Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients
Abstract
:1. Introduction
2. Results
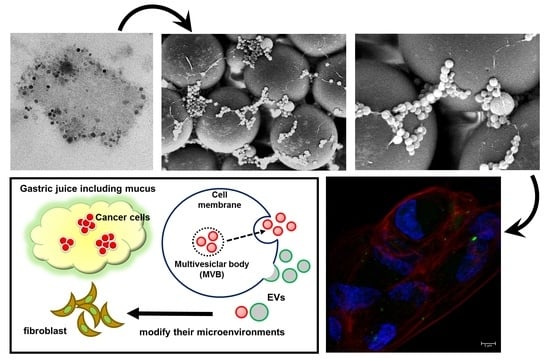
2.1. A Preprocessing Step was Established to Isolate Extracellular Vesicles (EVs) from Gastric Juice (GJ)
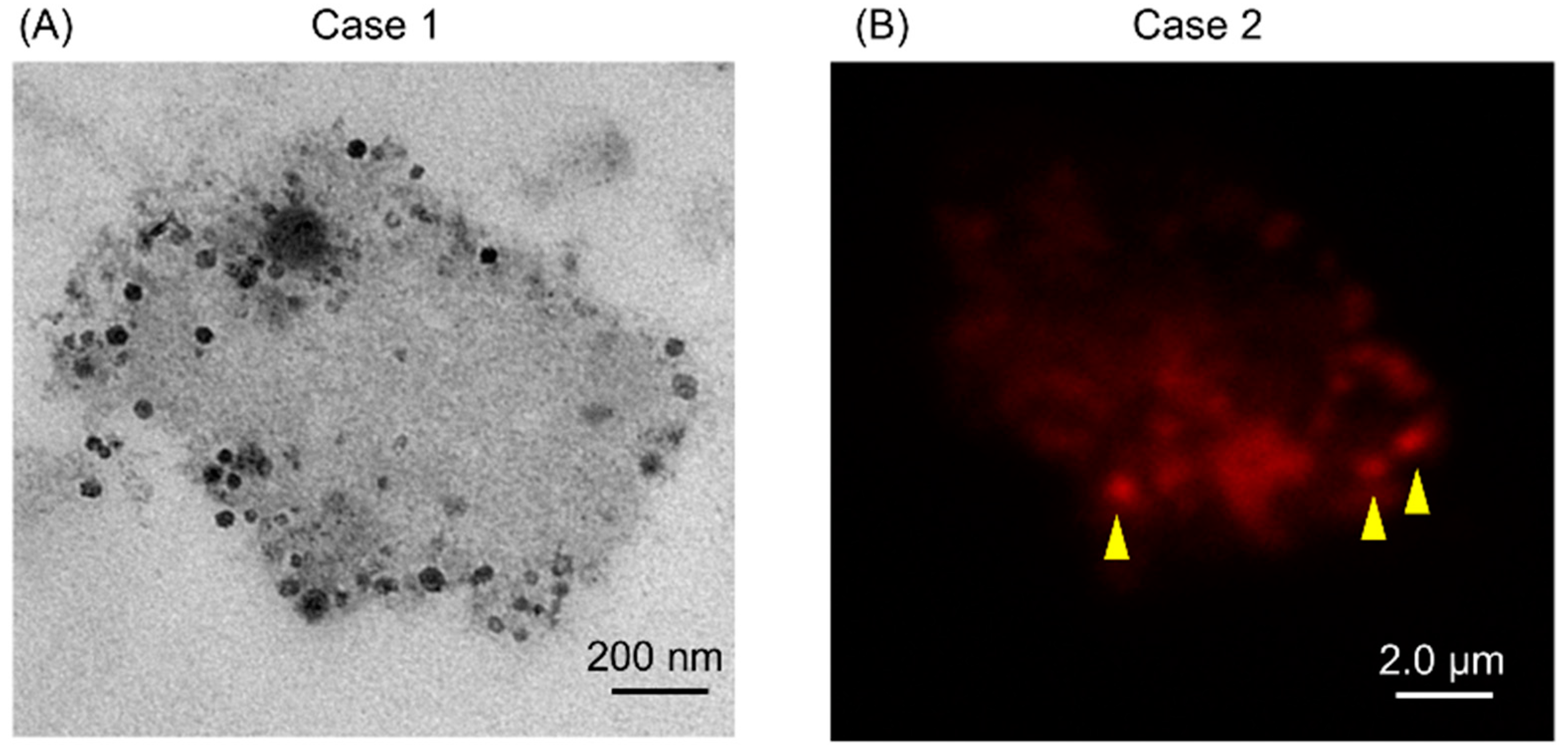
2.2. Isolates from GJ of Gastric Cancer (GC) Patients Contained EVs
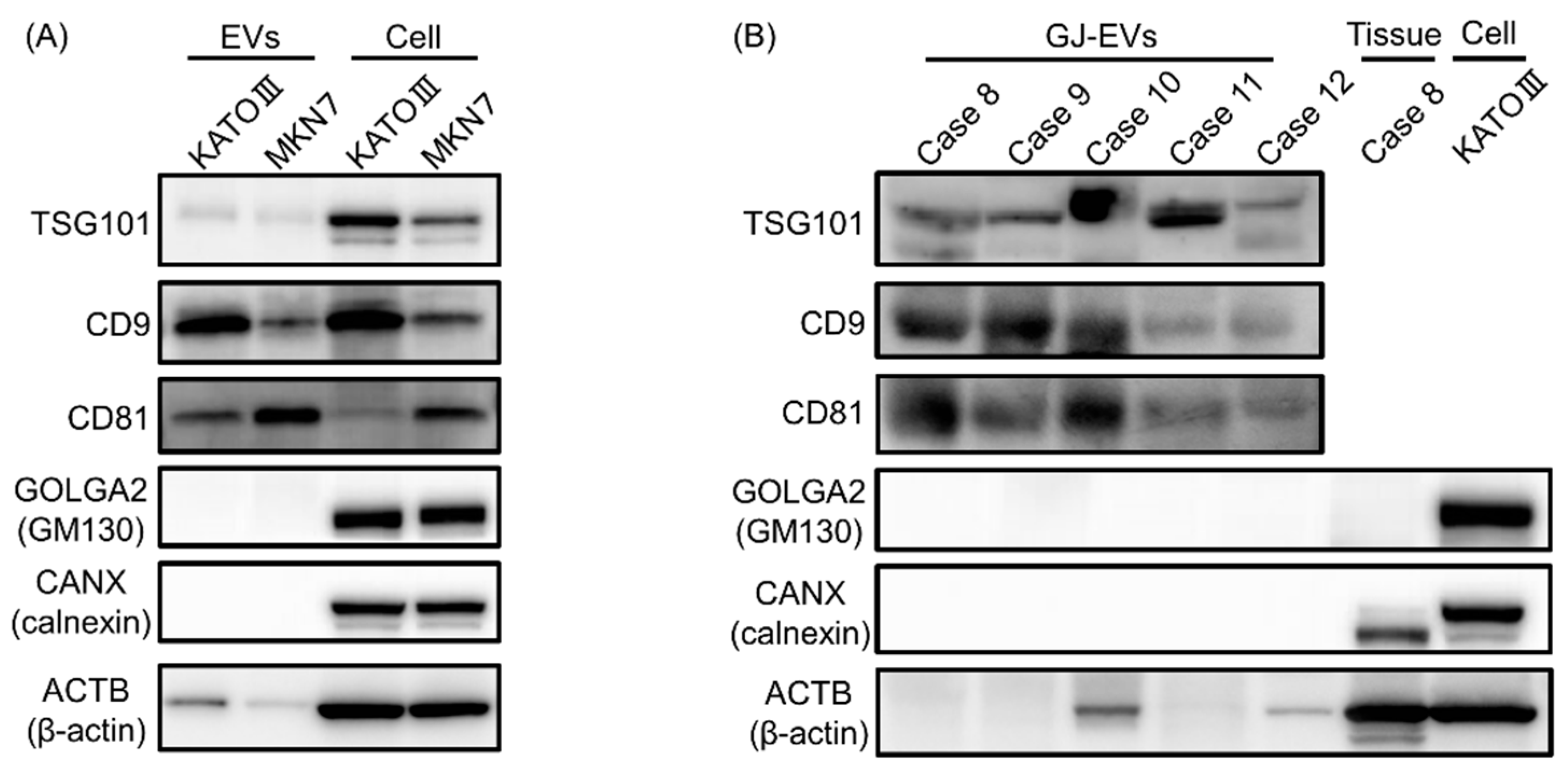
2.3. Protein Markers of Extracellular Vesicles (EVs) were Detected in GJ-EVs
2.4. EVs from GJ Contained Several MicroRNAs
2.5. GJ-EVs from GC Patients Promoted Tissue Fibrosis
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Cell Lines and Cell Culture
4.3. Isolation of Cancer-Cell-Line-Derived EVs
4.4. Sample Preparation for Isolation of EVs
4.5. Electron Microscopic Analysis
4.6. Nano Tracking Analysis
4.7. Western Blot Analysis
4.8. Bioanalyzer
4.9. Real-Time Quantitative Reverse Transcription PCR
4.10. Cell Viability Experiment
4.11. Immunofluorescent Microscopy
4.12. EVs-Incorporation Experiment
4.13. Terminology
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFs | cancer-associated fibroblasts |
| EVs | extracellular vesicles |
| GC | gastric cancer |
| GJ | gastric juice |
| HRP | horseradish peroxidase |
| MIR | microRNA gene symbol for humans |
| miRNA | microRNA |
| MVBs | multivesicular bodies |
| MVs | microvesicles |
| NTA | nano tracking system |
| RT-PCR | reverse transcription polymerase chain reaction |
| SEM | scanning electron microscope |
| snoRNA | small nucleolar RNA |
| TEM | transmission electron microscope |
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trams, E.G.; Lauter, C.J.; Salem, N.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegtel, D.M.; Cosmopoulos, K.; Thorley-Lawson, D.A.; van Eijndhoven, M.A.; Hopmans, E.S.; Lindenberg, J.L.; de Gruijl, T.D.; Wurdinger, T.; Middeldorp, J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. USA 2010, 107, 6328–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Yoshioka, Y.; Ochiya, T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016, 107, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [Green Version]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filen, J.J.; Lahesmaa, R.; Norman, M.; Neve, E.P.; Scheynius, A.; Gabrielsson, S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef]
- Ogawa, Y.; Miura, Y.; Harazono, A.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yamaguchi, T.; Toda, T.; Endo, T.; Tsubuki, M.; et al. Proteomic analysis of two types of exosomes in human whole saliva. Biol. Pharm. Bull. 2011, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; McKinney, K.Q.; Pavlopoulos, A.J.; Han, M.H.; Kim, S.H.; Kim, H.J.; Hwang, S. Exosomal proteome analysis of cerebrospinal fluid detects biosignatures of neuromyelitis optica and multiple sclerosis. Clin. Chim. Acta. 2016, 462, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, H.; Kuranaga, Y.; Kumazaki, M.; Sugito, N.; Yoshikawa, Y.; Takai, T.; Taniguchi, K.; Ito, Y.; Akao, Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. J. Immunol. 2017, 199, 1505–1515. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshioka, Y.; Yamamoto, Y.; Ochiya, T. How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell Mol. Life Sci. 2017, 74, 697–713. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Yang, Y.; Gong, L.; Xiao, B.; Liu, X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Sun, W.; Zhang, Q.; Gu, T.; Li, G. Plasma exosomes as novel biomarker for the early diagnosis of gastric cancer. Cancer Biomark. 2018, 21, 805–812. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Buschmann, D.; Kirchner, B.; Hermann, S.; Marte, M.; Wurmser, C.; Brandes, F.; Kotschote, S.; Bonin, M.; Steinlein, O.K.; Pfaffl, M.W.; et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J. Extracell. Vesicles 2018, 7, 1481321. [Google Scholar] [CrossRef]
- Andreu, Z.; Rivas, E.; Sanguino-Pascual, A.; Lamana, A.; Marazuela, M.; Gonzalez-Alvaro, I.; Sanchez-Madrid, F.; de la Fuente, H.; Yanez-Mo, M. Comparative analysis of EV isolation procedures for miRNAs detection in serum samples. J. Extracell. Vesicles. 2016, 5, 31655. [Google Scholar] [CrossRef] [Green Version]
- Endzelins, E.; Berger, A.; Melne, V.; Bajo-Santos, C.; Sobolevska, K.; Abols, A.; Rodriguez, M.; Santare, D.; Rudnickiha, A.; Lietuvietis, V.; et al. Detection of circulating miRNAs: comparative analysisof extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer 2017, 17, 730. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Phillips, S.; Kaimal, V.; Mao, Y.; Hua, W.; Yang, I.; Fu, C.C.; Nolan, J.; et al. miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J. Neurooncol. 2015, 123, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crossland, R.E.; Norden, J.; Kralj Juric, M.; Pearce, K.F.; Lendrem, C.; Bibby, L.A.; Collin, M.; Greinix, H.T.; Dickinson, A.M. Serum and Extracellular Vesicle MicroRNAs miR-423, miR-199, and miR-93* As Biomarkers for Acute Graft-versus-Host Disease. Front. Immunol. 2017, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Watanabe, Y.; Oikawa, R.; Morita, R.; Yoshida, Y.; Maehata, T.; Yasuda, H.; Itoh, F. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker For Early Detection of Gastric Cancer in an H. pylori-Independent Manner. Clin. Transl. Gastroenterol 2016, 7, e184. [Google Scholar] [CrossRef]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef]
- Lobb, R.; Moller, A. Size Exclusion Chromatography: A Simple and Reliable Method for Exosome Purification. Methods Mol. Biol. 2017, 1660, 105–110. [Google Scholar] [PubMed]
- Allen, A.; Carroll, N.J. Adherent and soluble mucus in the stomach and duodenum. Dig. Dis. Sci. 1985, 30, 55S–62S. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, L.F.; Wang, R.F. Role of cancer-associated fibroblasts in invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015, 21, 9717–9726. [Google Scholar] [CrossRef]
- Zhi, K.; Shen, X.; Zhang, H.; Bi, J. Cancer-associated fibroblasts are positively correlated with metastatic potential of human gastric cancers. J. Exp. Clin. Cancer Res. 2010, 29, 66. [Google Scholar] [CrossRef] [Green Version]
- Yashiro, M.; Hirakawa, K. Cancer-stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron. 2010, 3, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Satoyoshi, R.; Kuriyama, S.; Aiba, N.; Yashiro, M.; Tanaka, M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene 2015, 34, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Fuyuhiro, Y.; Yashiro, M.; Noda, S.; Kashiwagi, S.; Matsuoka, J.; Doi, Y.; Kato, Y.; Hasegawa, T.; Sawada, T.; Hirakawa, K. Upregulation of cancer-associated myofibroblasts by TGF-beta from scirrhous gastric carcinoma cells. Br. J. Cancer 2011, 105, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Tsujimura, N.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Nakagawa, Y.; Naoe, T.; Akao, Y. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim. Biophys. Acta 2014, 1839, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Kuranaga, Y.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Akao, Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-beta1-mediated suppression. Oncotarget 2016, 7, 27033–27043. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Khoo, F.; Kumazaki, M.; Shinohara, H.; Miki, K.; Yamada, N. Extracellular disposal of tumor-suppressor miRs-145 and -34a via microvesicles and 5-FU resistance of human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Iwatsuki, A.; Sugito, N.; Shinohara, H.; Kuranaga, Y.; Oshikawa, Y.; Tajirika, T.; Futamura, M.; Yoshida, K.; Uchiyama, K.; et al. Oncogene RNA helicase DDX6 promotes the process of c-Myc expression in gastric cancer cells. Mol. Carcinog. 2018, 57, 579–589. [Google Scholar] [CrossRef]
- Desvignes, T.; Batzel, P.; Berezikov, E.; Eilbeck, K.; Eppig, J.T.; McAndrews, M.S.; Singer, A.; Postlethwait, J.H. miRNA Nomenclature: A View Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends Genet. 2015, 31, 613–626. [Google Scholar] [CrossRef] [Green Version]






| Case No. | Mean (nm) | Mode (nm) |
|---|---|---|
| 3 | 225 ± 93 | 140 |
| 4 | 228 ± 86 | 184 |
| 5 | 270 ± 106 | 244 |
| 6 | 291 ± 138 | 212 |
| Case | RNA Area | RNA Concentration | RNA Ratio | RNA Integrity Number (RIN) |
|---|---|---|---|---|
| 13 | 39.2 | 293.2 | 0 | 2.6 |
| 14 | 33.2 | 248.3 | 0 | 2.6 |
| 15 | 1276.9 | 9552.4 | 0 | 2.6 |
| 16 | 16 | 119.7 | 0 | 2.7 |
| Gene | Case 13 | Case 14 | Case 15 | Case 16 |
|---|---|---|---|---|
| RNU6-6P | 34.47 ± 0.21 | 35.60 ± 0.26 | 35.82 ± 0.34 | 34.54 ± 0.17 |
| MIRLET7A1-5p | 28.11 ± 0.06 | 29.23 ± 0.12 | 26.07 ± 0.28 | 29.11 ± 0.16 |
| MIR16-5p | 24.79 ± 0.01 | 25.46 ± 0.04 | 14.14 ± 0.17 | 26.27 ± 0.11 |
| MIR103a-3p | 34.50 ± 0.18 | 32.80 ± 0.24 | 24.73 ± 0.15 | 31.90 ± 0.14 |
| MIR191-5p | 27.07 ± 0.02 | 27.22 ± 0.06 | 21.23 ± 0.27 | 29.46 ± 0.22 |
| MIR423-5p | 30.84 ± 0.47 | 34.46 ± 0.29 | 27.82 ± 0.08 | 34.56 ± 0.45 |
| Case | Age | Sex a | Type b | Size c | Pathology d | T e | N f | M g | Stage h |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | M | 1 | 45 × 28 | tub1, tub2 > pap | T2 | N1 | M0 | pStageIIA |
| 2 | 78 | M | 3 | 47 × 42 | por2 > tub2 | T3 | N3a | M0 | pStageIIIB |
| 3 | 78 | M | 3 | 40 × 50 | tub1, tub2, por | T3 | N2 | M0 | pStageIIIA |
| 4 | 66 | M | 0-IIc | 5 × 5 | tub2, por > sig | T1a | N0 | M0 | pStageIA |
| 5 | 72 | F | 3 | 40 × 30 | por2, por1 | T3 | N1 | M0 | ypStageIIB |
| 6 | 36 | F | 4 | 49 × 38 | por2, sig > tub2 | T1b | N1 | M0 | ypStageIB |
| 7 | 84 | M | 4 | 34 × 33 | por2 >> tub2 | T3 | N1 | M0 | ypStageIIB |
| 8 | 71 | M | 3 | 52 × 42 | por1 > sig | T3 | N0 | M0 | ypStageIIA |
| 9 | 56 | F | 3 | 20 × 20 | por2 > sig | T3 | N0 | M0 | ypStageIIA |
| 10 | 75 | M | 0-IIc | 66 × 46 | tub2, por2 > tub1 | T3 | N3a | M0 | pStageIIIB |
| 11 | 43 | F | 3 | 35 × 34 | sig | T3 | N1 | M0 | ypStageIIB |
| 12 | 37 | F | 4 | - | por, sig | T3 | N0 | M1 | sStageIV |
| 13 | 77 | F | 3 | 122 × 92 | tub1, pap, tub2, por2 | T4a | N3b | M0 | pStageIIIC |
| 14 | 54 | F | 4 | 49 × 46 | por2 > sig | T4a | N0 | M0 | pStageIIB |
| 15 | 50 | M | 4 | - | por, sig | T4a | N2 | M1 | sStageIV |
| 16 | 76 | M | 0-IIa + IIc | 1.2 × 1.2 | por2 > tub2 > tub1 | T1b | N0 | M0 | pStageIA |
| 17 | 78 | M | 0-I + Iia | 53 × 35 | tub1, pap > tub2 | T1a | N0 | M0 | pStageIA |
| 18 | 77 | F | 3 | - | tub2, por | T4a | N1 | M1 | pStageIV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagota, S.; Taniguchi, K.; Lee, S.-W.; Ito, Y.; Kuranaga, Y.; Hashiguchi, Y.; Inomata, Y.; Imai, Y.; Tanaka, R.; Tashiro, K.; et al. Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients. Int. J. Mol. Sci. 2019, 20, 953. https://doi.org/10.3390/ijms20040953
Kagota S, Taniguchi K, Lee S-W, Ito Y, Kuranaga Y, Hashiguchi Y, Inomata Y, Imai Y, Tanaka R, Tashiro K, et al. Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients. International Journal of Molecular Sciences. 2019; 20(4):953. https://doi.org/10.3390/ijms20040953
Chicago/Turabian StyleKagota, Shuji, Kohei Taniguchi, Sang-Woong Lee, Yuko Ito, Yuki Kuranaga, Yasuyuki Hashiguchi, Yosuke Inomata, Yoshiro Imai, Ryo Tanaka, Keitaro Tashiro, and et al. 2019. "Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients" International Journal of Molecular Sciences 20, no. 4: 953. https://doi.org/10.3390/ijms20040953
APA StyleKagota, S., Taniguchi, K., Lee, S.-W., Ito, Y., Kuranaga, Y., Hashiguchi, Y., Inomata, Y., Imai, Y., Tanaka, R., Tashiro, K., Kawai, M., Akao, Y., & Uchiyama, K. (2019). Analysis of Extracellular Vesicles in Gastric Juice from Gastric Cancer Patients. International Journal of Molecular Sciences, 20(4), 953. https://doi.org/10.3390/ijms20040953