Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results
Abstract
:1. Introduction
2. Results
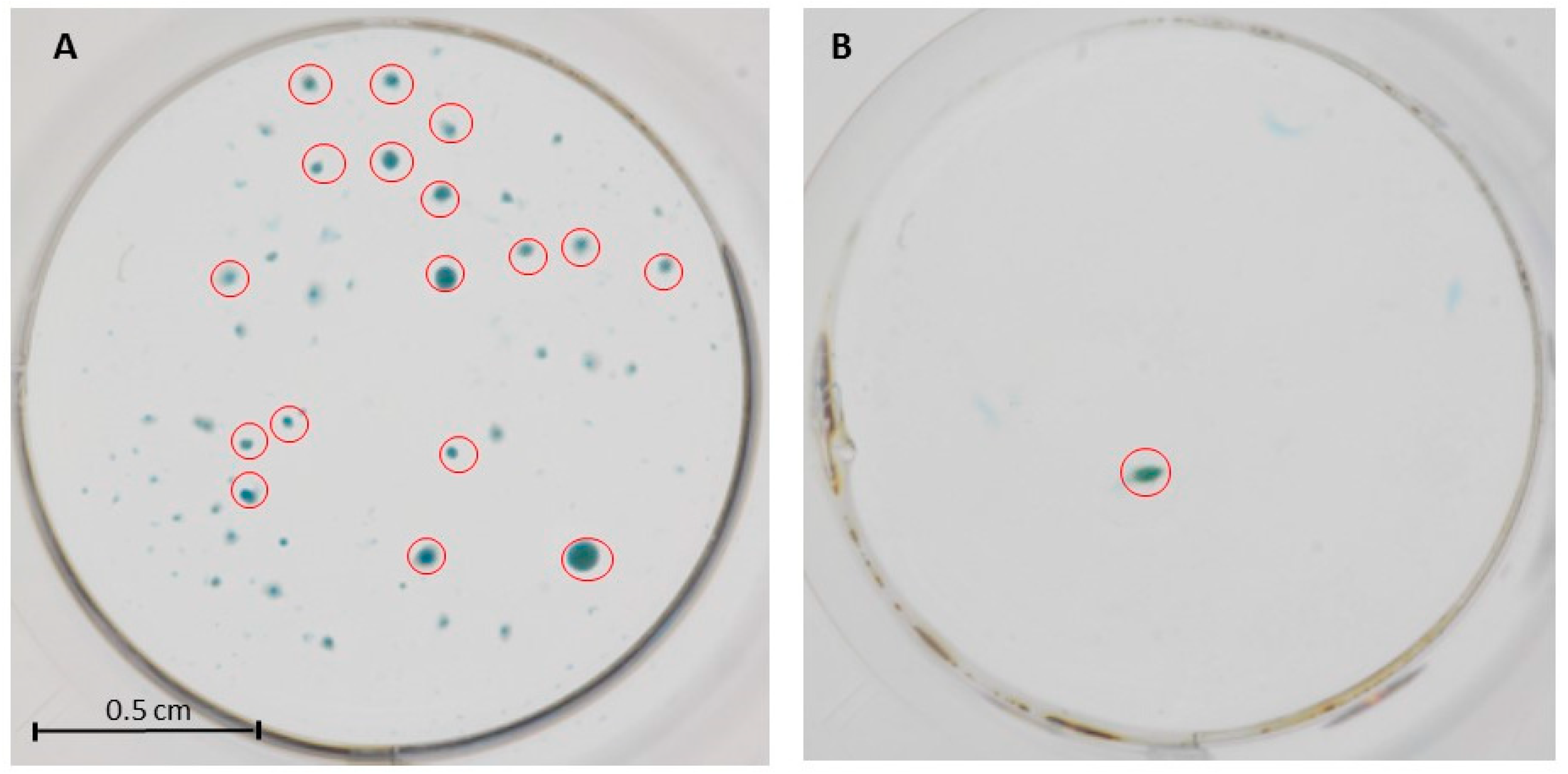
2.1. Process Validation Batches
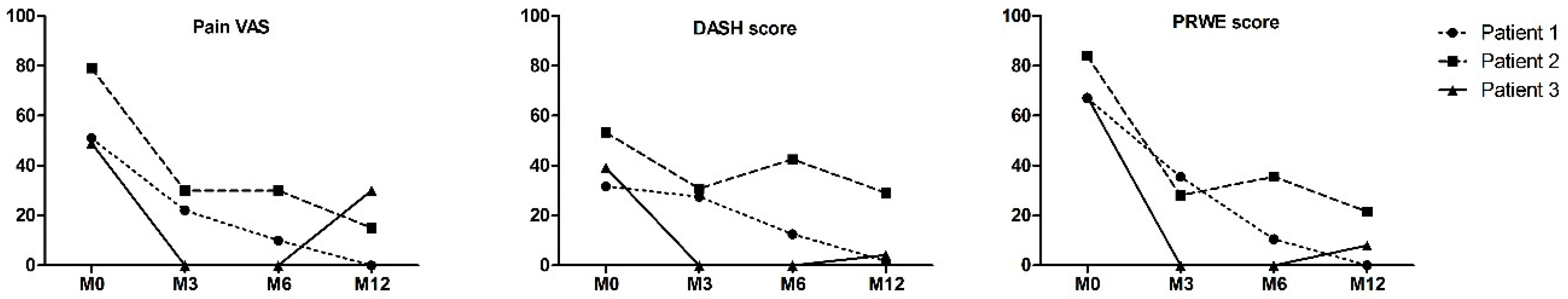
2.2. Preliminary Data from Clinical Trials
3. Discussion
4. Material and Methods
4.1. Obtention of Human Tissues and Regulatory Approval
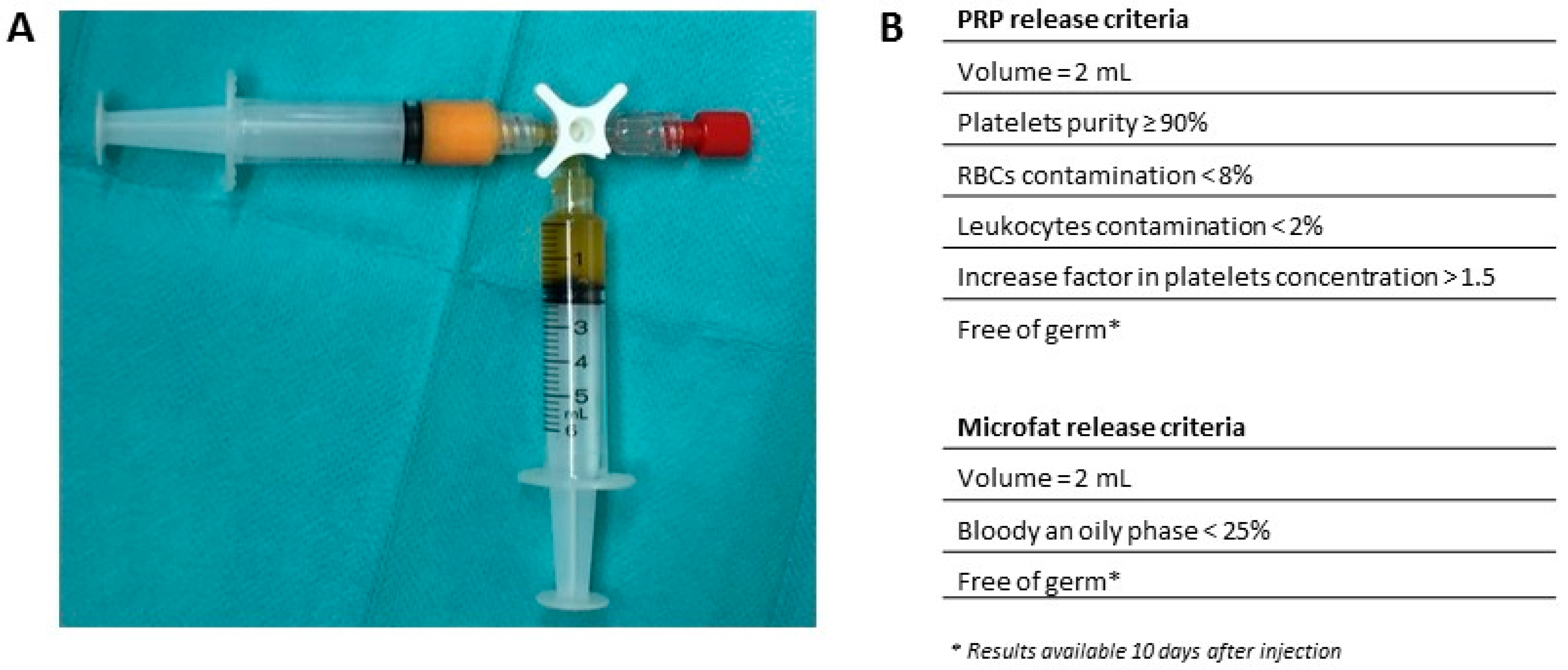
4.2. PRP Preparation
4.2.1. Blood and PRP Cell Counting
4.2.2. Platelets Aggregation Test
4.3. Microfat Preparation
4.3.1. Macroscopic Assessment of Microfat
4.3.2. Microbiological Assay
4.4. Microfat-PRP Mixture
4.4.1. Stromal Vascular Fraction (SVF) Extraction
4.4.2. Chondrocytes Differentiation
4.4.3. Microfat-PRP Growth Factors Release Measurement
4.5. Patients
4.5.1. Surgical Procedure and Injection
4.5.2. Clinical Assessment
4.6. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laulan, J.; Marteau, E.; Bacle, G. Wrist osteoarthritis. Orthop. Traumatol. Surg. Res. OTSR 2015, 101 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, H.K.; Ballet, F.L. The SLAC wrist: Scapholunate advanced collapse pattern of degenerative arthritis. J. Hand Surg. 1984, 9, 358–365. [Google Scholar] [CrossRef]
- Weiss, K.E.; Rodner, C.M. Osteoarthritis of the wrist. J. Hand Surg. 2007, 32, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Shidara, K.; Wise, B.L. Osteoarthritis year in review 2016: Clinical. Osteoarthr. Cartil. 2017, 25, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Lue, S.; Koppikar, S.; Shaikh, K.; Mahendira, D.; Towheed, T.E. Systematic review of non-surgical therapies for osteoarthritis of the hand: An update. Osteoarthr. Cartil. 2017, 25, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Fuchsberger, T.; Boesch, C.E.; Tonagel, F.; Fischborn, T.; Schaller, H.E.; Gonser, P. Patient Rated Long-Term Results after Complete Denervation of the Wrist. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Rudolf, K.-D.; Partecke, B.-D. Long-term results following denervation of the wrist in patients with stages II and III SLAC-/SNAC-wrist. Handchir. Mikrochir. Plast. Chir. Organ. 2006, 38, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, M.; Kluge, S.; Bickert, B.; Germann, G. Subjective and objective outcomes after total wrist arthrodesis in patients with radiocarpal arthrosis or Kienböck’s disease. Chir. Main 2000, 19, 223–231. [Google Scholar] [CrossRef]
- Aita, M.A.; Nakano, E.K.; de Schaffhausser, H.L.; Fukushima, W.Y.; Fujiki, E.N. Randomized clinical trial between proximal row carpectomy and the four-corner fusion for patients with stage II SNAC. Rev. Bras. Ortop. 2016, 51, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Dacho, A.K.; Baumeister, S.; Germann, G.; Sauerbier, M. Comparison of proximal row carpectomy and midcarpal arthrodesis for the treatment of scaphoid nonunion advanced collapse (SNAC-wrist) and scapholunate advanced collapse (SLAC-wrist) in stage II. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Elias, M.; Lluch, A.; Ferreres, A.; Papini-Zorli, I.; Rahimtoola, Z.O. Treatment of radiocarpal degenerative osteoarthritis by radioscapholunate arthrodesis and distal scaphoidectomy. J. Hand Surg. 2005, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Le Nen, D.; Richou, J.; Simon, E.; Le Bourg, M.; Nabil, N.; de Bodman, C.; Bacle, G.; Saint-Cast, Y.; Obert, L.; Saraux, A.; et al. The arthritic wrist. I–The degenerative wrist: Surgical treatment approaches. Orthop. Traumatol. Surg. Res. 2011, 97, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.M.; Chung, K.C. A Systematic Review of Total Wrist Arthroplasty Compared with Total Wrist Arthrodesis for Rheumatoid Arthritis. Plast. Reconstr. Surg. 2008, 122, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhang, C.; Tuan, R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res. Ther. 2014, 16, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.-L.; Zhou, A.-G.; Zhang, H.; Zhang, J. Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, A.; Leroux, T.; Wasserstein, D.; Marks, P.; Theodoropoulos, J.; Ogilvie-Harris, D.; Gandhi, R.; Takhar, K.; Lum, G.; Chahal, J. The Efficacy of Platelet-Rich Plasma in the Treatment of Symptomatic Knee Osteoarthritis: A Systematic Review with Quantitative Synthesis. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Laudy, A.B.M.; Bakker, E.W.P.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 657–672. [Google Scholar] [CrossRef] [PubMed]
- English, A.; Jones, E.A.; Corscadden, D.; Henshaw, K.; Chapman, T.; Emery, P.; McGonagle, D. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology 2007, 46, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maumus, M.; Manferdini, C.; Toupet, K.; Peyrafitte, J.-A.; Ferreira, R.; Facchini, A.; Gabusi, E.; Bourin, P.; Jorgensen, C.; Lisignoli, G.; et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res. 2013, 11, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic Potential of Multipotential Cells from Human Adipose Tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.S.; Desouches, C.; Gay, A.M.; Hautier, A.; Magalon, G. Development of micro-injection as an innovative autologous fat graft technique: The use of adipose tissue as dermal filler. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Bembo, F.; Eraud, J.; Philandrianos, C.; Bertrand, B.; Silvestre, A.; Veran, J.; Sabatier, F.; Magalon, G.; Magalon, J. Combined use of platelet rich plasma & micro-fat in sport and race horses with degenerative joint disease: Preliminary clinical study in eight horses. Muscles Ligaments Tendons J. 2016, 6, 198–204. [Google Scholar] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off. J. Eur. Union 2007, L 324, 121–137. [Google Scholar]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and Its Shortened Version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walenkamp, M.M.J.; de Muinck Keizer, R.-J.; Goslings, J.C.; Vos, L.M.; Rosenwasser, M.P.; Schep, N.W.L. The Minimum Clinically Important Difference of the Patient-rated Wrist Evaluation Score for Patients with Distal Radius Fractures. Clin. Orthop. Relat. Res. 2015, 473, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Deloach, J.; Porucznik, C.A.; Powell, A.P. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J. Shoulder Elbow Surg. 2009, 18, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Hung, M.; Keener, J.D.; Bowen, R.C.; McAllister, J.; Chen, W.; Ebersole, G.; Granger, E.K.; Chamberlain, A.M. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J. Shoulder Elbow Surg. 2017, 26, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Chang, J.-J.; Lee, J.H.; Lee, S.H. Safety reporting on implantation of autologous adipose tissue-derived stem cells with platelet-rich plasma into human articular joints. BMC Musculoskelet. Disord. 2013, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed. Res. Int. 2014, 2014, 370621. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Rennekampff, H.O.; Groddeck, R.; Allert, S. Autologous Fat Transfer for Thumb Carpometacarpal Joint Osteoarthritis: A Prospective Study. Plast. Reconstr. Surg. 2017, 140, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Kemper, R.; Wirth, J.; Baur, E.-M. Arthroscopic Synovectomy Combined with Autologous Fat Grafting in Early Stages of CMC Osteoarthritis of the Thumb. J. Wrist Surg. 2018, 7, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Z.; Opländer, C.; Almakadi, S.; Fritz, A.; Vogt, M.; Pallua, N. Conventional vs. micro-fat harvesting: How fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use; Guidance for Industry and Food and Drug Administration Staff; Availability, Federal Register, 82 (221/Friday, November 17); Food and Drug Administration: Silver Spring, MD, USA, 2017; pp. 54290–54292.
- Mojallal, A.; Lequeux, C.; Shipkov, C.; Rifkin, L.; Rohrich, R.; Duclos, A.; Brown, S.; Damour, O. Stem cells, mature adipocytes, and extracellular scaffold: What does each contribute to fat graft survival? Aesthet. Plast. Surg. 2011, 35, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Borrone, A.; Follenzi, A.; Messaggio, F.; Tremolada, C.; Cannas, M. Human Lipoaspirate as Autologous Injectable Active Scaffold for One-Step Repair of Cartilage Defects. Cell Transplant. 2016, 25, 1043–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Graiet, H.; Lokchine, A.; Francois, P.; Velier, M.; Grimaud, F.; Loyens, M.; Berda-Haddad, Y.; Veran, J.; Dignat-George, F.; Sabatier, F.; et al. Use of platelet-rich plasma in regenerative medicine: Technical tools for correct quality control. BMJ Open Sport Exerc. Med. 2018, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Sautereau, N.; Daumas, A.; Truillet, R.; Jouve, E.; Magalon, J.; Veran, J.; Casanova, D.; Frances, Y.; Magalon, G.; Granel, B. Efficacy of Autologous Microfat Graft on Facial Handicap in Systemic Sclerosis Patients. Plast. Reconstr. Surg. Glob. Open 2016, 4, e660. [Google Scholar] [CrossRef] [PubMed]
- Khuu, H.M.; Stock, F.; McGann, M.; Carter, C.S.; Atkins, J.W.; Murray, P.R.; Read, E.J. Comparison of automated culture systems with a CFR/USP-compliant method for sterility testing of cell-therapy products. Cytotherapy. 2004, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
| (A) | T0 | T + 3 h |
| Platelets Purity (%) | 97.4 ± 0.2 | 97.4 ± 0.1 |
| RBCs Contamination (%) | 2.5 ± 0.1 | 2.5 ± 0.1 |
| Leukocytes Contamination (%) | 0.04 ± 0.04 | 0.03 ± 0.03 |
| Potential Dose of Injected Platelets (106/ 2 mL) | 775.3 ± 35.8 | 766.7 ± 25.8 |
| Increase Factor in Platelets | 2.0 ± 0.4 | 2.0 ± 0.4 |
| Platelets Aggregation Response (%) | ||
| ADP 5 µM | 74.2 ± 9.8 | 69.0 ± 2.6 |
| ADP 2.5 µM | 55.5 ± 7.5 | 47.8 ± 1.9 |
| Arachidonic Acid 0.5 mg/ mL | 91.6 ± 3.5 | 87.5 ± 1.6 |
| Collagen 3.3 µg/mL | 90.1 ± 2.9 | 91.1 ± 2.4 |
| Ristocetin 1.25 mg/mL | 97.7 ± 2.5 | 96.9 ± 4.0 |
| Microbiological Assay | Free of germ (3/3) | Free of germ (3/3) |
| (B) | T0 | T + 3 h |
| Macroscopical Aspect | Absence of blood and oily phase (3/3) | Absence of blood and oily phase (3/3) |
| Microbiological Assay | Free of germ (3/3) | Free of germ (3/3) |
| (C) | T0 | - |
| SVF Viability (%) | 75.7 ± 1.3 | - |
| SVF Viable Nucleated Cells (x106 / 4 mL) | 1.3 ± 0.6 | - |
| Chondrocytes Differentiation * (Micromass Formed/Control Group) | 17/1 10/0 | - |
| GF Release (pg/mL) | ||
| VEGF | 22.0 ± 30.1 | - |
| PDGF | 394.0 ± 128.1 | - |
| FGF2 | 171.8 ± 83.1 | - |
| TGF-β1 | ND | - |
| IL1-β | 0.2 ± 0.1 | - |
| IL-1RA | 82.0 ± 73.3 | - |
| IL-1RA/IL1-β ratio | 443.3 ± 348.0 | - |
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Gender | Female | Female | Male |
| Age, years | 65 | 62 | 59 |
| Dominant hand | Right | Right | Right |
| Injured wrist | Left | Left | Left |
| Etiology | Radius fracture | SLAC | SLAC |
| Kellgren Lawrence grade | 4 | 4 | 4 |
| Seat(s) of osteoarthritis | RC | RC and IC | RC |
| Volume of microfat PRP injected (mL) | 2.7 | 4.0 | 3.8 |
| Dose of injected platelets (106) | 720.9 | 674.0 | 708.7 |
| Platelets purity (%) | 96.0 | 94.2 | 97.4 |
| PRP microbiological assay | Free of germ | Free of germ | Free of germ |
| Microfat microbiological assay | Free of germ | Free of germ | Free of germ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayoly, A.; Iniesta, A.; Curvale, C.; Kachouh, N.; Jaloux, C.; Eraud, J.; Vogtensperger, M.; Veran, J.; Grimaud, F.; Jouve, E.; et al. Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results. Int. J. Mol. Sci. 2019, 20, 1111. https://doi.org/10.3390/ijms20051111
Mayoly A, Iniesta A, Curvale C, Kachouh N, Jaloux C, Eraud J, Vogtensperger M, Veran J, Grimaud F, Jouve E, et al. Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results. International Journal of Molecular Sciences. 2019; 20(5):1111. https://doi.org/10.3390/ijms20051111
Chicago/Turabian StyleMayoly, Alice, Aurélie Iniesta, Caroline Curvale, Najib Kachouh, Charlotte Jaloux, Julia Eraud, Marie Vogtensperger, Julie Veran, Fanny Grimaud, Elisabeth Jouve, and et al. 2019. "Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results" International Journal of Molecular Sciences 20, no. 5: 1111. https://doi.org/10.3390/ijms20051111
APA StyleMayoly, A., Iniesta, A., Curvale, C., Kachouh, N., Jaloux, C., Eraud, J., Vogtensperger, M., Veran, J., Grimaud, F., Jouve, E., Casanova, D., Sabatier, F., Legré, R., & Magalon, J. (2019). Development of Autologous Platelet-Rich Plasma Mixed-Microfat as an Advanced Therapy Medicinal Product for Intra-Articular Injection of Radio-Carpal Osteoarthritis: From Validation Data to Preliminary Clinical Results. International Journal of Molecular Sciences, 20(5), 1111. https://doi.org/10.3390/ijms20051111





