Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs
Abstract
1. Introduction
2. Results
2.1. Similar Absolute Hardness of Zonal and Non-Zonal Constructs Before Implantation
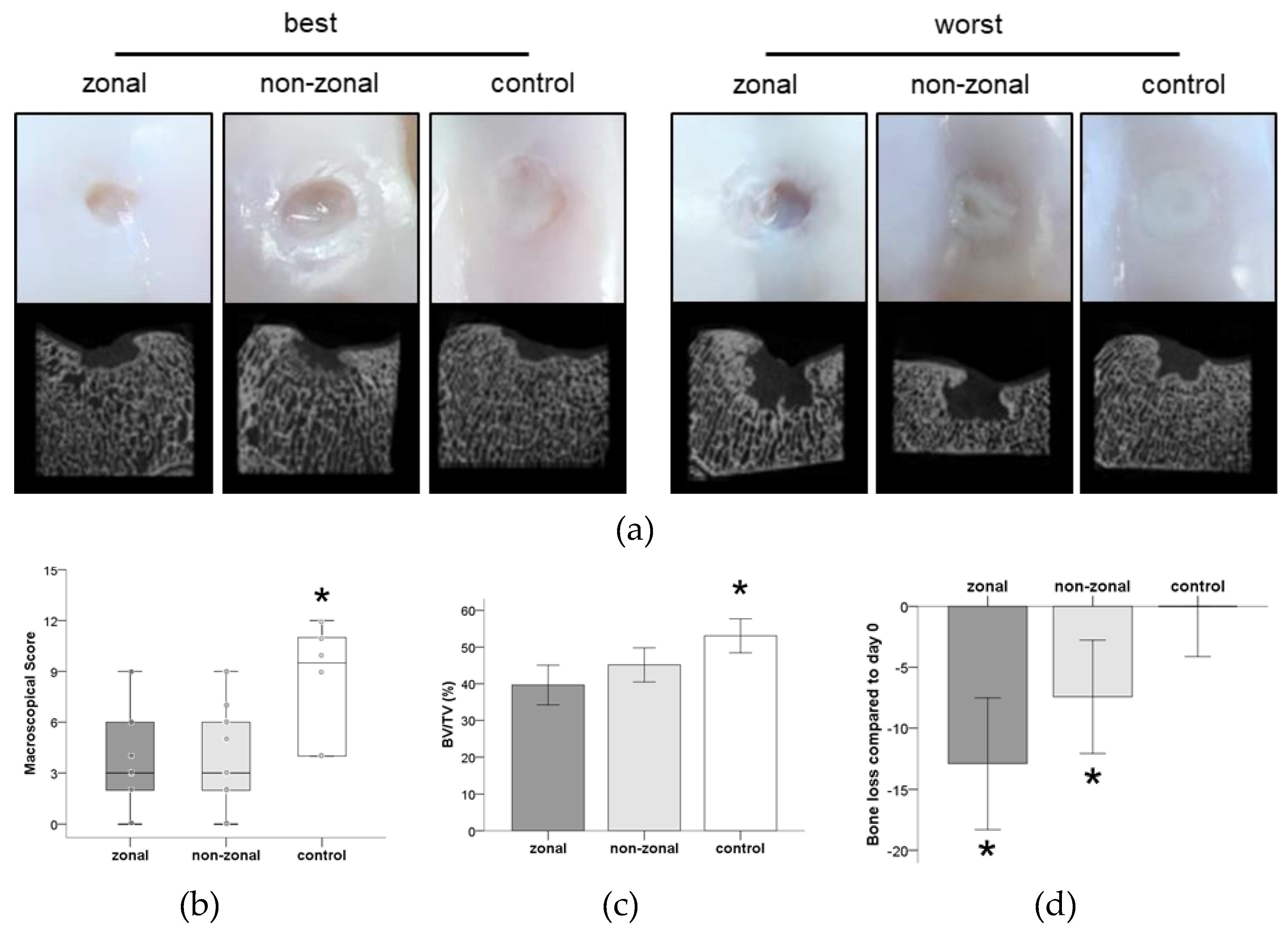
2.2. Inferior Gross Appearance and Substantial Subchondral Bone Changes in Implant-Treated Defects
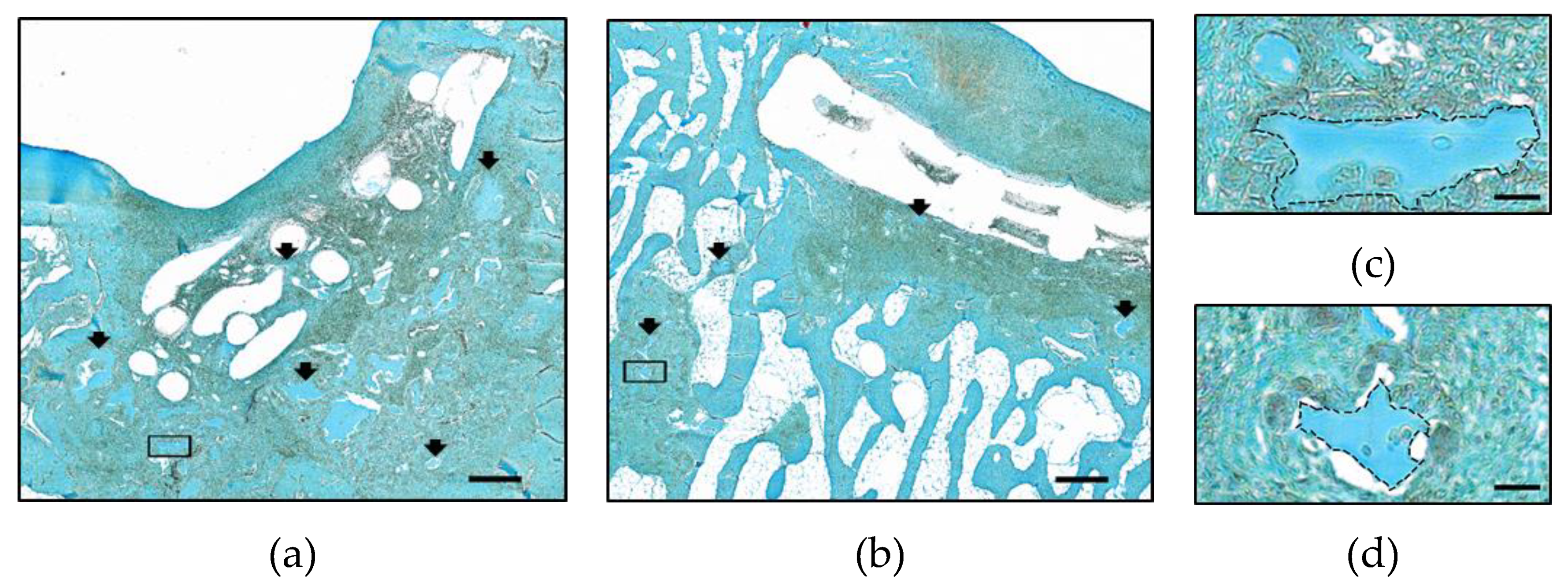
2.3. Implant Retention and Dislocation into Subchondral Bone
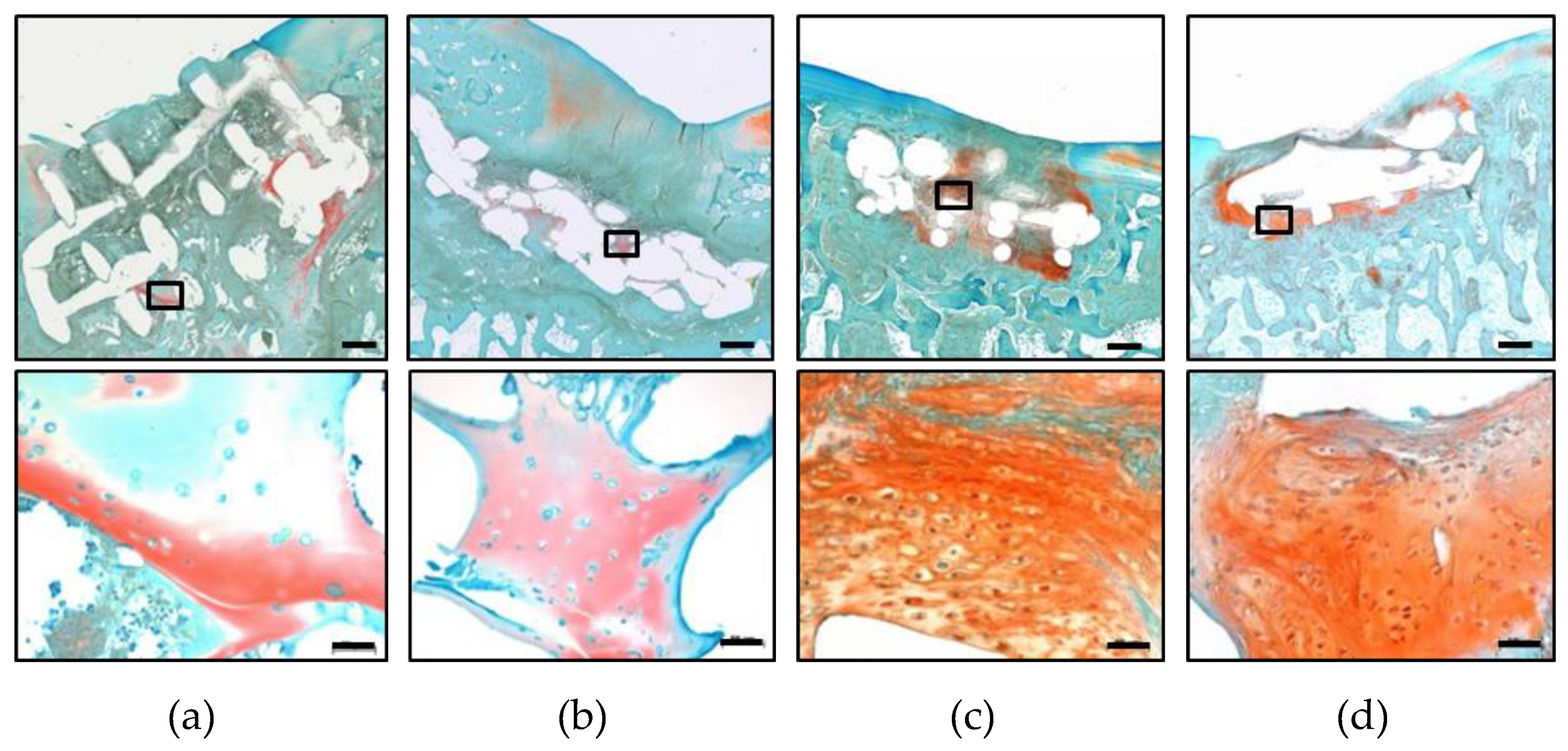
2.4. Hydrogel Persistence and Cell Differentiation in Zonal Versus Non-Zonal Implants
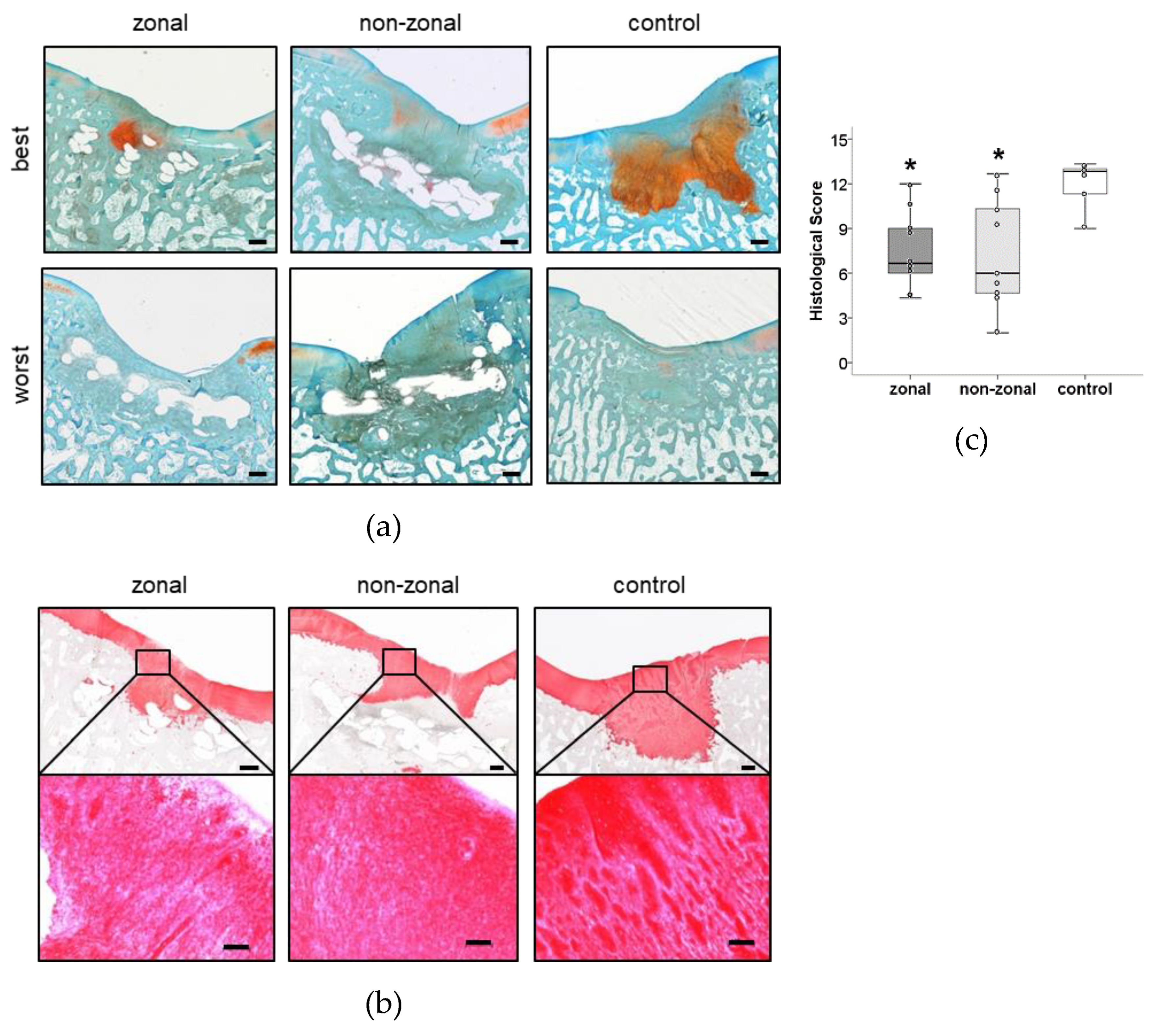
2.5. No Benefit of Zonal or Non-Zonal Design on the Quality of Repair Tissue Compared to Empty Controls
3. Discussion
4. Materials and Methods
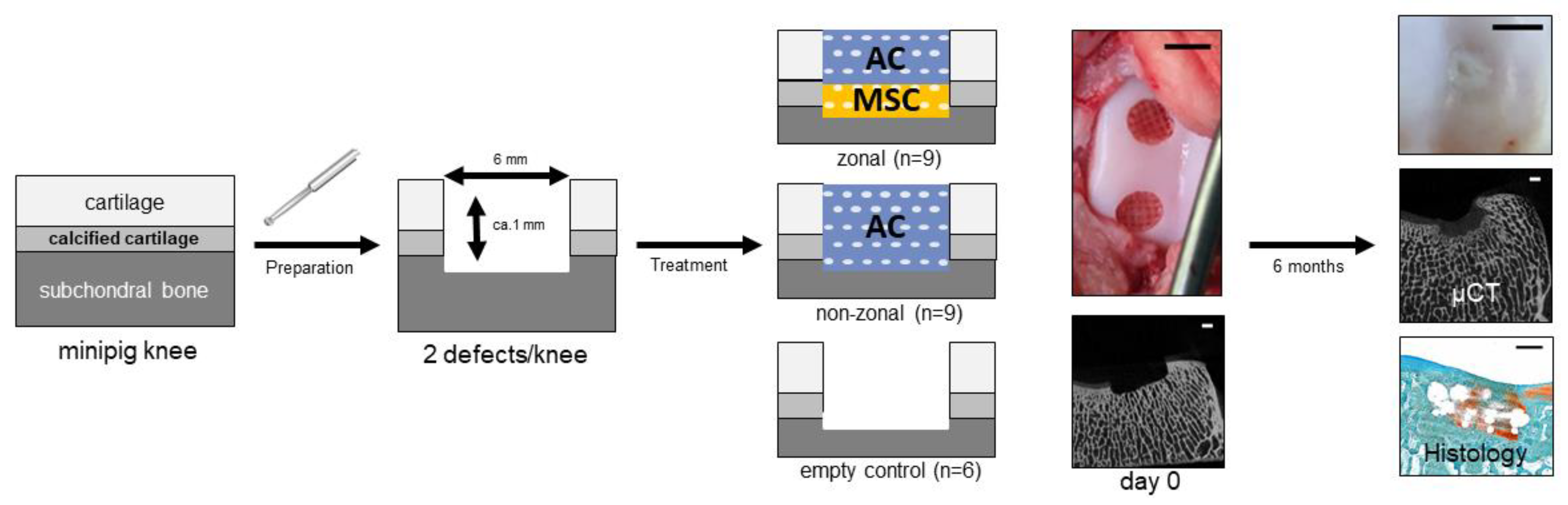
4.1. Animals
4.2. Isolation and Culture of Porcine Articular Chondrocytes
4.3. Isolation and Culture of Porcine Mesenchymal Stromal Cells
4.4. Preparation of Zonal and Non-Zonal Tissue Engineering Constructs
4.5. Biomechanical Testing of Zonal and Non-Zonal Constructs before Implantation
4.6. Orthotopic Transplantation
4.7. Macroscopic Evaluation by ICRS Score
4.8. Micro-CT Analysis
4.9. Histology
4.10. Modified O’Driscoll Score
5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| µCT | X-ray microtomography |
| AC | Articular cartilage |
| ACECM | Articular cartilage extracellular matrix |
| BV | Bone volume |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FCS | Fetal calf serum |
| FGF | Fibroblast growth factor |
| ICRS | International Cartilage Repair Society |
| MACI | Matrix-induced autologous chondrocyte implantation |
| MACT | Matrix-associated autologous chondrocyte transplantation |
| MMP | Matrixmetalloprotease |
| MSC | Mesenchymal stromal cells |
| MWU | Mann–Whitney-U test |
| N | Number |
| n.a. | Not applicable |
| PCL | Polycaprolactone |
| PEG | Poly(ethylene glycol) |
| PLGA | Poly(lactid-co-glycolid) |
| SD | Standard deviation |
| TE | Tissue engineering |
| TV | Total volume |
| VLRH | Very low rubber hardness |
| VOI | Volume of interest |
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Allen, K.D.; Golightly, Y.M. State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 276–283. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Brittberg, M. Autologous chondrocyte implantation—Technique and long-term follow-up. Injury 2008, 39 (Suppl. 1), S40–S49. [Google Scholar] [CrossRef]
- Behrens, P.; Bitter, T.; Kurz, B.; Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 2006, 13, 194–202. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef]
- Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed. Mater. Res. A 2018, 106, 2762–2776. [Google Scholar] [CrossRef]
- Longley, R.; Ferreira, A.M.; Gentile, P. Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. Int. J. Mol. Sci. 2018, 19, 1755. [Google Scholar] [CrossRef]
- Cheng, C.; Conte, E.; Pleshko-Camacho, N.; Hidaka, C. Differences in matrix accumulation and hypertrophy in superficial and deep zone chondrocytes are controlled by bone morphogenetic protein. Matrix Biol. 2007, 26, 541–553. [Google Scholar] [CrossRef]
- Clearfield, D.; Nguyen, A.; Wei, M. Biomimetic multidirectional scaffolds for zonal osteochondral tissue engineering via a lyophilization bonding approach. J. Biomed. Mater. Res. A 2018, 106, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Groen, W.M.; Diloksumpan, P.; van Weeren, P.R.; Levato, R.; Malda, J. From intricate to integrated: Biofabrication of articulating joints. J. Orthop. Res. 2017, 35, 2089–2097. [Google Scholar] [PubMed]
- Kandel, R.A.; Grynpas, M.; Pilliar, R.; Lee, J.; Wang, J.; Waldman, S.; Zalzal, P.; Hurtig, M. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials 2006, 27, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Ramesh, A.; Brady, R.T.; Brama, P.A.J.; Kearney, C.; Gleeson, J.P.; O’Brien, F.J. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 2016, 87, 69–81. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Thompson, E.; Matsiko, A.; Schepens, A.; Gleeson, J.P.; O’Brien, F.J. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater. 2016, 32, 149–160. [Google Scholar] [CrossRef]
- Guo, W.; Zheng, X.; Zhang, W.; Chen, M.; Wang, Z.; Hao, C.; Huang, J.; Yuan, Z.; Zhang, Y.; Wang, M.; et al. Mesenchymal Stem Cells in Oriented PLGA/ACECM Composite Scaffolds Enhance Structure-Specific Regeneration of Hyaline Cartilage in a Rabbit Model. Stem Cells Int. 2018, 2018, 6542198. [Google Scholar] [CrossRef]
- Yin, L.; Wu, Y.; Yang, Z.; Denslin, V.; Ren, X.; Tee, C.A.; Lai, Z.; Lim, C.T.; Han, J.; Lee, E.H. Characterization and application of size-sorted zonal chondrocytes for articular cartilage regeneration. Biomaterials 2018, 165, 66–78. [Google Scholar] [CrossRef]
- Kunisch, E.; Knauf, A.K.; Hesse, E.; Freudenberg, U.; Werner, C.; Bothe, F.; Diederichs, S.; Richter, W. StarPEG/heparin-hydrogel based in vivo engineering of stable bizonal cartilage with a calcified bottom layer. Biofabrication 2018, 11, 015001. [Google Scholar] [CrossRef]
- Marionneaux, A.; Walters, J.; Guo, H.; Mercuri, J. Tailoring the subchondral bone phase of a multi-layered osteochondral construct to support bone healing and a cartilage analog. Acta Biomater. 2018, 78, 351–364. [Google Scholar] [CrossRef]
- Chen, T.; Bai, J.; Tian, J.; Huang, P.; Zheng, H.; Wang, J. A single integrated osteochondral in situ composite scaffold with a multi-layered functional structure. Colloids Surf. B Biointerfaces 2018, 167, 354–363. [Google Scholar] [CrossRef]
- Redler, I.; Mow, V.C.; Zimny, M.L.; Mansell, J. The ultrastructure and biomechanical significance of the tidemark of articular cartilage. Clin. Orthop. Relat. Res. 1975, 112, 357–362. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Allan, K.S.; Pilliar, R.M.; Wang, J.; Grynpas, M.D.; Kandel, R.A. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007, 13, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Sharma, B.; Williams, C.G.; Ruffner, M.A.; Malik, A.; McFarland, E.G.; Elisseeff, J.H. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage 2003, 11, 653–664. [Google Scholar] [CrossRef]
- Klein, T.J.; Rizzi, S.C.; Reichert, J.C.; Georgi, N.; Malda, J.; Schuurman, W.; Crawford, R.W.; Hutmacher, D.W. Strategies for zonal cartilage repair using hydrogels. Macromol. Biosci. 2009, 9, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B Rev. 2009, 15, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Sarem, M.; Arya, N.; Heizmann, M.; Neffe, A.T.; Barbero, A.; Gebauer, T.P.; Martin, I.; Lendlein, A.; Shastri, V.P. Interplay between stiffness and degradation of architectured gelatin hydrogels leads to differential modulation of chondrogenesis in vitro and in vivo. Acta Biomater. 2018, 69, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, K.; Schell, H.; Kleemann, R.U.; Schill, A.; Weiler, A.; Duda, G.N.; Epari, D.R. Influence of scaffold stiffness on subchondral bone and subsequent cartilage regeneration in an ovine model of osteochondral defect healing. Am. J. Sports Med. 2008, 36, 2379–2391. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.A.D.; Vindas Bolanos, R.A.; Brommer, H.; Castilho, M.; Ribeiro, A.; van Loon, J.; Mensinga, A.; van Rijen, M.H.P.; Malda, J.; van Weeren, R. Fixation of Hydrogel Constructs for Cartilage Repair in the Equine Model: A Challenging Issue. Tissue Eng. Part C Methods 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Farrell, M.J.; Steinberg, D.R.; Burdick, J.A.; Mauck, R.L. Enhanced nutrient transport improves the depth-dependent properties of tri-layered engineered cartilage constructs with zonal co-culture of chondrocytes and MSCs. Acta Biomater. 2017, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cigan, A.D.; Durney, K.M.; Nims, R.J.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. Nutrient Channels Aid the Growth of Articular Surface-Sized Engineered Cartilage Constructs. Tissue Eng. Part A 2016, 22, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Angione, S.L.; Ng, K.W.; Lima, E.G.; Williams, D.Y.; Mao, D.Q.; Ateshian, G.A.; Hung, C.T. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage 2009, 17, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Nims, R.J.; Cigan, A.D.; Albro, M.B.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. Matrix Production in Large Engineered Cartilage Constructs Is Enhanced by Nutrient Channels and Excess Media Supply. Tissue Eng. Part C Methods 2015, 21, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Brehm, W.; Aklin, B.; Yamashita, T.; Rieser, F.; Trub, T.; Jakob, R.P.; Mainil-Varlet, P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: Implantation method and short-term results. Osteoarthritis Cartilage 2006, 14, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Gotterbarm, T.; Breusch, S.J.; Schneider, U.; Jung, M. The minipig model for experimental chondral and osteochondral defect repair in tissue engineering: Retrospective analysis of 180 defects. Lab. Anim. 2008, 42, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Barron, V.; Neary, M.; Mohamed, K.M.; Ansboro, S.; Shaw, G.; O’Malley, G.; Rooney, N.; Barry, F.; Murphy, M. Evaluation of the Early In Vivo Response of a Functionally Graded Macroporous Scaffold in an Osteochondral Defect in a Rabbit Model. Ann. Biomed. Eng. 2016, 44, 1832–1844. [Google Scholar] [CrossRef]
- Von Rechenberg, B.; Akens, M.K.; Nadler, D.; Bittmann, P.; Zlinszky, K.; Kutter, A.; Poole, A.R.; Auer, J.A. Changes in subchondral bone in cartilage resurfacing—An experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage 2003, 11, 265–277. [Google Scholar] [CrossRef]
- Fisher, M.B.; Belkin, N.S.; Milby, A.H.; Henning, E.A.; Bostrom, M.; Kim, M.; Pfeifer, C.; Meloni, G.; Dodge, G.R.; Burdick, J.A.; et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: Implications for tissue engineering. Tissue Eng. Part A 2015, 21, 850–860. [Google Scholar] [CrossRef]
- Lopa, S.; Madry, H. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng. Part A 2014, 20, 2052–2076. [Google Scholar] [CrossRef]
- Lofvall, H.; Newbould, H.; Karsdal, M.A.; Dziegiel, M.H.; Richter, J.; Henriksen, K.; Thudium, C.S. Osteoclasts degrade bone and cartilage knee joint compartments through different resorption processes. Arthritis Res. Ther. 2018, 20, 67. [Google Scholar] [CrossRef]
- Getgood, A.; Henson, F.; Skelton, C.; Herrera, E.; Brooks, R.; Fortier, L.A.; Rushton, N. The Augmentation of a Collagen/Glycosaminoglycan Biphasic Osteochondral Scaffold with Platelet-Rich Plasma and Concentrated Bone Marrow Aspirate for Osteochondral Defect Repair in Sheep: A Pilot Study. Cartilage 2012, 3, 351–363. [Google Scholar] [CrossRef] [PubMed]
- McCarrel, T.M.; Pownder, S.L.; Gilbert, S.; Koff, M.F.; Castiglione, E.; Saska, R.A.; Bradica, G.; Fortier, L.A. Two-Year Evaluation of Osteochondral Repair with a Novel Biphasic Graft Saturated in Bone Marrow in an Equine Model. Cartilage 2017, 8, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Freudenberg, U.; Niemietz, T.; Greth, C.; Weisser, M.; Hagmann, S.; Binner, M.; Werner, C.; Richter, W. Peptide-functionalized starPEG/heparin hydrogels direct mitogenicity, cell morphology and cartilage matrix distribution in vitro and in vivo. J. Tissue Eng. Regen. Med. 2018, 12, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Blanke, M.; Carl, H.D.; Klinger, P.; Swoboda, B.; Hennig, F.; Gelse, K. Transplanted chondrocytes inhibit endochondral ossification within cartilage repair tissue. Calcif. Tissue Int. 2009, 85, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Orth, P.; Cucchiarini, M.; Kaul, G.; Ong, M.F.; Graber, S.; Kohn, D.M.; Madry, H. Temporal and spatial migration pattern of the subchondral bone plate in a rabbit osteochondral defect model. Osteoarthritis Cartilage 2012, 20, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, M.V.; Chwalek, K.; Prokoph, S.; Zieris, A.; Levental, K.R.; Freudenberg, U.; Werner, C. Defined polymer-peptide conjugates to form cell-instructive starPEG-heparin matrices in situ. Adv. Mater. 2013, 25, 2606–2610. [Google Scholar] [CrossRef] [PubMed]
- Krase, A.; Abedian, R.; Steck, E.; Hurschler, C.; Richter, W. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthritis Cartilage 2014, 22, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kaszap, B.; Redohl, A.; Steck, E.; Breusch, S.; Richter, W.; Gotterbarm, T. Enhanced early tissue regeneration after matrix-assisted autologous mesenchymal stem cell transplantation in full thickness chondral defects in a minipig model. Cell Transplant. 2009, 18, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Mainil-Varlet, P.; Aigner, T.; Brittberg, M.; Bullough, P.; Hollander, A.; Hunziker, E.; Kandel, R.; Nehrer, S.; Pritzker, K.; Roberts, S.; et al. Histological assessment of cartilage repair: A report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J. Bone Joint Surg. Am. 2003, 85-A (Suppl. 2), 45–57. [Google Scholar] [CrossRef]
- O’Driscoll, S.W.; Keeley, F.W.; Salter, R.B. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J. Bone Joint Surg. Am. 1988, 70, 595–606. [Google Scholar] [CrossRef]

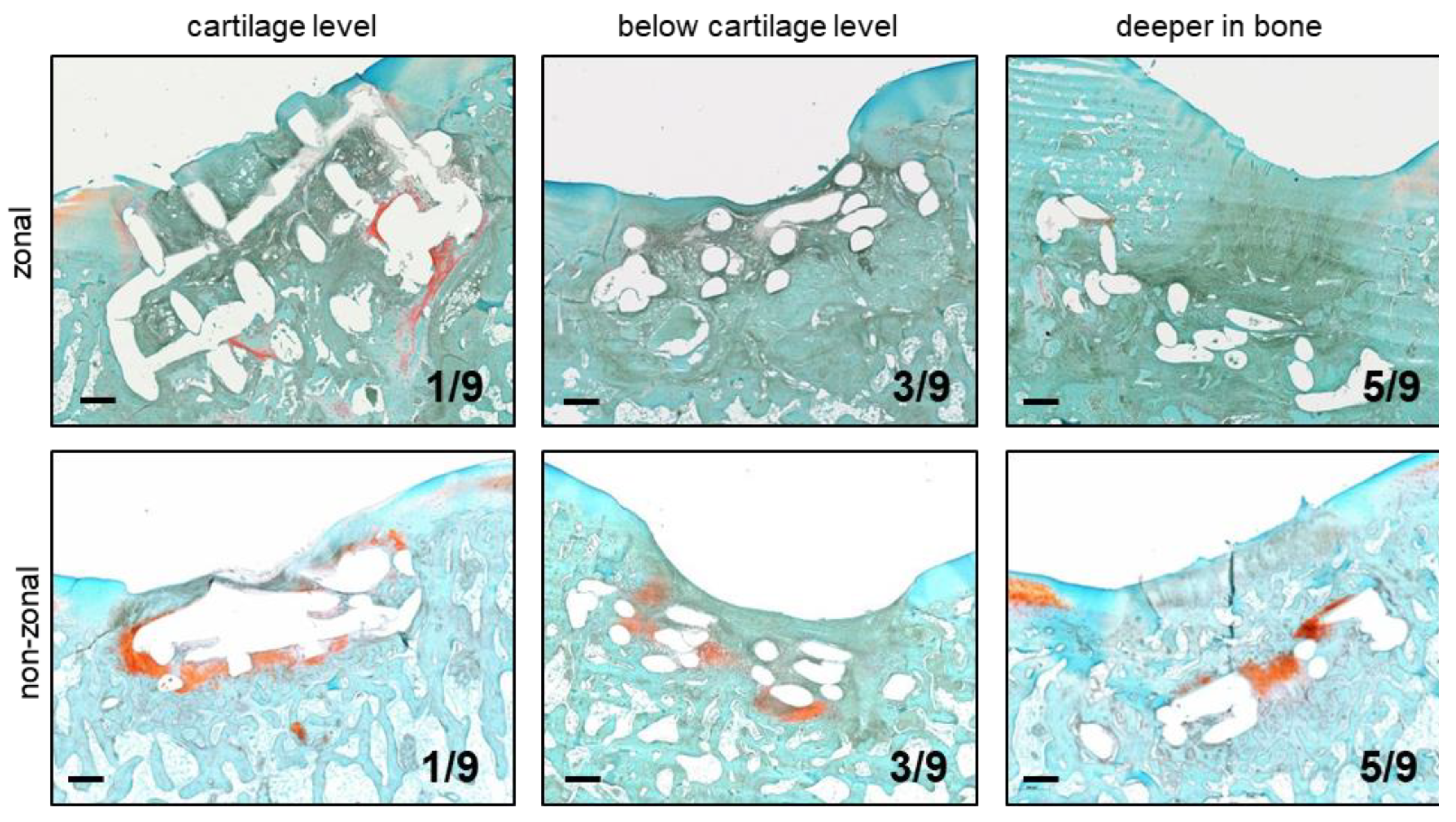
| Group | Total | Differentiation in Hydrogel | Differentiation Connected to PCL | Non-Connected Differentiation |
|---|---|---|---|---|
| zonal | 4/9 | 2 | 2 | 1 |
| non-zonal | 7/9 | 3 | 2 | 3 |
| empty control | 4/6 | n.a. 1 | n.a. 1 | 4 |
| Characteristic | Grading | Score |
|---|---|---|
| Degree of defect repair | In level with surrounding cartilage | 4 |
| 75% repair of defect depth | 3 | |
| 50% repair of defect depth | 2 | |
| 25% repair of defect depth | 1 | |
| 0% repair of defect depth | 0 | |
| Integration to border zone | Complete integration with surrounding cartilage | 4 |
| Demarcating border <1 mm | 3 | |
| 3/4th of graft integrated, 1/4th with a notable border >1 mm width | 2 | |
| 1/2 of graft integrated with surrounding cartilage, 1/2 with a notable border >1 mm | 1 | |
| From no contact to 1/4th of graft integrated with surrounding cartilage | 0 | |
| Macroscopic appearance | Intact smooth surface | 4 |
| Fibrillated surface | 3 | |
| Small, scattered fissures or cracks | 2 | |
| Several, small or few but large fissures | 1 | |
| Total degeneration of grafted area | 0 | |
| Total | 12 |
| Characteristic | Criterion | Score Range |
|---|---|---|
| Nature of predominant tissue | Morphology | 0–4 |
| Staining of matrix | 0–3 | |
| Structural characteristics | Surface | 0–1 |
| Lateral integration | 0–2 | |
| Basal integration | 0–2 | |
| Subchondral bone | 0–3 | |
| Total | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bothe, F.; Deubel, A.-K.; Hesse, E.; Lotz, B.; Groll, J.; Werner, C.; Richter, W.; Hagmann, S. Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs. Int. J. Mol. Sci. 2019, 20, 653. https://doi.org/10.3390/ijms20030653
Bothe F, Deubel A-K, Hesse E, Lotz B, Groll J, Werner C, Richter W, Hagmann S. Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs. International Journal of Molecular Sciences. 2019; 20(3):653. https://doi.org/10.3390/ijms20030653
Chicago/Turabian StyleBothe, Friederike, Anne-Kathrin Deubel, Eliane Hesse, Benedict Lotz, Jürgen Groll, Carsten Werner, Wiltrud Richter, and Sebastien Hagmann. 2019. "Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs" International Journal of Molecular Sciences 20, no. 3: 653. https://doi.org/10.3390/ijms20030653
APA StyleBothe, F., Deubel, A.-K., Hesse, E., Lotz, B., Groll, J., Werner, C., Richter, W., & Hagmann, S. (2019). Treatment of Focal Cartilage Defects in Minipigs with Zonal Chondrocyte/Mesenchymal Progenitor Cell Constructs. International Journal of Molecular Sciences, 20(3), 653. https://doi.org/10.3390/ijms20030653





