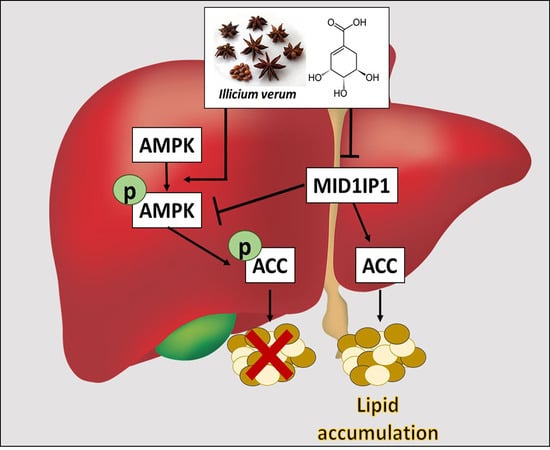
Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC
Abstract
1. Introduction
2. Results
2.1. Shikimic Acid Exerted Weak Cytotoxicity in HepG2 and Huh7 Cells and 3T3-L1 Cells
2.2. Shikimic Acid Reduced the Number of Lipid Droplets in HCCs
2.3. MID1IP1 Depletion Suppressed Proliferation and the Expression of SREBP-1c and FAS in HepG2 Cells
2.4. Shikimic Acid Downregulated MID1IP1 Expression Level by Phosphorylation of AMPK in HCCs and Adipocytes
2.5. Pivotal Role of MID1IP1 in Shikimic Acid Regulated Lipogenesis in HepG2 and AML-12 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents, Antibodies and Plasmids
4.2. Cell Lines and Culture
4.3. Cell Viability Assay
4.4. Adipogenic Differentiation Induction
4.5. Oil-Red-O Staining
4.6. Western Blotting
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. RNA Interference
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MID1IP1 | MIG12, Midline-1-interacting G12-like protein |
| AMPK | Adenosine 3′,5′-cyclic monophosphate (cAMP)-activated protein kinaseivated protein kinase |
| SREBP-1c | Sterol regulatory element-binding protein 1 |
| FAS | Fatty acid synthase |
| ACC | Acetyl-coA carboxylase |
| LXR-α | Liver X receptor alpha |
| HCC | Human Hepatocellular Carcinoma |
References
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Geisler, C.E.; Renquist, B.J. Hepatic lipid accumulation: Cause and consequence of dysregulated glucoregulatory hormones. J. Endocrinol. 2017, 234, R1–R21. [Google Scholar] [CrossRef]
- de Castro, G.S.; Calder, P.C. Non-alcoholic fatty liver disease and its treatment with n-3 polyunsaturated fatty acids. Clin. Nutr. 2018, 37, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hebrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. (Oxf) 2009, 196, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, L.; Zhang, X.; Li, X.; Yu, H. De novo lipogenesis and desaturation of fatty acids during adipogenesis in bovine adipose-derived mesenchymal stem cells. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 23–31. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Wang, L.; Cai, Y.; Zhong, X.; He, X.; Hu, L.; Tian, S.; Wu, M.; Hui, L.; et al. Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J. 2017, 36, 1330–1347. [Google Scholar] [CrossRef]
- Aipoalani, D.L.; O’Callaghan, B.L.; Mashek, D.G.; Mariash, C.N.; Towle, H.C. Overlapping roles of the glucose-responsive genes, S14 and S14R, in hepatic lipogenesis. Endocrinology 2010, 151, 2071–2077. [Google Scholar] [CrossRef]
- Kim, C.W.; Moon, Y.A.; Park, S.W.; Cheng, D.; Kwon, H.J.; Horton, J.D. Induced polymerization of mammalian acetyl-CoA carboxylase by MIG12 provides a tertiary level of regulation of fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 9626–9631. [Google Scholar] [CrossRef]
- Inoue, J.; Yamasaki, K.; Ikeuchi, E.; Satoh, S.; Fujiwara, Y.; Nishimaki-Mogami, T.; Shimizu, M.; Sato, R. Identification of MIG12 as a mediator for stimulation of lipogenesis by LXR activation. Mol. Endocrinol. 2011, 25, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.W.; Hu, W.T.; Huang, B.K.; Qin, L.P. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011, 136, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Bongaerts, J.; Bovenberg, R.; Kremer, S.; Muller, U.; Orf, S.; Wubbolts, M.; Raeven, L. Metabolic engineering for microbial production of shikimic acid. Metab. Eng. 2003, 5, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.M.; Estevez, R.J. A short overview on the medicinal chemistry of (-)-shikimic acid. Mini Rev. Med. Chem. 2012, 12, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L. Shikimic acid from Artemisia absinthium inhibits protein glycation in diabetic rats. Int. J. Biol. Macromol. 2018, 122, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Rawat, G.; Yadav, S.; Saxena, R.K. Shikimic acid, a base compound for the formulation of swine/avian flu drug: Statistical optimization, fed-batch and scale up studies along with its application as an antibacterial agent. Antonie Van Leeuwenhoek 2015, 107, 419–431. [Google Scholar] [CrossRef]
- Rabelo, T.K.; Guimaraes, A.G.; Oliveira, M.A.; Gasparotto, J.; Serafini, M.R.; de Souza Araujo, A.A.; Quintans-Junior, L.J.; Moreira, J.C.F.; Gelain, D.P. Shikimic acid inhibits LPS-induced cellular pro-inflammatory cytokines and attenuates mechanical hyperalgesia in mice. Int. Immunopharmacol. 2016, 39, 97–105. [Google Scholar] [CrossRef]
- Sun, J.Y.; You, C.Y.; Dong, K.; You, H.S.; Xing, J.F. Anti-inflammatory, analgesic and antioxidant activities of 3,4-oxo-isopropylidene-shikimic acid. Pharm. Biol. 2016, 54, 2282–2287. [Google Scholar] [CrossRef]
- Veach, D.; Hosking, H.; Thompson, K.; Santhakumar, A.B. Anti-platelet and anti-thrombogenic effects of shikimic acid in sedentary population. Food Funct. 2016, 7, 3609–3616. [Google Scholar] [CrossRef]
- Doege, H.; Grimm, D.; Falcon, A.; Tsang, B.; Storm, T.A.; Xu, H.; Ortegon, A.M.; Kazantzis, M.; Kay, M.A.; Stahl, A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J. Biol. Chem. 2008, 283, 22186–22192. [Google Scholar] [CrossRef]
- Hunt, G.B.; Luff, J.A.; Daniel, L.; Van den Bergh, R. Evaluation of hepatic steatosis in dogs with congenital portosystemic shunts using Oil Red O staining. Vet. Pathol. 2013, 50, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J.; Semczuk, A. Liver X receptor (LXR) and the reproductive system—A potential novel target for therapeutic intervention. Pharmacol. Rep. 2010, 62, 15–27. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Beaven, S.W.; Wroblewski, K.; Wang, J.; Hong, C.; Bensinger, S.; Tsukamoto, H.; Tontonoz, P. Liver X receptor signaling is a determinant of stellate cell activation and susceptibility to fibrotic liver disease. Gastroenterology 2011, 140, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Park, H.; Kaushik, V.K.; Dean, D.; Constant, S.; Prentki, M.; Saha, A.K. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol. Scand. 2003, 178, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Pan, D.A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002, 30, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Colbert, C.L.; Kim, C.W.; Moon, Y.A.; Henry, L.; Palnitkar, M.; McKean, W.B.; Fitzgerald, K.; Deisenhofer, J.; Horton, J.D.; Kwon, H.J. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18820–18825. [Google Scholar] [CrossRef] [PubMed]






| Sense | Antisense | |
|---|---|---|
| MID1IP1 | 5′GGC GAC ACC TTT CCT GGA CT3′ | 5′GAT GGC TGA GGG TGC TCT GT3′ |
| SREBP-1c | 5′CCA TGG ATG CAC TTT CGA A3′ | 5′CCA GCA TAG GGT GGG TCA A3′ |
| FAS | 5′GCT GCT CCA CGA ACT CAA ACA CCG3′ | 5′CGG TAC GCG ACG GCT GCC TG3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.-J.; Kim, S.-H. Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. https://doi.org/10.3390/ijms20030582
Kim MJ, Sim DY, Lee HM, Lee H-J, Kim S-H. Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. International Journal of Molecular Sciences. 2019; 20(3):582. https://doi.org/10.3390/ijms20030582
Chicago/Turabian StyleKim, Moon Joon, Deok Yong Sim, Hye Min Lee, Hyo-Jung Lee, and Sung-Hoon Kim. 2019. "Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC" International Journal of Molecular Sciences 20, no. 3: 582. https://doi.org/10.3390/ijms20030582
APA StyleKim, M. J., Sim, D. Y., Lee, H. M., Lee, H.-J., & Kim, S.-H. (2019). Hypolipogenic Effect of Shikimic Acid Via Inhibition of MID1IP1 and Phosphorylation of AMPK/ACC. International Journal of Molecular Sciences, 20(3), 582. https://doi.org/10.3390/ijms20030582