IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling
Abstract
1. Introduction
2. Results
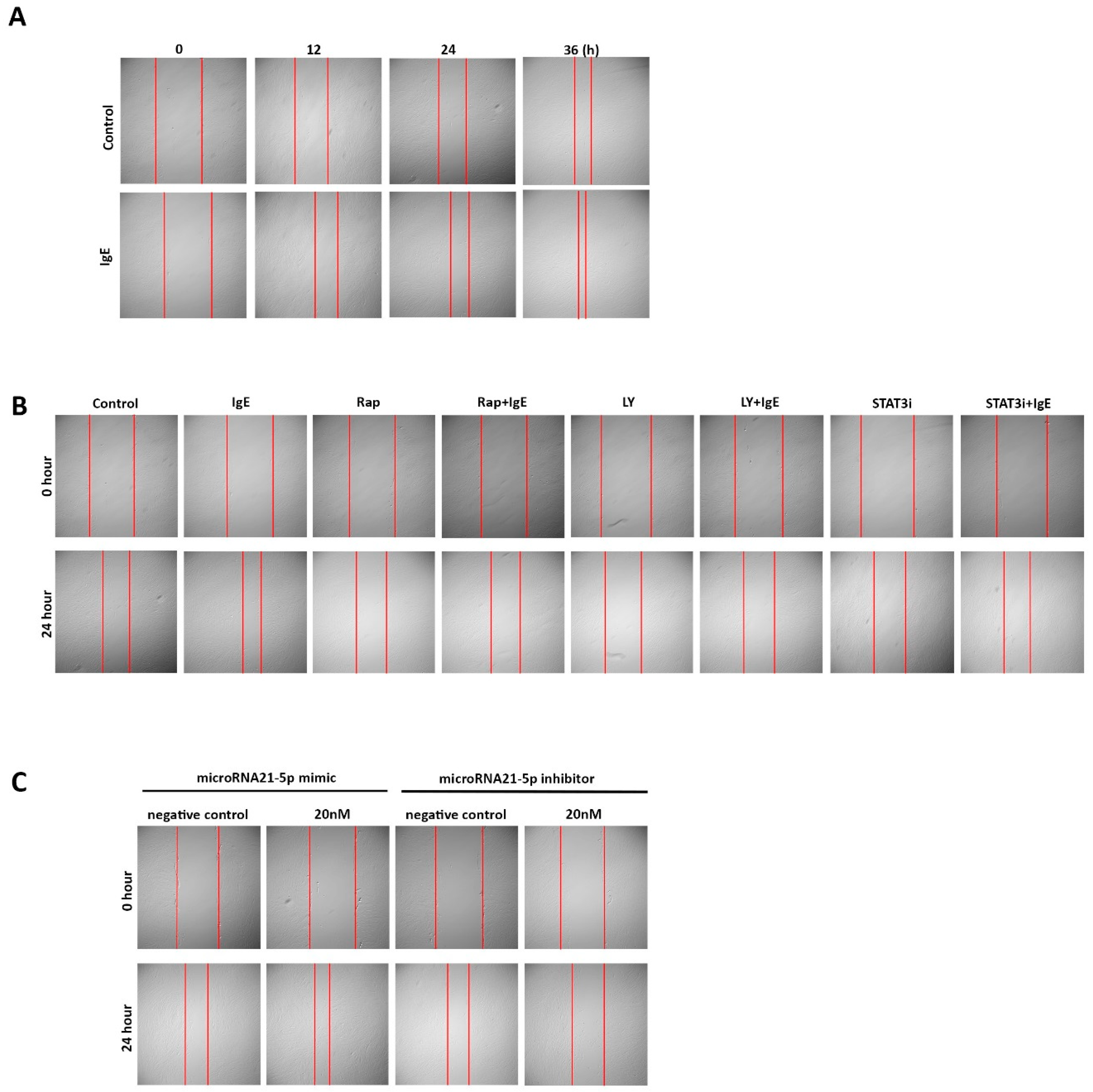
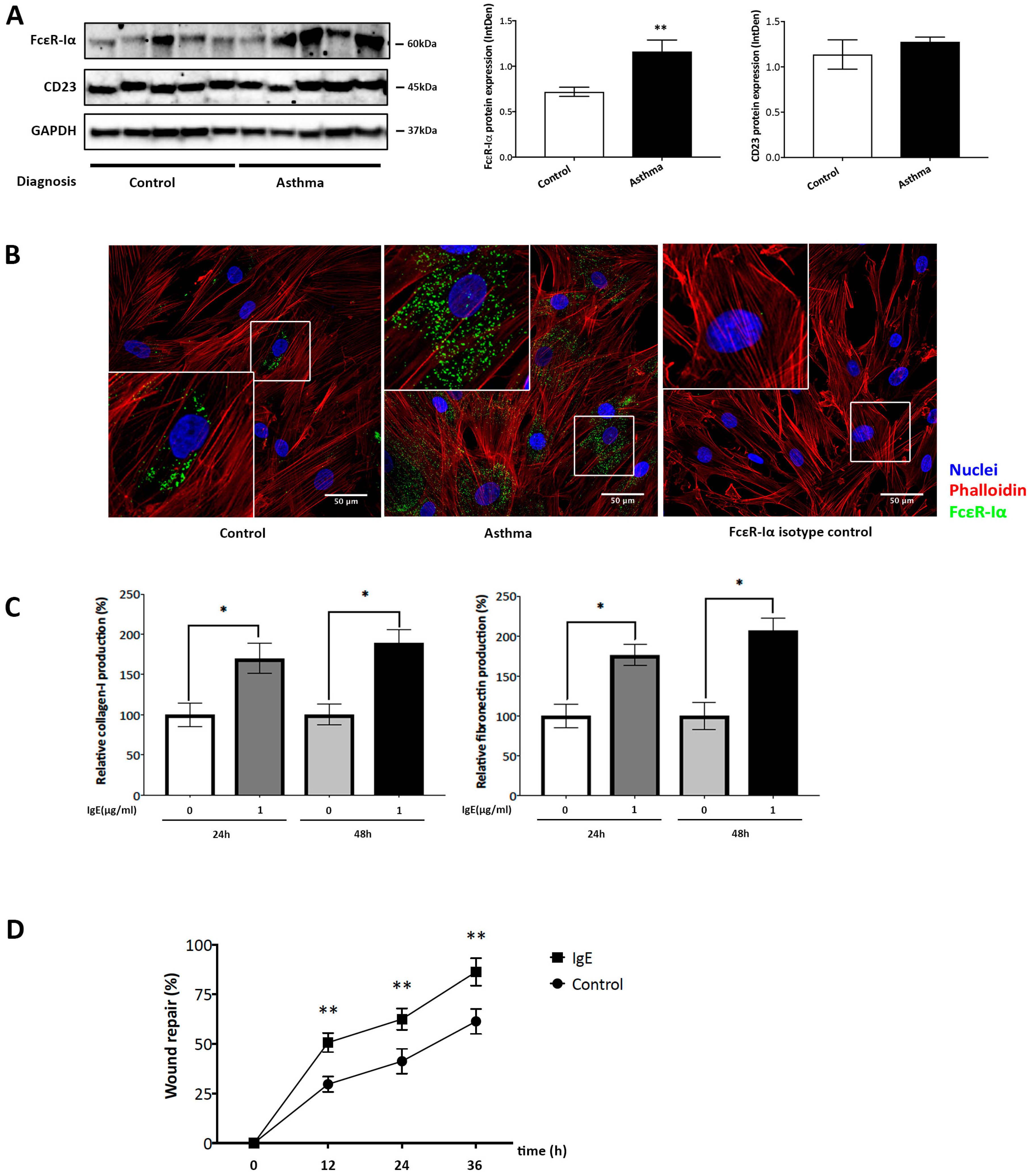
2.1. IgE Stimulated ECM Deposition and Migration in ASMC from Asthmatic Patients
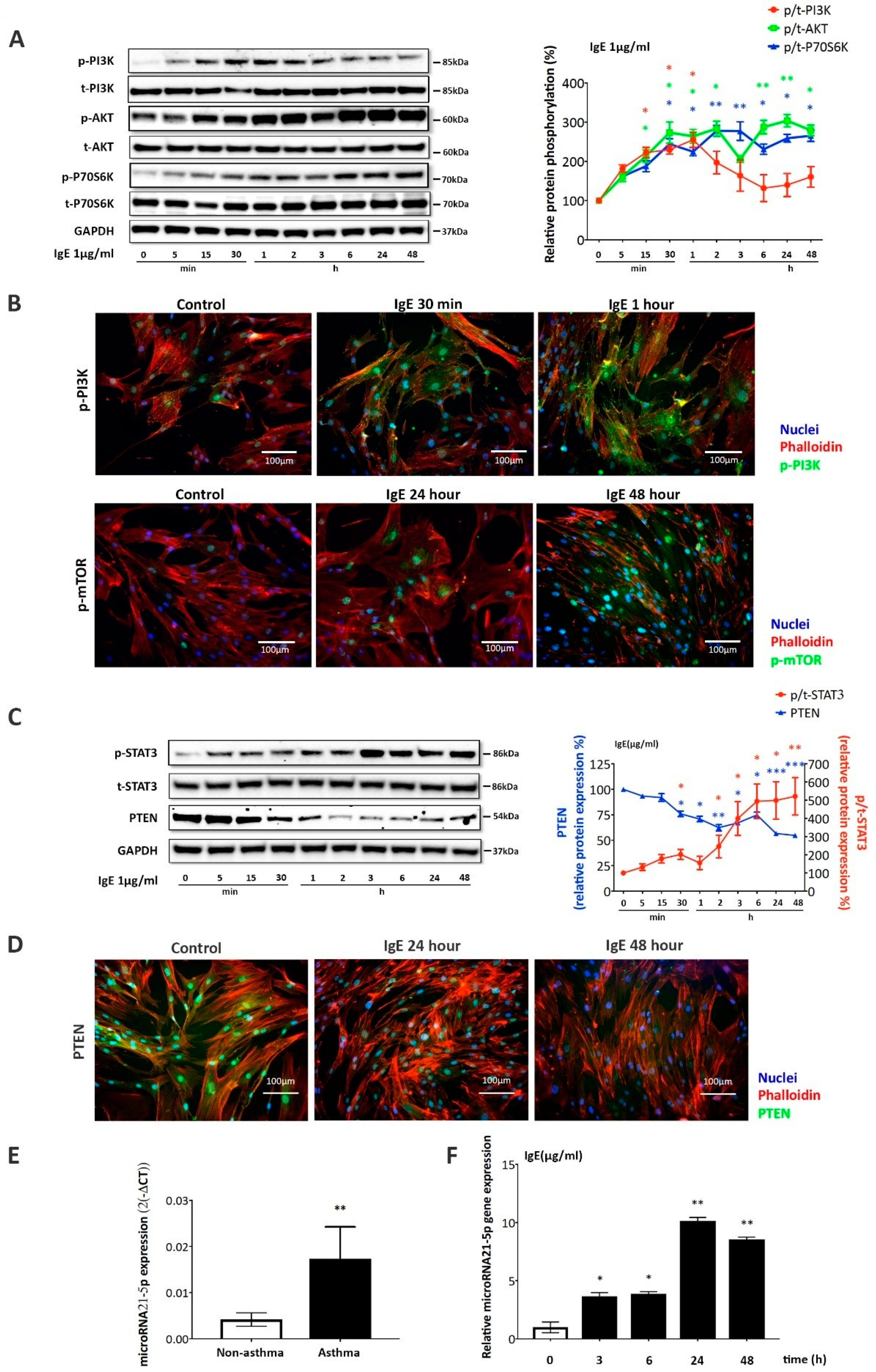
2.2. IgE Upregulated the Expression of Mitochondria-Related Genes and Proteins in ASMC
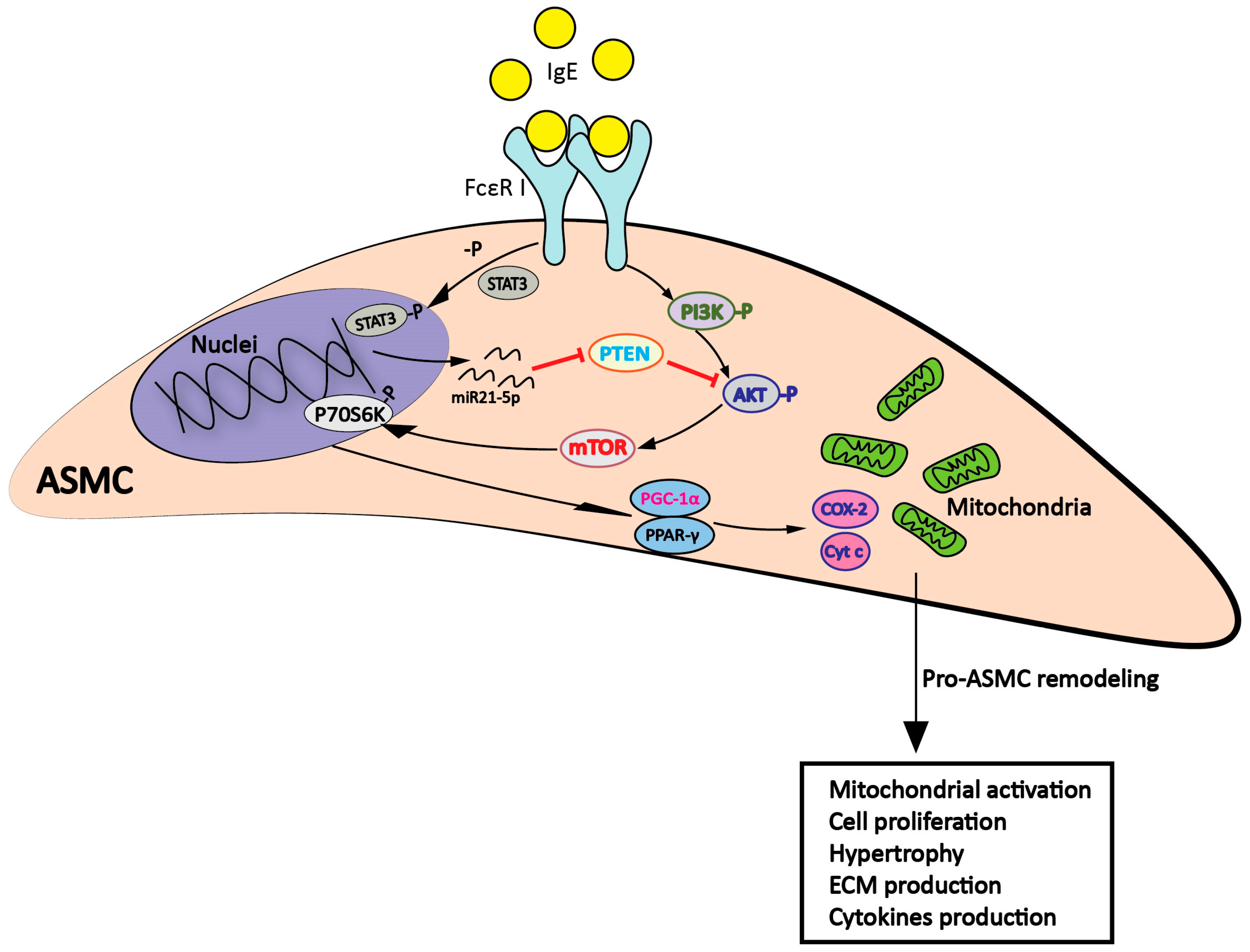
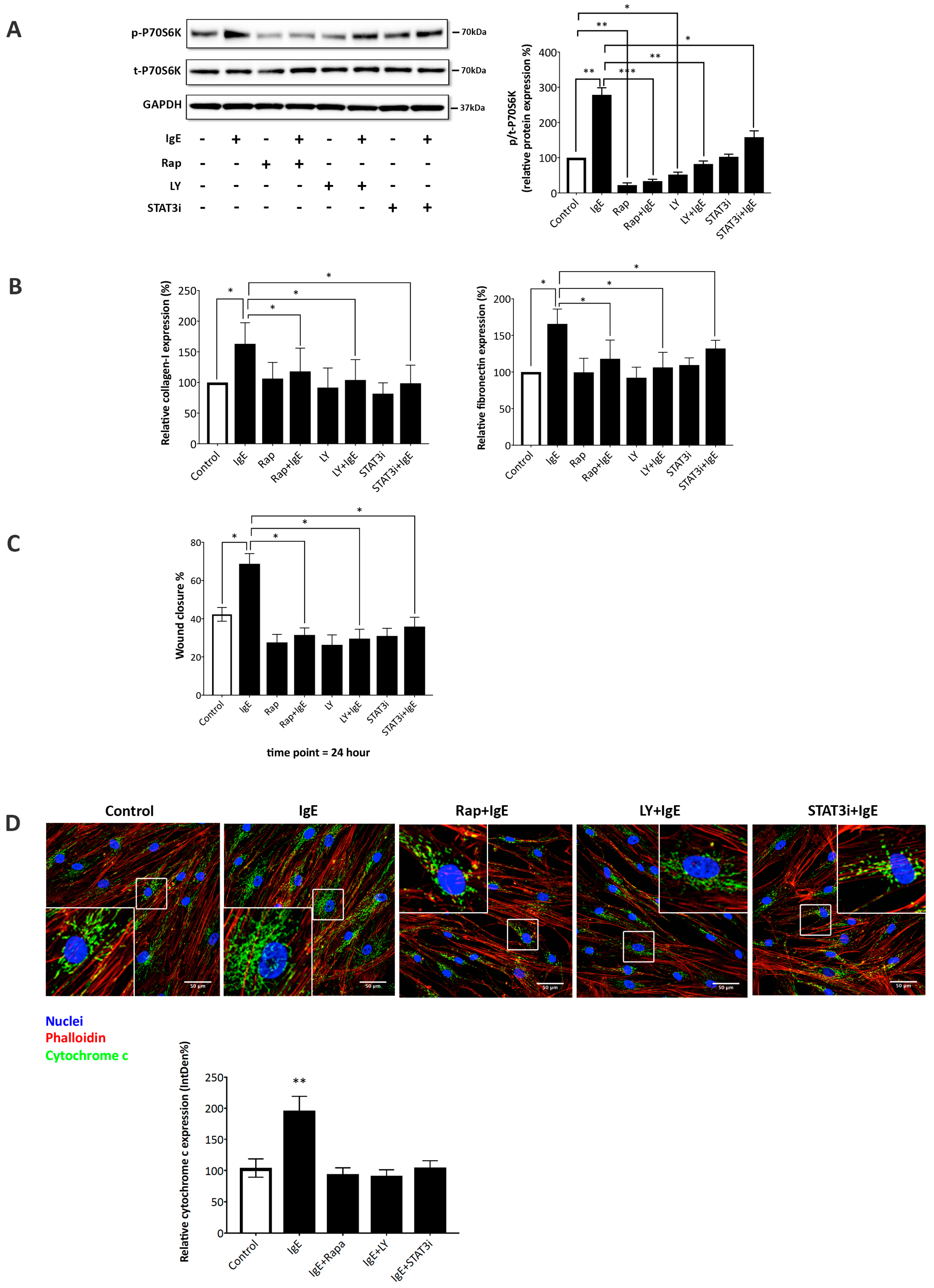
2.3. IgE Acted via PI3K→Akt→p70s6k and STAT3→miR-21→PTEN Signaling Pathways
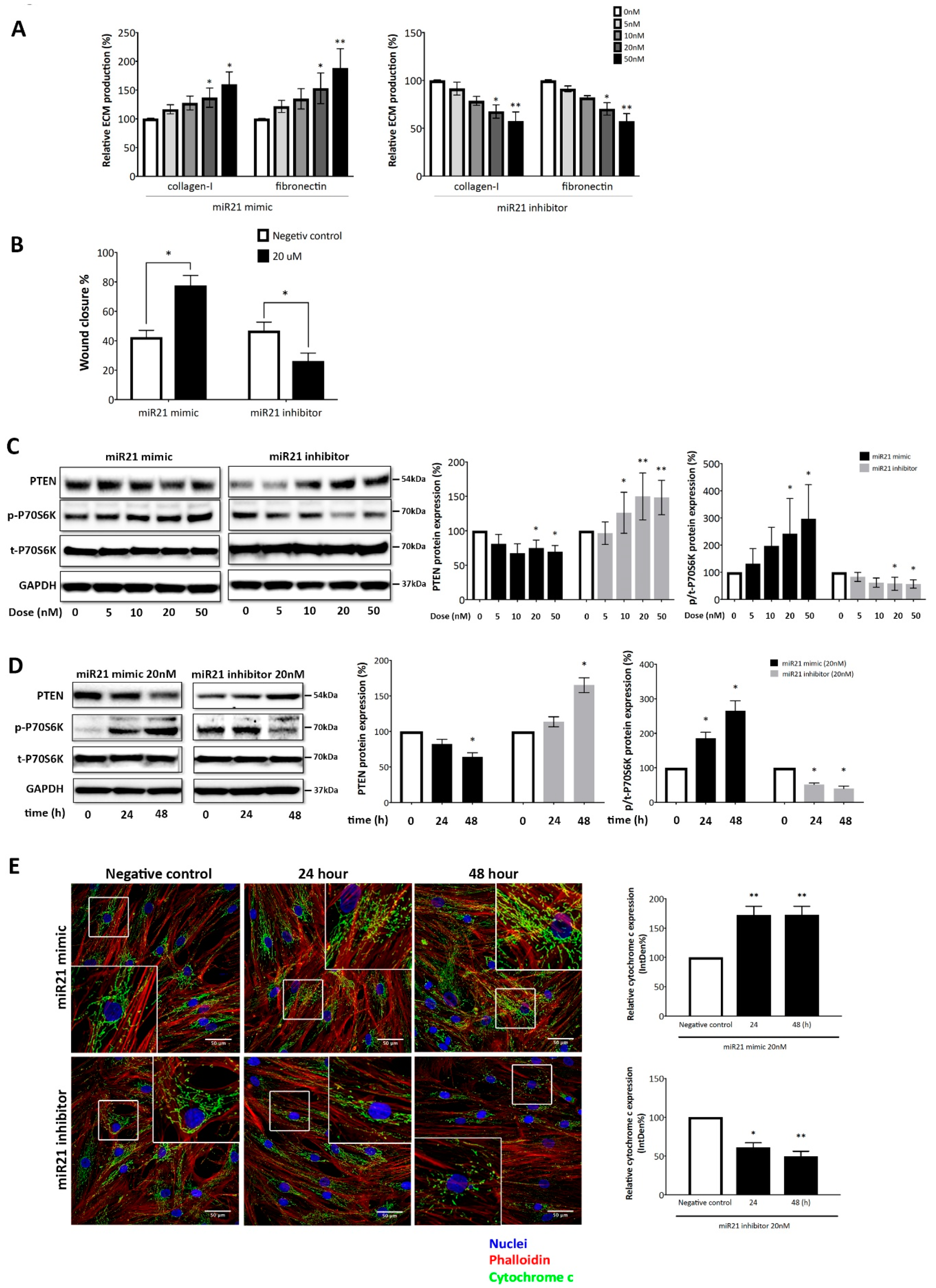
2.4. MicroRNA-21-5p is Essential for IgE-Induced ASMC Remodeling
3. Discussion
4. Materials and Methods
4.1. Tissue Biopsies and Primary Cell Cultures
4.2. ASMC Treatments
4.3. Real-Time Quantitative PCR (RT-qPCR)
4.4. Primer Sequence Details
4.5. Western Blot
4.6. Immunofluorescence
4.7. Confocal Microscopy
4.8. Collagen Type-I and Fibronectin ELISA
4.9. Cell Migration Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASMC | airway smooth muscle cells |
| MAPK | mitogen activated protein kinases |
| STAT3 | signal transducer and activator of transcription |
| PTEN | phosphatase and tensin homolog gene |
| COX-2 | cytochrome c Oxidase Subunit 2 |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor-γ |
| PGC-1α | Peroxisome Proliferator-Activated Receptor γ Coactivator-1α |
Appendix A
References
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; Frey, U. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Halayko, A.J.; Gosens, R.; Panettieri, R.A., Jr.; Camoretti-Mercado, B.; Penn, R.B. An Official American Thoracic Society Research Statement: Current Challenges Facing Research and Therapeutic Advances in Airway Remodeling. Am. J. Respir. Crit. Care Med. 2017, 195, e4–e19. [Google Scholar] [CrossRef] [PubMed]
- Noble, P.B.; Pascoe, C.D.; Lan, B.; Ito, S.; Kistemaker, L.E.; Tatler, A.L.; Pera, T.; Brook, B.S.; Gosens, R.; West, A.R. Airway smooth muscle in asthma: Linking contraction and mechanotransduction to disease pathogenesis and remodelling. Pulm. Pharmacol. Ther. 2014, 29, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Mitochondrial Dysfunction in Airway Disease. Chest 2017, 152, 618–626. [Google Scholar] [CrossRef]
- Rufo, J.; Taborda-Barata, L.; Lourenco, O. Serum biomarkers in elderly asthma. J. Asthma 2013, 50, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, N.; Nabe, T.; Yoshino, S. IgE/antigen-mediated enhancement of IgE production is a mechanism underlying the exacerbation of airway inflammation and remodelling in mice. Immunology 2015, 144, 107–115. [Google Scholar] [CrossRef]
- Samitas, K.; Delimpoura, V.; Zervas, E.; Gaga, M. Anti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: Current knowledge and future perspectives. Eur. Respir. Rev. 2015, 24, 594–601. [Google Scholar] [CrossRef]
- Roth, M.; Zhong, J.; Zumkeller, C.; S’ng, C.T.; Goulet, S.; Tamm, M. The role of IgE-receptors in IgE-dependent airway smooth muscle cell remodelling. PLoS ONE 2013, 8, e56015. [Google Scholar] [CrossRef]
- Redhu, N.S.; Gounni, A.S. The high affinity IgE receptor (FcepsilonRI) expression and function in airway smooth muscle. Pulm. Pharmacol. Ther. 2013, 26, 86–94. [Google Scholar] [CrossRef]
- Roth, M.; Zhao, F.; Zhong, J.; Lardinois, D.; Tamm, M. Serum IgE Induced Airway Smooth Muscle Cell Remodeling Is Independent of Allergens and Is Prevented by Omalizumab. PLoS ONE 2015, 10, e0136549. [Google Scholar] [CrossRef]
- Redhu, N.S.; Shan, L.; Al-Subait, D.; Ashdown, H.L.; Movassagh, H.; Lamkhioued, B.; Gounni, A.S. IgE induces proliferation in human airway smooth muscle cells: Role of MAPK and STAT3 pathways. Allergy Asthma Clin Immunol 2013, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Radinger, M.; Gilfillan, A.M. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Krymskaya, V.P. Targeting the phosphatidylinositol 3-kinase pathway in airway smooth muscle: Rationale and promise. BioDrugs 2007, 21, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Trian, T.; Benard, G.; Begueret, H.; Rossignol, R.; Girodet, P.O.; Ghosh, D.; Ousova, O.; Vernejoux, J.M.; Marthan, R.; Tunon-de-Lara, J.M.; Berger, P. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 2007, 204, 3173–3181. [Google Scholar] [CrossRef]
- Aravamudan, B.; Kiel, A.; Freeman, M.; Delmotte, P.; Thompson, M.; Vassallo, R.; Sieck, G.C.; Pabelick, C.M.; Prakash, Y.S. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L840–L854. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Allegra, A.; Pioggia, G.; Picardi, G.; Musolino, C.; Gangemi, S. MicroRNA Profiling in Asthma: Potential Biomarkers and Therapeutic Targets. Am. J. Respir. Cell Mol. Biol. 2017, 57, 642–650. [Google Scholar] [CrossRef]
- Makiguchi, T.; Yamada, M.; Yoshioka, Y.; Sugiura, H.; Koarai, A.; Chiba, S.; Fujino, N.; Tojo, Y.; Ota, C.; Kubo, H.; Kobayashi, S. Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 110. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Shi, H.; Xu, J.; Zhang, D.; Wu, Y.; Zhou, S.; Sun, X. MiR-21 modulates human airway smooth muscle cell proliferation and migration in asthma through regulation of PTEN expression. Exp. Lung. Res. 2015, 41, 535–545. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, H.; Sun, Q.; Zhu, W.; Xu, J.; Gao, N.; Zhang, R.; Liu, L.; Wu, X.; Yang, X.; Meng, L. Extracellular microRNA-21 and microRNA-26a increase in body fluids from rats with antigen induced pulmonary inflammation and children with recurrent wheezing. BMC Pulm. Med. 2016, 16, 50. [Google Scholar] [CrossRef]
- Hammad Mahmoud Hammad, R.; Hamed, D.; Eldosoky, M.; Ahmad, A.; Osman, H.M.; Abd Elgalil, H.M.; Osman, H.M.; Abd Elgalil, H.M.; Mahmoud Hassan, M.M. Plasma microRNA-21, microRNA-146a and IL-13 expression in asthmatic children. Innate Immun. 2018, 24, 171–179. [Google Scholar] [CrossRef]
- Green, D.E.; Murphy, T.C.; Kang, B.Y.; Bedi, B.; Yuan, Z.; Sadikot, R.T.; Hart, C.M. Peroxisome proliferator-activated receptor-gamma enhances human pulmonary artery smooth muscle cell apoptosis through microRNA-21 and programmed cell death 4. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L371–L383. [Google Scholar] [CrossRef] [PubMed]
- Tao Song, J.; Hu, B.; yan Qu, H.; long Bi, C.; zhen Huang, X.; Zhang, M. Mechanical stretch modulates microRNA 21 expression, participating in proliferation and apoptosis in cultured human aortic smooth muscle cells. PLoS ONE 2012, 7, e47657. [Google Scholar]
- Berair, R.; Brightling, C.E. Asthma therapy and its effect on airway remodelling. Drugs 2014, 74, 1345–1369. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.; Pereira, C.; Teixeira, L.; Canelas, A.; Tavares, B.; Loureiro, G.; Calado, G.; Ribeiro, C.; Chieira, C. Thoracic high resolution computed tomography (HRCT) in asthma. Eur. Ann Allergy Clin. Immunol. 2009, 41, 139–145. [Google Scholar] [PubMed]
- Elliot, J.G.; Jones, R.L.; Abramson, M.J.; Green, F.H.; Mauad, T.; McKay, K.O.; Bai, T.R.; James, A.L. Distribution of airway smooth muscle remodelling in asthma: Relation to airway inflammation. Respirology 2015, 20, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Harker, J.A. Allergic Airway Disease: More than Meets the IgE? Am. J. Respir. Cell Mol. Biol. 2017, 57, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.; Burton, O.T. IgE and Mast Cells: The Endogenous Adjuvant. Oettgen HC, Burton OT. Adv. Immunol. 2015, 127, 203–256. [Google Scholar]
- Greer, A.M.; Wu, N.; Putnam, A.L.; Woodruff, P.G.; Wolters, P.; Kinet, J.P.; Shin, J.S. Serum IgE clearance is facilitated by human FcεRI internalization. J. Clin. Investig. 2014, 124, 1187–1198. [Google Scholar] [CrossRef]
- Girodet PO Allard, B.; Thumerel, M.; Begueret, H.; Dupin, I.; Ousova, O.; Lassalle, R.; Maurat, E.; Ozier, A.; Trian, T.; Marthan, R. Bronchial Smooth Muscle Remodeling in Nonsevere Asthma. Am. J. Respir. Crit. Care Med. 2016, 193, 627–633. [Google Scholar] [CrossRef]
- Sakai, K.; Yokoyama, A.; Kohno, N.; Hamada, H.; Hiwada, K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int. Arch Allergy Immunol. 2001, 126, 126–134. [Google Scholar] [CrossRef]
- Riccio, A.M.; Dal Negro, R.W.; Micheletto, C.; De Ferrari, L.; Folli, C.; Chiappori, A.; Canonica, G.W. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int. J. Immunopathol. Pharmacol. 2012, 25, 475–484. [Google Scholar] [CrossRef]
- Kuprys-Lipinska, I.; Molinska, K.; Kuna, P. The effect of omalizumab on eosinophilic inflammation of the respiratory tract in patients with allergic asthma. Pneumonol. Alergol. Pol. 2016, 84, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Milgrom, H.; Berger, W.; Nayak, A.; Gupta, N.; Pollard, S.; McAlary, M.; Taylor, A.F.; Rohane, P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001, 108, E36. [Google Scholar] [CrossRef] [PubMed]
- Lemanske, R.F., Jr.; Nayak, A.; McAlary, M.; Everhard, F.; Fowler-Taylor, A.; Gupta, N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics 2002, 110, e55. [Google Scholar] [CrossRef] [PubMed]
- Lezmi, G.; Gosset, P.; Deschildre, A.; Abou-Taam, R.; Mahut, B.; Beydon, N.; de Blic, J. Airway Remodeling in Preschool Children with Severe Recurrent Wheeze. Am. J. Respir. Crit. Care Med. 2015, 192, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Grainge, C.L.; Lau, L.C.; Ward, J.A.; Dulay, V.; Lahiff, G.; Wilson, S.; Holgate, S.; Davies, D.E.; Howarth, P.H. Effect of bronchoconstriction on airway remodeling in asthma. N. Engl. J. Med. 2011, 364, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Regulation of PPARgamma function by TNF-alpha. Biochem. Biophys. Res. Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Rohas, L.M.; St-Pierre, J.; Uldry, M.; Jäger, S.; Handschin, C.; Spiegelman, B.M. A fundamental system of cellular energy homeostasis regulated by PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 7933–7938. [Google Scholar] [CrossRef]
- Kupr, B.; Handschin, C. Complex Coordination of Cell Plasticity by a PGC-1alpha-controlled Transcriptional Network in Skeletal Muscle. Front. Physiol. 2015, 6, 325. [Google Scholar] [CrossRef]
- Kleniewska, P.; Pawliczak, R. The participation of oxidative stress in the pathogenesis of bronchial asthma. Biomed. Pharmacother. 2017, 94, 100–108. [Google Scholar] [CrossRef]
- Perez de Obanos, M.P.; López-Zabalza, M.J.; Arriazu, E.; Modol, T.; Prieto, J.; Herraiz, M.T.; Iraburu, M.J. Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim. Biophys. Acta 2007, 1773, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Amanso, A.; Abrahao, T.B.; Lassegue, B.; Griendling, K.K. Polymerase delta-interacting protein 2 regulates collagen accumulation via activation of the Akt/mTOR pathway in vascular smooth muscle cells. J. Mol. Cell Cardiol. 2016, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Das, F.; Ghosh-Choudhury, N.; Venkatesan, B.; Kasinath, B.S.; Ghosh Choudhury, G. PDGF receptor-beta uses Akt/mTORC1 signaling node to promote high glucose-induced renal proximal tubular cell collagen I (alpha2) expression. Am. J. Physiol. Renal Physiol. 2017, 313, F291–F307. [Google Scholar] [CrossRef]
- Sawant, D.V.; Yao, W.; Wright, Z.; Sawyers, C.; Tepper, R.S.; Gupta, S.K.; H Kaplan, M.; L Dent, A. Serum MicroRNA-21 as a Biomarker for Allergic Inflammatory Disease in Children. Microrna 2015, 4, 36–40. [Google Scholar] [CrossRef]
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; Sunkara, K.P. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase-mediated suppression of histone deacetylase 2. J. Allergy Clin. Immunol. 2017, 139, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Erlich, T.H.; Yagil, Z.; Kay, G.; Peretz, A.; Migalovich-Sheikhet, H.; Tshori, S.; Nechushtan, H.; Levi-Schaffer, F.; Saada, A.; Razin, E. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J. Allergy Clin. Immunol. 2014, 134, 460–469. [Google Scholar] [CrossRef] [PubMed]
- McHale, C.; Mohammed, Z.; Deppen, J.; Gomez, G. Interleukin-6 potentiates FcepsilonRI-induced PGD2 biosynthesis and induces VEGF from human in situ-matured skin mast cells. Biochim. Biophys. Acta 2018, 1862, 1069–1078. [Google Scholar] [CrossRef]
- Johnson, P.R.; Armour, C.L.; Carey, D.; Black, J.L. Heparin and PGE2 inhibit DNA synthesis in human airway smooth muscle cells in culture. Am. J. Physiol. 1995, 269, L514–L519. [Google Scholar] [CrossRef]
- Lambers, C.; Roth, M.; Zhong, J.; Campregher, C.; Binder, P.; Burian, B.; Petkov, V.; Block, L.H. The interaction of endothelin-1 and TGF-beta1 mediates vascular cell remodeling. PLoS ONE 2013, 8, e73399. [Google Scholar] [CrossRef]

| Samples | Age | Gender | Smoking | FEV1% |
|---|---|---|---|---|
| Control 1 | 68 | Female | N.A. | 101 |
| Control 2 | 75 | Male | N.A. | 97 |
| Control 3 | 81 | Male | N.A. | 89 |
| Control 4 | 32 | Female | 0 pack/year | 94 |
| Control 5 | 65 | Female | 30 pack/year | 89 |
| Asthma 1 | 46 | Female | 10 pack/year | 67 |
| Asthma 2 | 79 | Female | 0 pack/year | 102 |
| Asthma 3 | 74 | Female | 20 pack/year | 49 |
| Asthma 4 | 52 | Female | 0 pack/year | 92 |
| Asthma 5 | 58 | Male | 10 pack/year | 82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Wang, X.; Sun, Q.; Papakonstantinou, E.; S’ng, C.; Tamm, M.; Stolz, D.; Roth, M. IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling. Int. J. Mol. Sci. 2019, 20, 875. https://doi.org/10.3390/ijms20040875
Fang L, Wang X, Sun Q, Papakonstantinou E, S’ng C, Tamm M, Stolz D, Roth M. IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling. International Journal of Molecular Sciences. 2019; 20(4):875. https://doi.org/10.3390/ijms20040875
Chicago/Turabian StyleFang, Lei, Xinggang Wang, Qingzhu Sun, Eleni Papakonstantinou, Chongteck S’ng, Michael Tamm, Daiana Stolz, and Michael Roth. 2019. "IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling" International Journal of Molecular Sciences 20, no. 4: 875. https://doi.org/10.3390/ijms20040875
APA StyleFang, L., Wang, X., Sun, Q., Papakonstantinou, E., S’ng, C., Tamm, M., Stolz, D., & Roth, M. (2019). IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling. International Journal of Molecular Sciences, 20(4), 875. https://doi.org/10.3390/ijms20040875






