; Forlenza, O.V.; de Paula, V.J.R.; Talib, L.L.; Denecke, B.; Eduardo, C.P.; Gattaz, W.F. The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. J. Neural Transm. 2008, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A. Dopaminergic transplants in experimental parkinsonism: cellular mechanisms of graft-induced functional recovery. Curr. Opin. Neurobiol. 1992, 2, 683–689. [Google Scholar] [CrossRef]
- Ganapathy, K.; Datta, I.; Bhonde, R. Astrocyte-Like Cells Differentiated from Dental Pulp Stem Cells Protect Dopaminergic Neurons Against 6-Hydroxydopamine Toxicity. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gnanasegaran, N.; Govindasamy, V.; Abu Kasim, N.H. Differentiation of stem cells derived from carious teeth into dopaminergic-like cells. Int. Endod. J. 2016, 49, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult Human Dental Pulp Stem Cells Differentiate Toward Functionally Active Neurons Under Appropriate Environmental Cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.U.; de Vellis, J. Stem cell-based cell therapy in neurological diseases: A review. J. Neurosci. Res. 2009, 87, 2183–2200. [Google Scholar] [CrossRef] [Green Version]
- Piccini, P.; Pavese, N.; Hagell, P.; Reimer, J.; Björklund, A.; Oertel, W.H.; Quinn, N.P.; Brooks, D.J.; Lindvall, O. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain 2005, 128, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.E.; Petit, G.H.; Brundin, P. Cell transplantation in Parkinsonʼs disease: problems and perspectives. Curr. Opin. Neurol. 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Barker, R.A.; Parmar, M. Neural grafting in Parkinson’s disease Problems and possibilities. Prog. Brain Res. 2010, 184, 265–294. [Google Scholar]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Bernhard, F.; Verleysdonk, S.; Buadze, M.; Lourhmati, A.; Klopfer, T.; Schaumann, F.; Schmid, B.; et al. Therapeutic Efficacy of Intranasally Delivered Mesenchymal Stem Cells in a Rat Model of Parkinson Disease. Rejuvenation Res. 2011, 14, 3–16. [Google Scholar] [CrossRef]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- De Almeida, F.M.; Marques, S.A.; Ramalho, B.D.S.; Rodrigues, R.F.; Cadilhe, D.V.; Furtado, D.; Kerkis, I.; Pereira, L.V.; Rehen, S.K.; Martinez, A.M.B. Human Dental Pulp Cells: A New Source of Cell Therapy in a Mouse Model of Compressive Spinal Cord Injury. J. Neurotrauma. 2011, 28, 1939–1949. [Google Scholar] [CrossRef]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and Characterization of Neural Crest-Derived Stem Cells from Dental Pulp of Neonatal Mice. PLoS ONE 2011, 6, e27526. [Google Scholar] [CrossRef]
- Victor, A.K.; Reiter, L.T. Dental pulp stem cells for the study of neurogenetic disorders. Hum. Mol. Genet. 2017, 26, R166–R171. [Google Scholar] [CrossRef]
- Wang, F.; Jia, Y.; Liu, J.; Zhai, J.; Cao, N.; Yue, W.; He, H.; Pei, X. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease: Therapeutical effects of hDPSCs on AD model. Cell Biol. Int. 2017, 41, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, V.; Abdullah, A.N.; Ronald, V.S.; Musa, S.; Ab Aziz, Z.A.C.; Zain, R.B.; Totey, S.; Bhonde, R.R.; Abu Kasim, N.H. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J. Endod. 2010, 36, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- McLaren, A. Ethical and social considerations of stem cell research. Nature 2001, 414, 129–131. [Google Scholar] [CrossRef]
- Mooney, D.J.; Vandenburgh, H. Cell Delivery Mechanisms for Tissue Repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhu, J.; Xu, G.; Liu, X. Intranasal delivery of stem cells to the brain. Expert Opin. Drug Deliv. 2011, 8, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Reitz, M.; Demestre, M.; Sedlacik, J.; Meissner, H.; Fiehler, J.; Kim, S.U.; Westphal, M.; Schmidt, N.O. Intranasal delivery of neural stem/progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl. Med. 2012, 1, 866–873. [Google Scholar] [CrossRef]
- Gnanasegaran, N.; Govindasamy, V.; Kathirvaloo, P.; Musa, S.; Abu Kasim, N.H. Effects of cell cycle phases on the induction of dental pulp stem cells toward dopaminergic-like cells: Growth phases influence differentiation of dental pulp stem cells toward dopaminergic like cells. J. Tissue Eng. Regen. Med. 2018, 12, e881–e893. [Google Scholar] [CrossRef]
- Gnanasegaran, N.; Govindasamy, V.; Simon, C.; Gan, Q.F.; Vincent-Chong, V.K.; Mani, V.; Krishnan Selvarajan, K.; Subramaniam, V.; Musa, S.; Abu Kasim, N.H. Effect of dental pulp stem cells in MPTP-induced old-aged mice model. Eur. J. Clin. Investig. 2017, 47, 403–414. [Google Scholar] [CrossRef]
- Fujii, H.; Matsubara, K.; Sakai, K.; Ito, M.; Ohno, K.; Ueda, M.; Yamamoto, A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015, 1613, 59–72. [Google Scholar] [CrossRef]
- Majumdar, D.; Kanafi, M.; Bhonde, R.; Gupta, P.; Datta, I. Differential Neuronal Plasticity of Dental Pulp Stem Cells from Exfoliated Deciduous and Permanent Teeth Towards Dopaminergic Neurons: Difference in da-neuronal differentiation. J. Cell. Physiol. 2016, 231, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair: DPSC Therapy for Neural and Retinal Repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Sun, Z.; Wang, X.; Yang, H.; Shi, S.; Wang, S. Stem Cells from Human-Exfoliated Deciduous Teeth Can Differentiate into Dopaminergic Neuron-Like Cells. Stem Cells Dev. 2010, 19, 1375–1383. [Google Scholar] [CrossRef]
- Smeyne, R.J.; Jackson-Lewis, V. The MPTP model of Parkinson’s disease. Mol. Brain Res. 2005, 134, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Balyasnikova, I.V.; Prasol, M.S.; Ferguson, S.D.; Han, Y.; Ahmed, A.U.; Gutova, M.; Tobias, A.L.; Mustafi, D.; Rincón, E.; Zhang, L.; et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Ekhator, O.R.; Ghisays, V. Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease. J. Vis. Exp. JoVE 2013. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhl, A.M.; Dirr, E.R.; Fleming, S.M. Olfactory assays for mouse models of neurodegenerative disease. J. Vis. Exp. JoVE 2014, e51804. [Google Scholar] [CrossRef]




| Gene Name | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Base Pair Size |
|---|---|---|---|
| SOX 2 | GGACAGTTACGCGCACATGA | AGCCGTTCATGTAGGTCTGC | 188 |
| OCT 4 | TCCCGAATGGAAAGGGGAGA | GGCTGAATACCTTCCCAAATAGA | 209 |
| NES | GTAGCTCCCAGAGAGGGGAA | CTCTAGAGGGCCAGGGACTT | 206 |
| NR4A2 | CGCCTGTAACTCGGCTGAA | AGTGTTGGTGAGGTCCATGC | 169 |
| TUBB3 | GCGAGATGTACGAAGACGAC | TTTAGACACTGCTGGCTTCG | 115 |
| NCAM | TCTGCTAGCTCGTCTACCCC | AGCTTAGGTGCACTGGGTTC | 110 |
| MAP2 | TAGAGGGTGTGATGGCTGAG | GGCAGAGGAAGGGATTTCTA | 183 |
| TH | TCATCACCTGGTCACCAAGTT | GGTCGCCGTGCCTGTACT | 125 |
| DAT | AAAGTCCTTTCCCGATGCGT | ATACCAGGACCCCCATCCTC | 111 |
| 18s rRNA | CGGCTACCATCCAAGGAA | GCTGGAATTACCGCGGCT | 186 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, C.; Gan, Q.F.; Kathivaloo, P.; Mohamad, N.A.; Dhamodharan, J.; Krishnan, A.; Sengodan, B.; Palanimuthu, V.R.; Marimuthu, K.; Rajandas, H.; et al. Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. Int. J. Mol. Sci. 2019, 20, 568. https://doi.org/10.3390/ijms20030568
Simon C, Gan QF, Kathivaloo P, Mohamad NA, Dhamodharan J, Krishnan A, Sengodan B, Palanimuthu VR, Marimuthu K, Rajandas H, et al. Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. International Journal of Molecular Sciences. 2019; 20(3):568. https://doi.org/10.3390/ijms20030568
Chicago/Turabian StyleSimon, Christopher, Quan Fu Gan, Premasangery Kathivaloo, Nur Afiqah Mohamad, Jagadeesh Dhamodharan, Arulmoli Krishnan, Bharathi Sengodan, Vasanth Raj Palanimuthu, Kasi Marimuthu, Heera Rajandas, and et al. 2019. "Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice" International Journal of Molecular Sciences 20, no. 3: 568. https://doi.org/10.3390/ijms20030568
APA StyleSimon, C., Gan, Q. F., Kathivaloo, P., Mohamad, N. A., Dhamodharan, J., Krishnan, A., Sengodan, B., Palanimuthu, V. R., Marimuthu, K., Rajandas, H., Ravichandran, M., & Parimannan, S. (2019). Deciduous DPSCs Ameliorate MPTP-Mediated Neurotoxicity, Sensorimotor Coordination and Olfactory Function in Parkinsonian Mice. International Journal of Molecular Sciences, 20(3), 568. https://doi.org/10.3390/ijms20030568






