X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions
Abstract
:1. Introduction
2. Results and Discussions
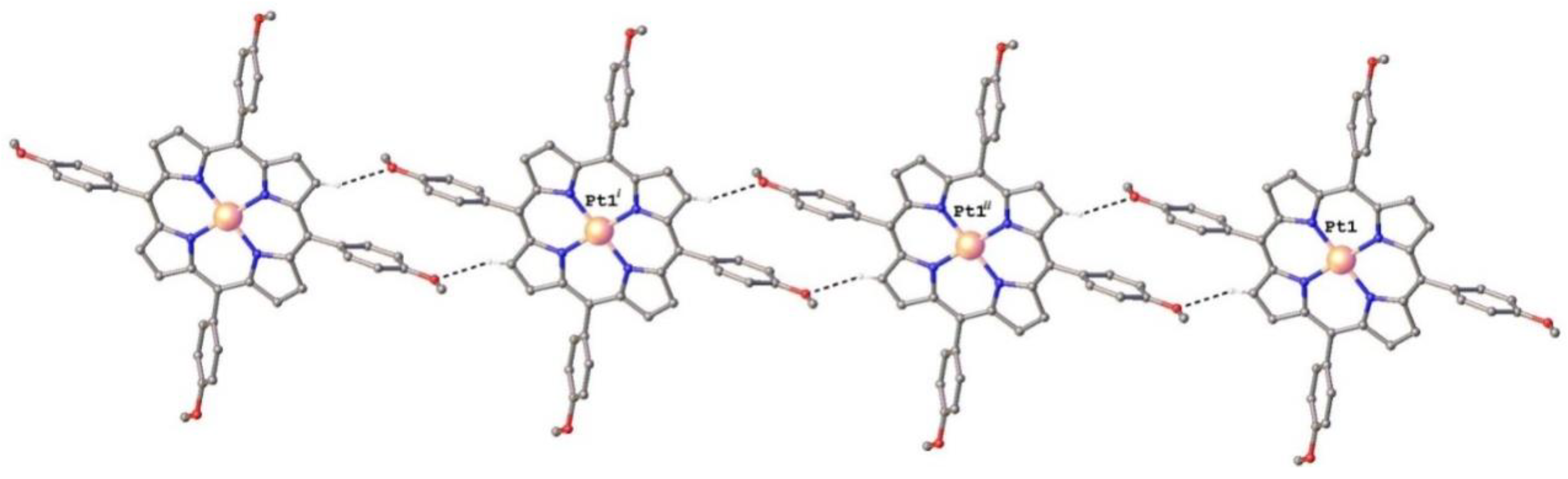
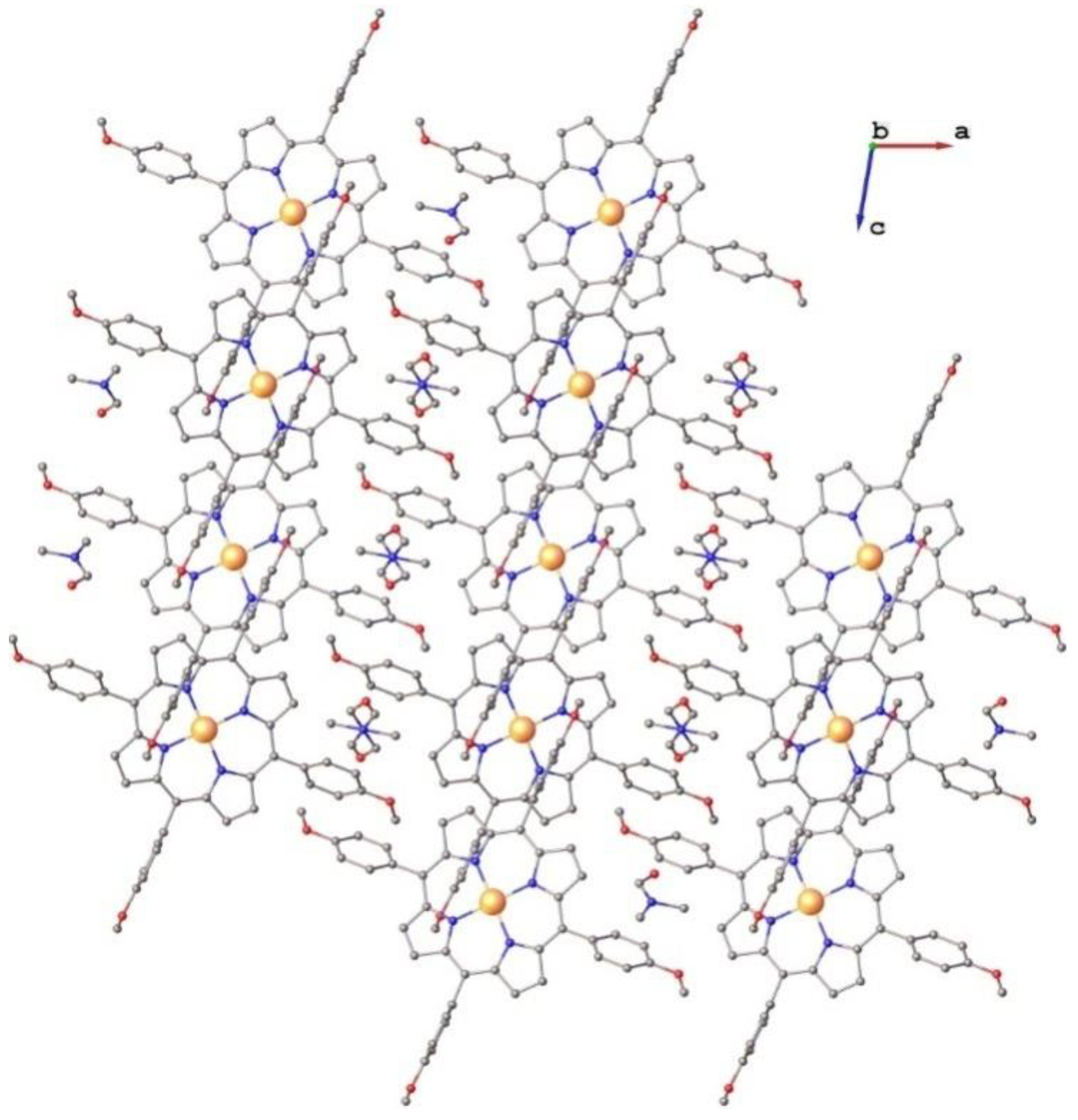
2.1. Structure of Pt(II)-5,10,15,20-tetra(4-methoxy-phenyl)-porphyrin (PtTMeOPP) Investigated by Single Crystal X-ray Diffraction
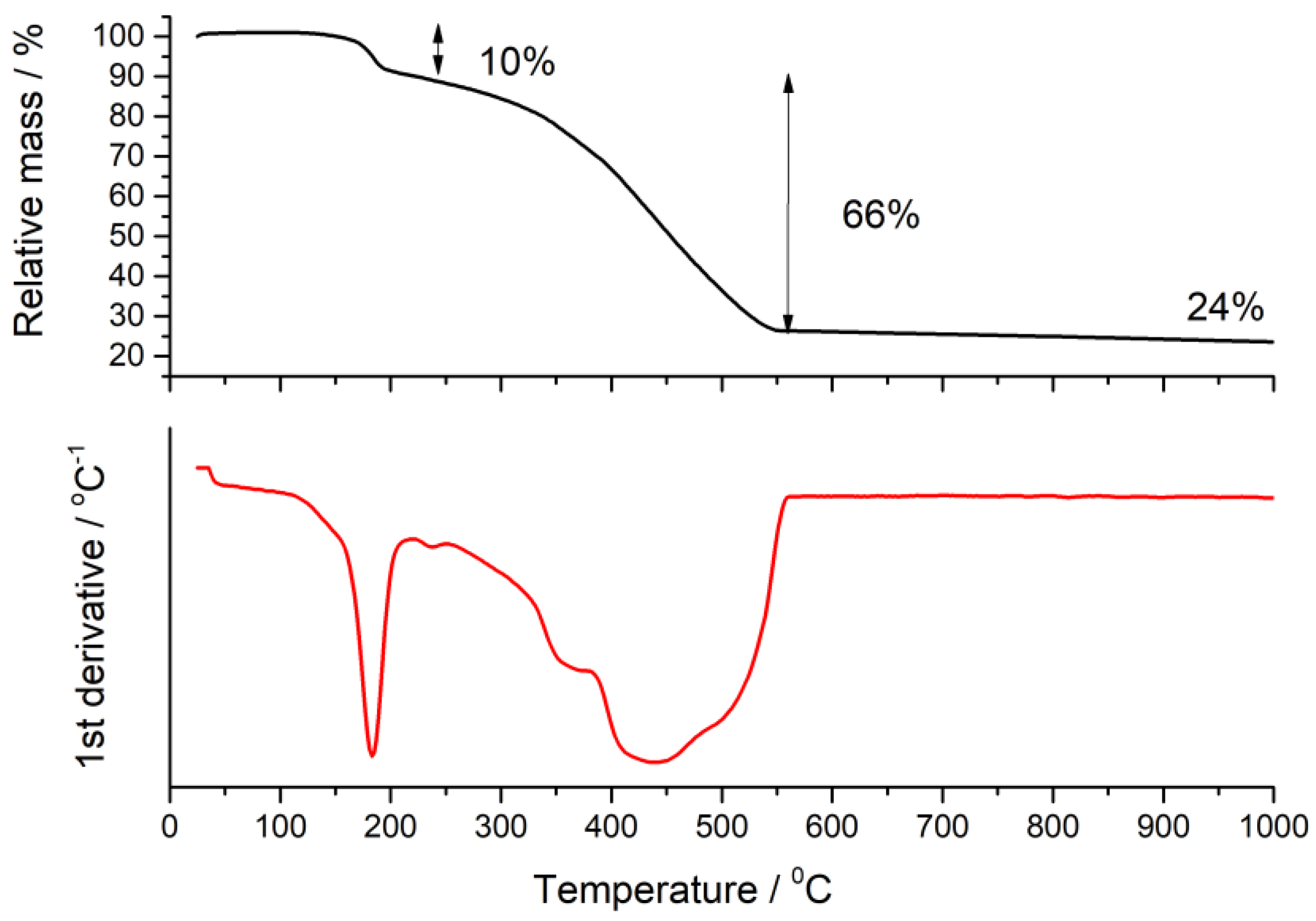
2.2. Thermogravimetric Analysis
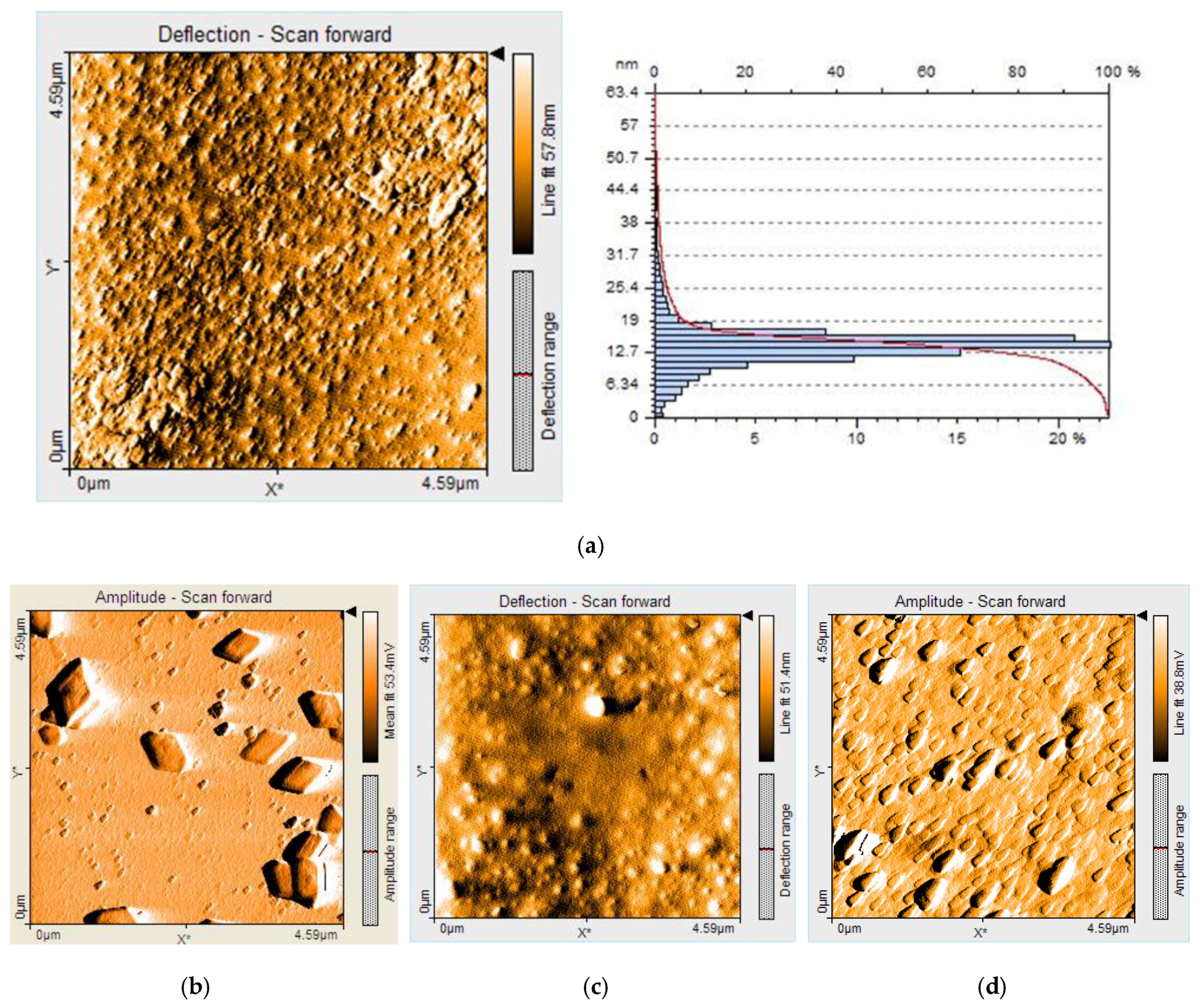
2.3. AFM Investigations
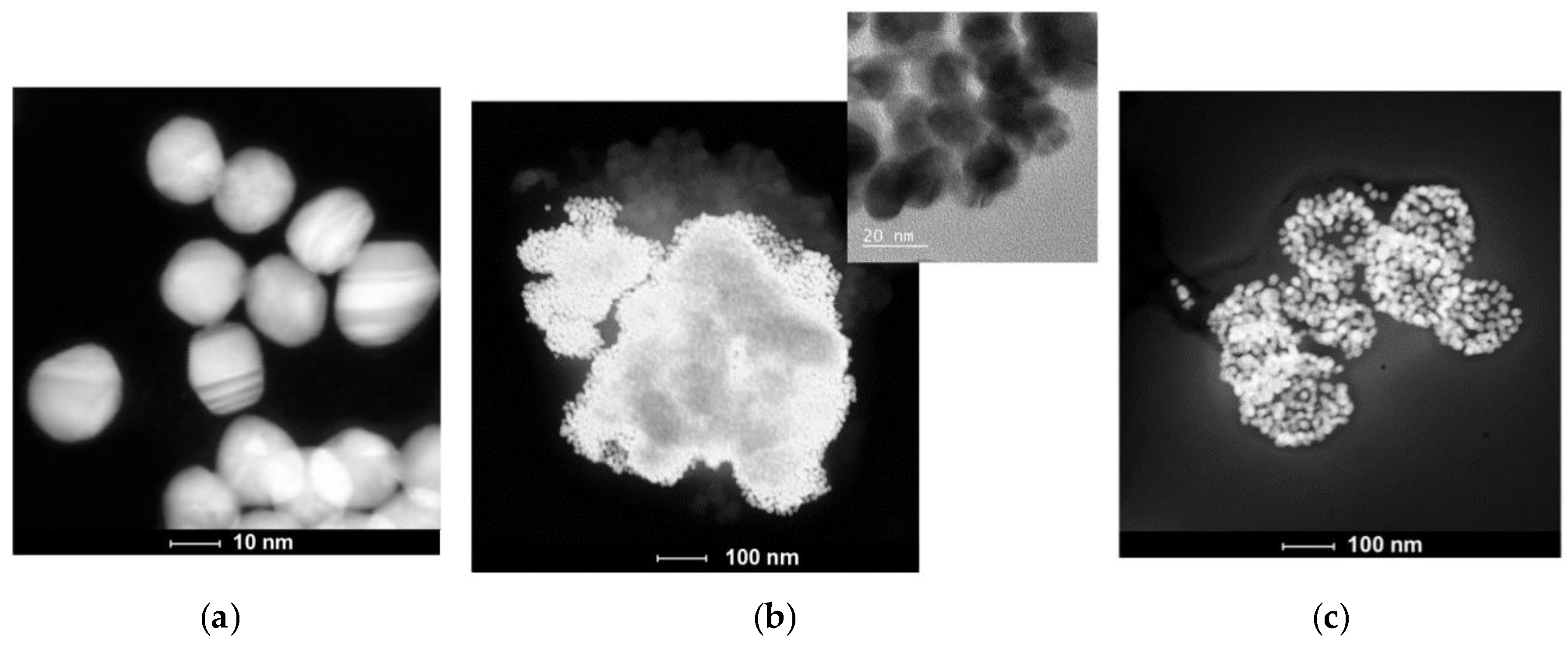
2.4. Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM) Investigations
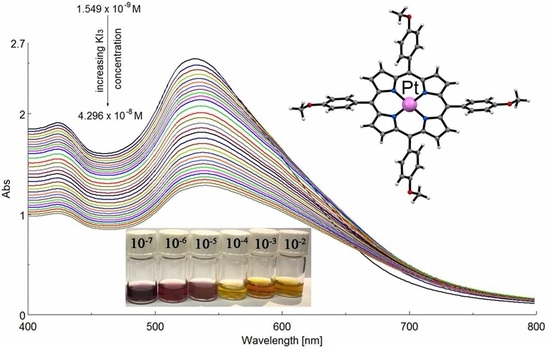
2.5. UV–Vis Spectrophotometric Detection of Triiodide Ion I3− Using as Sensitive Material AuNPs/PtTMeOPP Hybrid
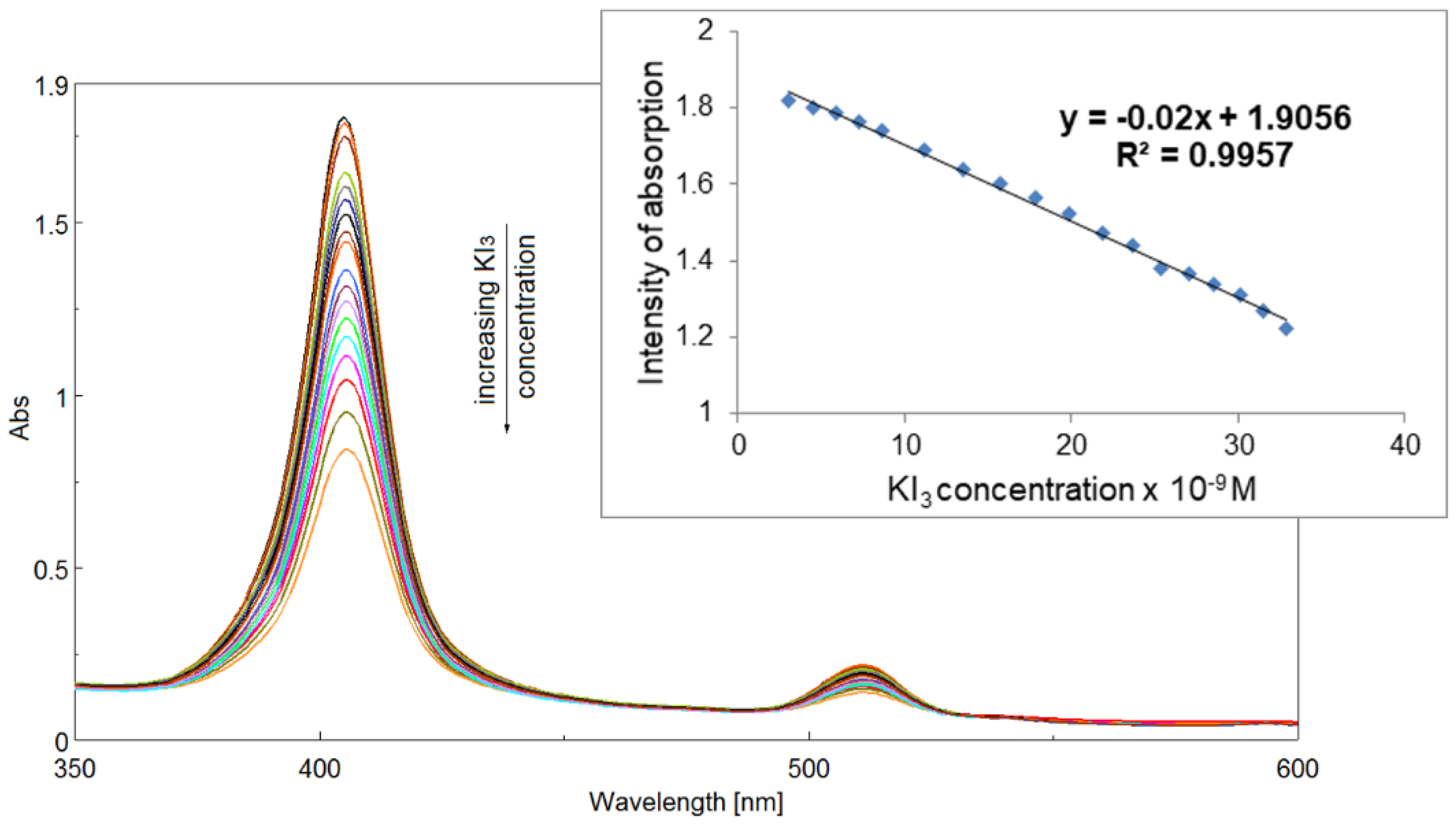
2.5.1. Obtaining the AuNPs/PtTMeOPP Hybrid Material
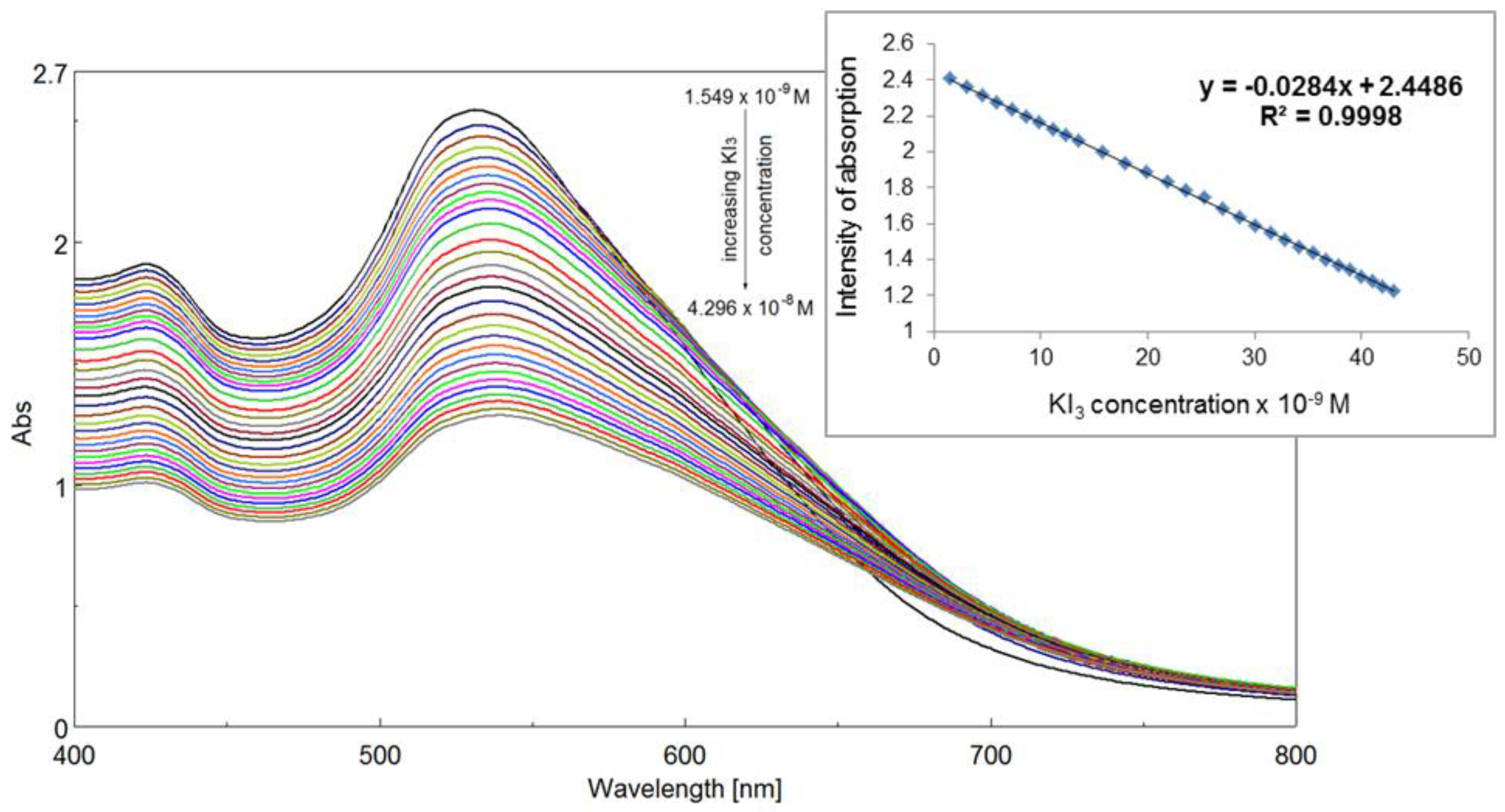
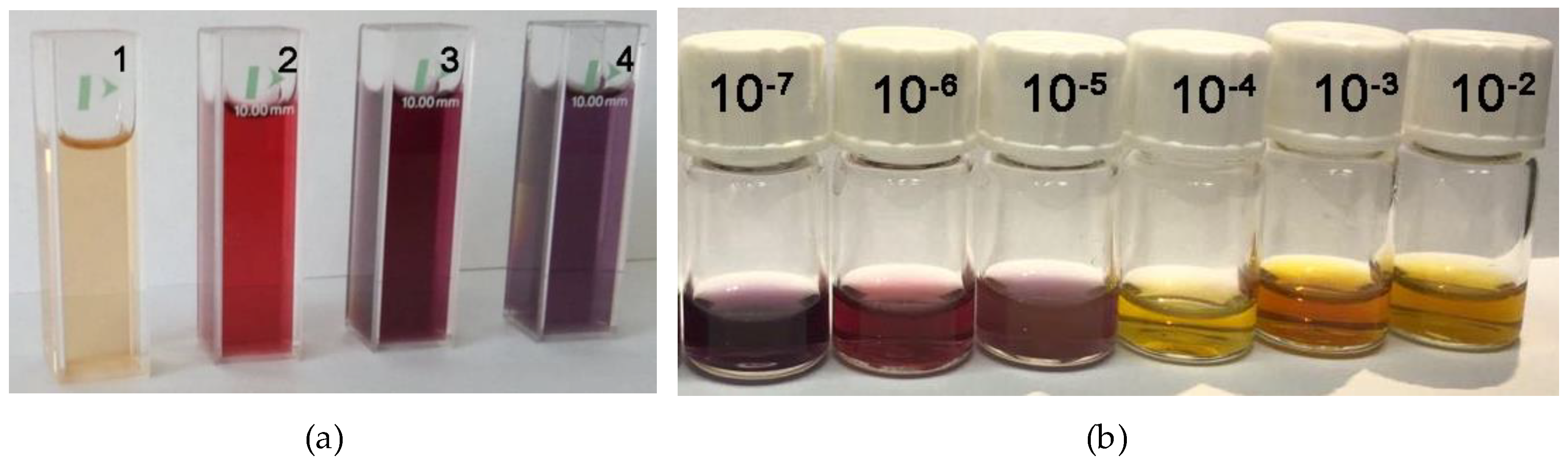
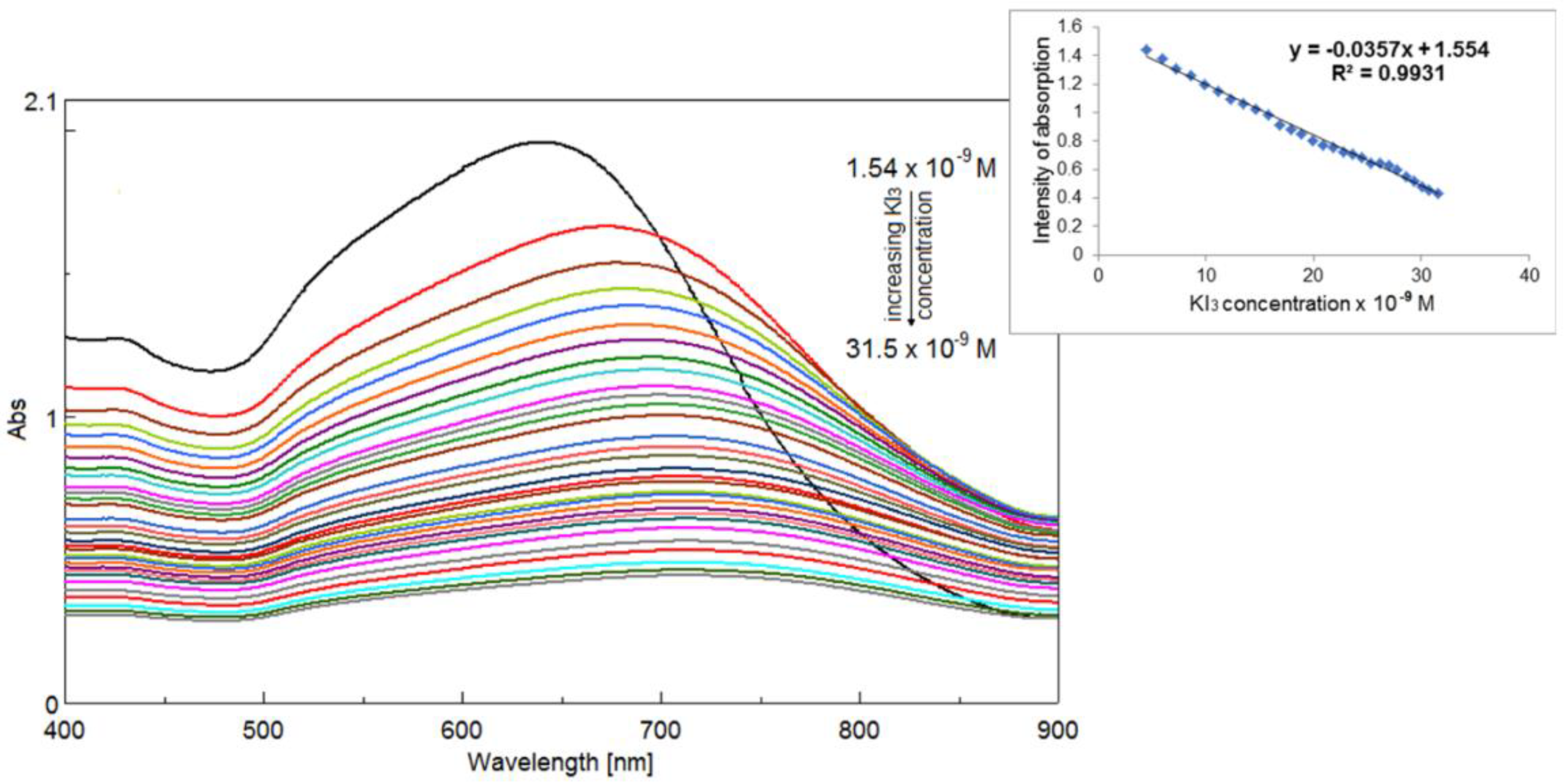
2.5.2. Detection of Triiodide Ion I3− Using As Sensitive Material AuNPs/PtTMeOPP Hybrid
2.5.3. Detection of Triiodide ion I3− Using As Sensitive Material AuNPs/PtTMeOPP Hybrid in Phosphate Buffer
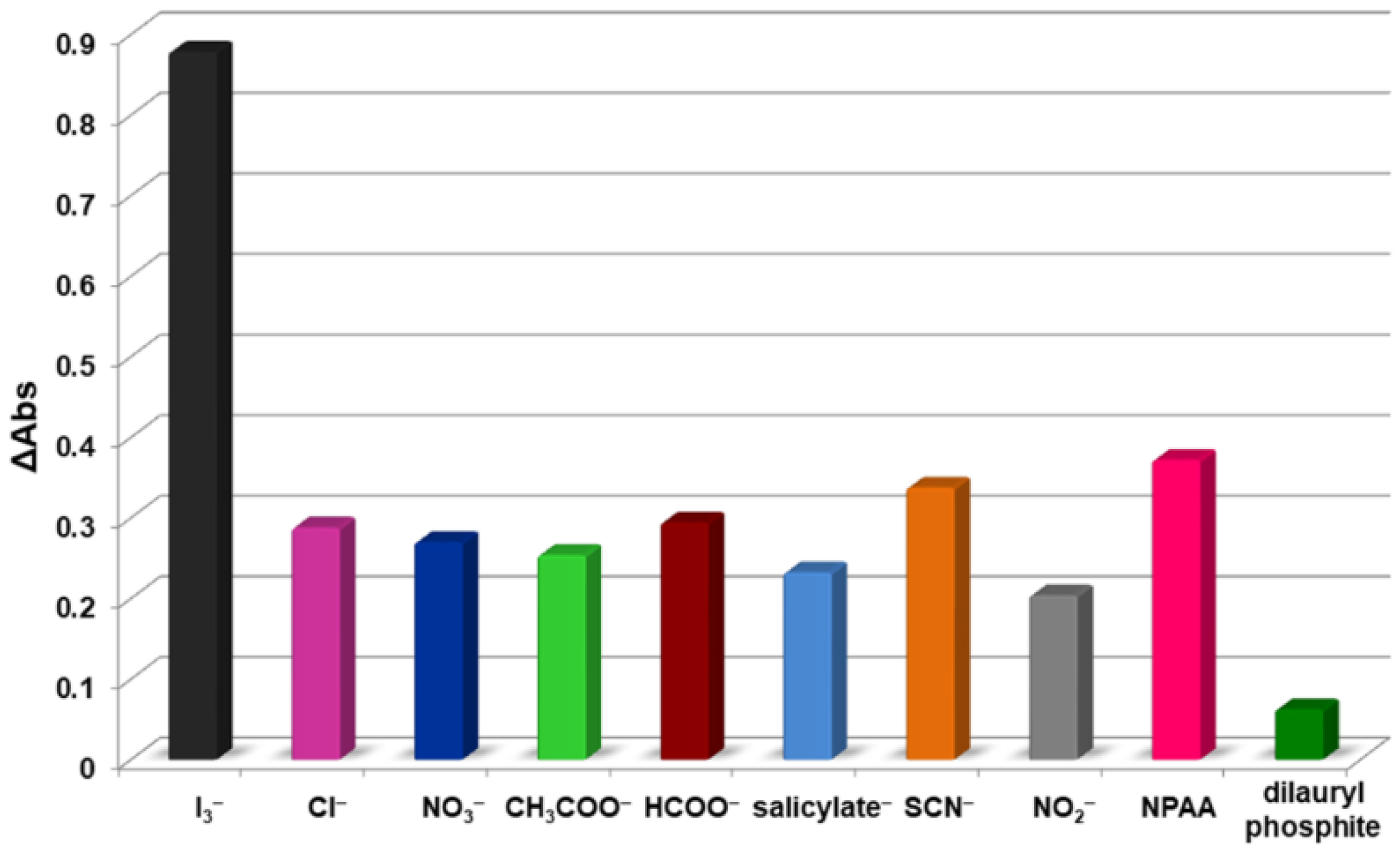
2.5.4. Interference Study
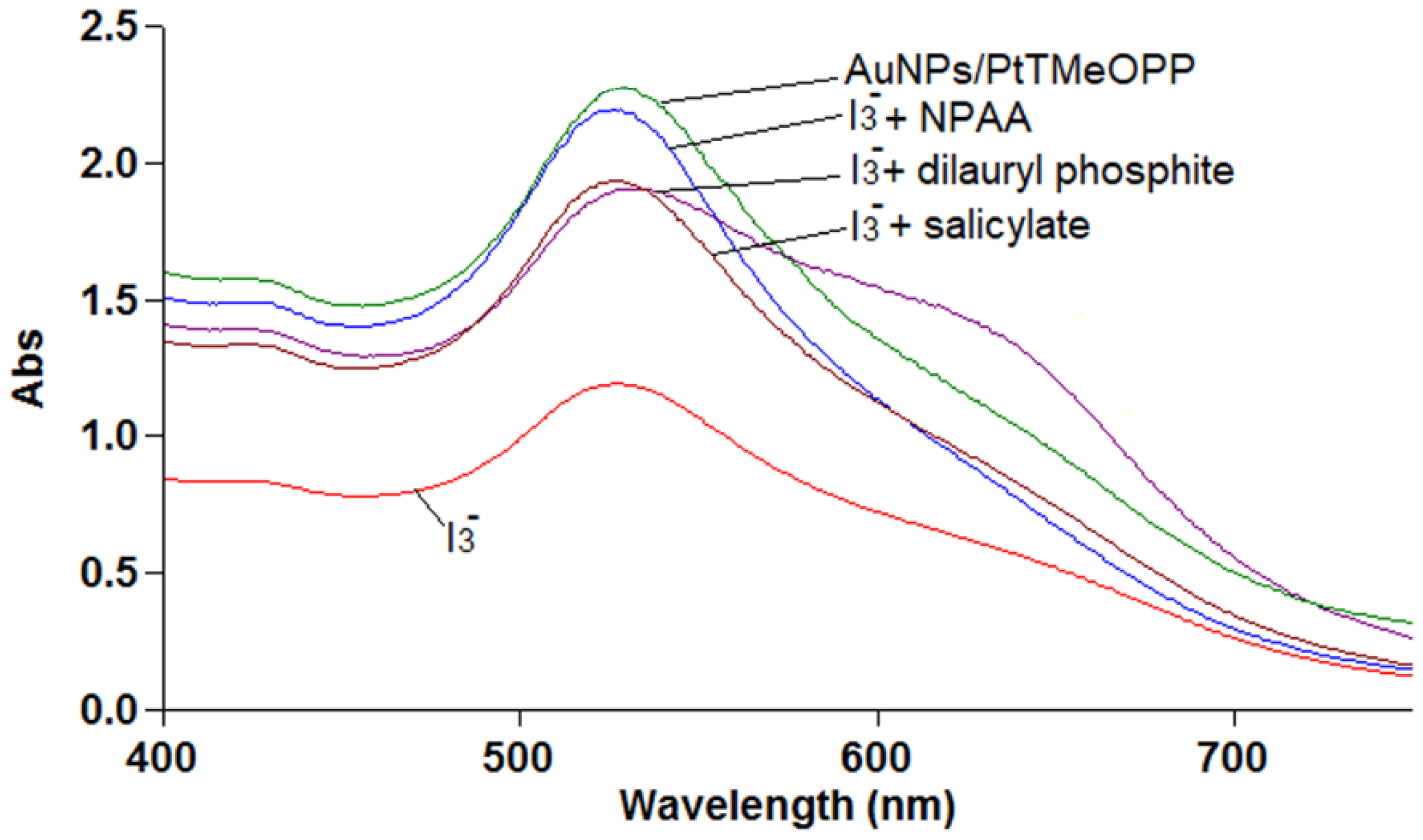
2.5.5. Preliminary Mechanism Investigations for I3− Detection
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.2.1. Synthesis of Pt(II) 5,10,15,20-tetra(4-methoxy-phenyl)-porphyrin
3.2.2. Single Crystal Preparation of Pt(II) 5,10,15,20-tetra(4-methoxy-phenyl)-porphyrin (PtTMeOPP)/DMF (2:1)
3.2.3. Synthesis of AuNPs
3.3. X-ray Crystallography
3.4. UV–Visible Spectral Studies
3.5. AFM, STEM, TEM Imaging
3.6. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EAR | The estimated average requirement |
| WHO | World Health Organization |
| FAO | Food and Agriculture Organization of the United Nations |
| PVC | Polyvinylchloride |
| NPOE | O-Nitrophenyloctylether |
| DOP | Dioctylphtalate |
| CSD | Cambridge Structural Database |
| PtTMeOPP | Pt(II) 5,10,15,20-tetra(4-methoxy-phenyl)-porphyrin |
| AuNPs | Gold nanoparticles |
| AFM | Atomic force microscopy |
| DMF | Dimethylformamide |
| GOF | Goodness of fit |
| TGA | Thermogravimetric analysis |
| THF | Tetrahydrofuran |
| NPAA | N-phenylantranilic acid |
References
- Hand, D.; Wilson, D. Iodine or Iodide? A laboratory evaluation of the content of powdered iodine supplements. J. Restor. Med. 2017, 6, 62–68. [Google Scholar] [CrossRef]
- Taylor, P.; Okosieme, O.; Premawardhana, L.; Lazarus, J.H. Iodine deficiency, Reference Module in Biomedical Sciences. In Encyclopedia of Endocrine Diseases, 2nd ed.; Cardiff University: Cardiff, UK, 2018; pp. 1–12. [Google Scholar]
- Mancini, F.R.; Rajaobelina, K.; Dow, C.; Habbal, T.; Affret, A.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.-C.; Fagherazzi, G. High iodine dietary intake is associated with type 2 diabetes among women of the E3N-EPIC cohort study. Clin. Nutr. 2018, 1–6. [Google Scholar] [CrossRef]
- Kramer, G.H. Retention of iodine in the body: Biological half-life of iodine in the human body. In Comprehensive Handbook of Iodine: Nutritional Biochemical, Pathological and Therapeutic Aspects; Preedy, V.R., Burrow, G.N., Watson, R.R., Eds.; Elsevier, Academic Press: Atlanta, GA, USA, 2009; pp. 184–191. ISBN 9780080920863. [Google Scholar]
- Farhadi, K.; Maleki, R.; Shamsipur, M. Triiodide ion-selective polymeric membrane electrode based on ketoconazole-triiodide ion pair. Electroanalysis 2002, 14, 760–766. [Google Scholar] [CrossRef]
- Pedreno, C.-S.; Ortuño, J.-A.; Hernandez, J. Determination of chlorine and dissolved oxygen in waters and of ascorbic acid in pharmaceuticals by iodimetric potentiometric titration using a plasticized poly(vinyl chloride) membrane electrode. J. Anal. Chim. Acta 1996, 333, 107–113. [Google Scholar] [CrossRef]
- Khayatian, G.; Rezatabar, H.; Karonian, F.S.; Salimi, A. Triiodide ion-selective electrode based on charge-transfer complex of 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo-[8.8.8]hexacosane. J. Chin. Chem. Soc. 2006, 53, 1133–1139. [Google Scholar] [CrossRef]
- Garg, A.; Tomar, M.; Gupta, V. Synthesis and characterization of thin films of bismuth triiodide for semiconductor radiation detectors. Conf. Pap. Sci. 2014, 2014. [Google Scholar] [CrossRef]
- Kireev, S.V.; Shnyrev, S.L. Study of molecular iodine, iodate ions, iodide ions and triiodide ions solutions absorption in the UV and visible light spectral bands. Laser Phys. 2015, 25, 075602. [Google Scholar] [CrossRef]
- La Rosa Novo, D.; Eisenhardt Mello, J.; Soares Rondan, F.; Schneider Henn, A.; Azevedo Mello, P.; Foster Mesko, M. Bromine and iodine determination in human saliva: Challenges in the development of an accurate method. Talanta 2019, 191, 415–421. [Google Scholar] [CrossRef]
- Khayatian, G.; Rezatabar, H.; Salimi, A. Triiodide Ion-selective Electrode based on 7,16-dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane. Anal. Sci. 2005, 21, 297–302. [Google Scholar] [CrossRef]
- Fagadar-Cosma, G.-R.; Vlascici, D.; Fagadar-Cosma, E. 5,10,15,20-Tetrakis(4-pyridyl)-21,23H-porphyrin -Zn(II)-iodide-selective ionophore in formulation of new polymeric membrane electrodes. J. Biol. Inorg. Chem. 2007, 12, S218. [Google Scholar]
- Vlascici, D.; Pica, E.M.; Fagadar-Cosma, E.; Cosma, V.; Bizerea, O. Thiocyanate and fluoride electrochemical sensors based on nanostructurated metalloporphyrn systems. J. Optoelectron. Adv. Mater. 2008, 10, 2303–2306. [Google Scholar]
- Vlascici, D.; Fagadar-Cosma, G.; Plesu, N.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric sensors for iodide and bromide based on Pt(II)-porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Shawkat, M.; Aly, S.M.; Alarousu, E.; Mohammed, O.F. Photoinduced triplet-state electron transfer of platinum porphyrin: A one-step direct method for sensing iodide to an unprecedented detection limit. J. Mater. Chem. A 2015, 3, 6733–6738. [Google Scholar] [CrossRef]
- Urbani, M.; Gratzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Hutter, L.H.; Muller, B.J.; Koren, K.; Borisov, S.M.; Klimant, I. Robust optical oxygen sensors based on polymer-bound NIR-emitting platinum(II)–benzoporphyrins. J. Mater. Chem. C 2014, 2, 7589–7598. [Google Scholar] [CrossRef]
- Arunkumar, C.; Kooriyaden, F.R.; Zhang, X.; Sujatha, S.; Zhao, J. Fluorinated meso-tetraarylPt(II)-porphyrins: Structure, photophysical, electrochemical and phosphorescent oxygen sensing studies. New J. Chem. 2017, 41, 4908–4917. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Shmilovits, M.; Diskin-Posner, Y.; Vinodu, M.; Goldberg, I. Crystal Engineering of “Porphyrin Sieves” Based on Coordination Polymers of Pd- and Pt-tetra(4-carboxyphenyl)porphyrin. Cryst. Growth Des. 2003, 3, 855–863. [Google Scholar] [CrossRef]
- Muniappan, S.; Lipstman, S.; George, S.; Goldberg, I. Porphyrin framework solids. synthesis and structure of hybrid coordination polymers of tetra(carboxyphenyl)porphyrins and lanthanide-bridging ions. Inorg. Chem. 2007, 46, 5544–5554. [Google Scholar] [CrossRef]
- Barron, P.M.; Son, H.-T.; Hu, C.; Choe, W. Highly tunable heterometallic frameworks constructed from paddle-wheel units and metalloporphyrins. Cryst. Growth Des. 2009, 9, 1960–1965. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Jiang, J. Porphyrin-cucurbituril organic molecular porous material: Structure and iodine adsorption properties. Inorg. Chem. Commun. 2013, 35, 156–159. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Sebarchievici, I.; Lascu, A.; Creanga, I.; Palade, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, G. Optical and electrochemical behavior of new nano-sized complexes based on gold-colloid and Co-porphyrin derivative in the presence of H2O2. J. Alloys Compd. 2016, 686, 896–904. [Google Scholar] [CrossRef]
- Lascu, A.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Hybrid Mn-porphyrin-nanogold nanomaterial applied for the spectrophotometric detection of β-carotene. J. Chem. Hindawi 2018. [Google Scholar] [CrossRef]
- Palade, A.; Lascu, A.; Fringu, I.; Salageanu, L.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, E. Comparative diclofenac detection for chronic toxicity levels using water soluble Zn-metalloporphyrin, gold nanoparticles and their hybrid. Farmacia 2018, 66, 468–476. [Google Scholar] [CrossRef]
- Cristescu, R.; Popescu, C.; Popescu, A.C.; Grigorescu, S.; Mihailescu, I.N.; Ciucu, A.A.; Iordache, S.; Andronie, A.; Stamatin, I.; Fagadar-Cosma, E.; et al. MAPLE deposition of Mn(III) metalloporphyrin thin films: Structural, topographical and electrochemical investigations. Appl. Surf. Sci. 2011, 257, 5293–5297. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and electrochemical mediated detection of ascorbic acid using manganese porphyrin and its gold hybrids. C. R. Chim. 2017, 21, 327–338. [Google Scholar] [CrossRef]
- Gu, S.; Ma, K.; Kong, J.; Al-Ghanim, K.A.; Mahboob, S.; Liu, Y.; Zhang, X. Functionalized polyethyleneimine-gold nanoparticles-porphyrin nanocomposite for electrochemical glucose biosensing. Int. J. Electrochem. Sc. 2017, 12, 5092–5103. [Google Scholar] [CrossRef]
- Mihailescu, G.; Olenic, L.; Garabagiu, S.; Blanita, G.; Fagadar-Cosma, E.; Biris, A. Coupling between plasmonic resonances in nanoparticles and porphyrins molecules. J. Nanosci. Nanotechnol. 2010, 10, 2527–2530. [Google Scholar] [CrossRef]
- Umemiya, M.; Sugiura, K.; Miyasaka, H.; Ishii, T.; Yamashita, M. Synthesis and structural determination of a porphyrinatoplatinum(II):meso-tetrakis(4-t-butylphenyl)porphyrinatoplatinum(II). Bull. Chem. Soc. Jpn. 2003, 76, 2123–2127. [Google Scholar] [CrossRef]
- Esipova, T.V.; Vinogradov, S.A. Synthesis of phosphorescent asymmetrically π-extended porphyrins for two-photon applications. J. Org. Chem. 2014, 79, 8812–8825. [Google Scholar] [CrossRef]
- Shmilovits, M.; Vinodu, M.; Goldberg, I. Porphyrinclathrates. Crystal structures of two unexpected products obtained by solvothermal reactions of Pt-tetra(4-carboxyphenyl)porphyrin with copper acetate. J. Incl. Phenom. Macrocycl. Chem. 2004, 48, 165–171. [Google Scholar] [CrossRef]
- Hazell, A. Structure of (5,10,15,20-tetraphenyl-21H,23H-porphinato)platinum(II), C44H28N4Pt. Acta Crystallogr. C 1984, 40, 751–753. [Google Scholar] [CrossRef]
- Milgrom, L.R.; Sheppard, R.N.; Slawin, A.M.Z.; Williams, D.J. X-ray crystal structure of 2,3,7,8,12,13,17,18-octaethylporphyrinatoplatinum(II) (PtOEP): Potential for correlation of meso-carbon bond-angle (Ĉm) with one-bond 13Cmeso-1Hmethine coupling constant in some diamagnetic metal complexes of OEP. Polyhedron 1988, 7, 57–61. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.R.; Kantardjieff, K.; Crundwell, G.; Mink, L.M. Dibromo-[5,10,15,20-tetrakis(4-methoxyphenyl)porphyrinato-k4N]platinum(IV) chloroform acetonitrile solvate. Acta Crystallogr. C 2002, 58, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Lascu, A.; Palade, A.; Fagadar-Cosma, G.; Creanga, I.; Ianasi, C.; Sebarchievici, I.; Birdeanu, M.; Fagadar-Cosma, E. Mesoporous manganese-porphyrin–silica hybrid nanomaterial sensitive to H2O2 fluorescent detection. Mater. Res. Bull. 2016, 74C, 325–332. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.-H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef]
- Wang, N.; Lin, H.; Li, X.; Lin, C.; Zhang, L.; Wu, J.; Dou, Y.; Li, J. Enhanced exchange current density and diffusion coefficient of iodide-based liquid electrolyte by layered α-zirconium phosphate. Electrochem. Commun. 2006, 8, 946–950. [Google Scholar] [CrossRef]
- Maiti, N.; Mazumdar, S.; Perisamy, N. J- and H-Aggregates of porphyrins with surfactants: Fluorescence, Stopped Flow and Electron Microscopy Studies. J. Porphyr. Phthalocyanines 1998, 2, 369–376. [Google Scholar] [CrossRef]
- Qiu, W.G.; Li, Z.F.; Bai, G.M.; Meng, S.N.; Dai, H.X.; He, H. Interaction of water-soluble cationic porphyrin with anionic surfactant. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 68, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Hao, X.; Zhang, D.; Jiang, Y. Reaction-based fluorescent turn-on probe for selective detection of thiophenols in aqueous solution and living cells. Dyes Pigments 2017, 142, 167–174. [Google Scholar] [CrossRef]
- Radwan, S.H.; Azzazy, H.M. Gold nanoparticles for molecular diagnostics. Expert Rev. Mol. Diagn. 2009, 9, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476–482. [Google Scholar] [CrossRef]
- Muthukumar, P.; Abraham John, S. Gold nanoparticles decorated on cobalt porphyrin-modified glassy carbon electrode for the sensitive determination of nitrite ion. J. Colloid Interface Sci. 2014, 421, 78–84. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis RED, Version 1.171.36.32; Oxford Diffraction Ltd.: Oxfordshire, UK, 2003.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]





| Compound | (PtTMeOPP)/DMF (2:1) |
|---|---|
| empirical formula | C49.5H40N4.5O4.5Pt |
| Fw | 964.95 |
| T [K] | 180 |
| Crystal system | monoclinic |
| space group | P21/c |
| a [Å] | 14.1668(15) |
| b [Å] | 9.6654(5) |
| c [Å] | 15.5836(10) |
| β [°] | 99.744(8) |
| V [Å3] | 2103.0(3) |
| Z | 2 |
| ρalcd [g·cm−3] | 1.524 |
| μ [mm−1] | 3.388 |
| Crystal size [mm] | 0.15 × 0.1 × 0.02 |
| 2Θ range | 4.98 to 50.052 |
| Reflections collected | 8329 |
| Independent reflections | 3712 [Rint = 0.0544] |
| Data/restraints/parameters | 3712/9/241 |
| R1[a] | 0.0495 |
| wR2[b] | 0.1214 |
| GOF [c] | 1.029 |
| Largest diff. peak/hole/e Å−3 | 1.33/−0.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.-F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions. Int. J. Mol. Sci. 2019, 20, 710. https://doi.org/10.3390/ijms20030710
Fagadar-Cosma E, Lascu A, Shova S, Zaltariov M-F, Birdeanu M, Croitor L, Balan A, Anghel D, Stamatin S. X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions. International Journal of Molecular Sciences. 2019; 20(3):710. https://doi.org/10.3390/ijms20030710
Chicago/Turabian StyleFagadar-Cosma, Eugenia, Anca Lascu, Sergiu Shova, Mirela-Fernanda Zaltariov, Mihaela Birdeanu, Lilia Croitor, Adriana Balan, Diana Anghel, and Serban Stamatin. 2019. "X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions" International Journal of Molecular Sciences 20, no. 3: 710. https://doi.org/10.3390/ijms20030710