Molecular Imaging of Vascular Calcification with 18F-Sodium-Fluoride in Patients Infected with Human Immunodeficiency Virus
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients Selection
4.2. HIV Related History and Immuno-Virological Parameters
4.3. Imaging Acquisition and Interpretation
4.3.1. Computer Tomography for Coronary Artery Calcium
4.3.2. Positron Emission Tomography with 18F-Sodium-Fluoride
4.3.3. Image Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hogg, R.S.; Eyawo, O.; Collins, A.B.; Zhang, W.; Jabbari, S.; Hull, M.W.; Lima, V.D.; Ahmed, T.; Kendall, C.E.; Althoff, K.N.; et al. Health-adjusted life expectancy in HIV-positive and HIV-negative men and women in British Columbia, Canada: A population-based observational cohort study. Lancet HIV 2017, 4, e270–e276. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Bidwell Goetz, M.; Leaf, D.; Oursler, K.A.; et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Grinspoon, S.K. Epidemiology of ischemic heart disease in HIV. Curr. Opin. HIV AIDS 2017, 12, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.; Manicardi, M.; Guaraldi, G.; Raggi, P. Cardiovascular disease in human immunodeficiency virus infected patients: A true or perceived risk? World J. Cardiol. 2015, 7, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Blau, M.; Nagler, W.; Bender, M.A. Fluorine-18: A new isotope for bone scanning. J. Nucl. Med. 1962, 3, 332–334. [Google Scholar] [PubMed]
- Derlin, T.; Richter, U.; Bannas, P.; Begemann, P.; Buchert, R.; Mester, J.; Klutmann, S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J. Nucl. Med. 2010, 51, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Wisotzki, C.; Richter, U.; Apostolova, I.; Bannas, P.; Weber, C.; Mester, J.; Klutmann, S. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: Correlation with atherogenic risk factors. J. Nucl. Med. 2011, 52, 362–368. [Google Scholar] [CrossRef]
- Oliveira-Santos, M.; Castelo-Branco, M.; Silva, R.; Gomes, A.; Chichorro, N.; Abrunhosa, A.; Donato, P.; de Lima, J.P.; Pego, M.; Gonçalves, L.; et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects—A subclinical atherosclerosis imaging study with 18F-NaF PET-CT. Atherosclerosis 2017, 260, 41–46. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yamamoto, H.; Toshimitsu, S.; Sasaki, K.; Senoo, A.; Kubo, Y.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. (18)F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis 2017, 263, 385–392. [Google Scholar] [CrossRef]
- Lee, J.M.; Bang, J.I.; Koo, B.K.; Hwang, D.; Park, J.; Zhang, J.; Yaliang, T.; Suh, M.; Paeng, J.C.; Shiono, Y.; et al. Clinical relevance of (18)F-sodium fluoride positron-emission tomography in noninvasive identification of high-risk plaque in patients with coronary artery disease. Circ. Cardiovasc. Imaging 2017, 10, e006704. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.; Calvert, P.A.; Craighead, F.H.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Jenkins, W.S.; Vesey, A.T.; Shah, A.S.; Pawade, T.A.; Chin, C.W.; White, A.C.; Fletcher, A.; Cartlidge, T.R.; Mitchell, A.J.; Pringle, M.A.; et al. Valvular (18)F-Fluoride and (18)F-Fluorodeoxyglucose Uptake Predict Disease Progression and Clinical Outcome in Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- Massera, D.; Trivieri, M.G.; Andrews, J.P.M.; Sartori, S.; Abgral, R.; Chapman, A.R.; Jenkins, W.S.A.; Vesey, A.T.; Doris, M.K.; Pawade, T.A.; et al. Disease Activity in Mitral Annular Calcification. Circ. Cardiovasc. Imaging 2019, 12, e008513. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Callister, T.Q.; Shaw, L.J. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Hokanson, J.E.; Nasir, K.; Shaw, L.J.; Kinney, G.L.; Chow, D.; Demoss, D.; Nuguri, V.; Nabavi, V.; Ratakonda, R.; et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc. Imaging 2010, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Young, R.; Lopez, V.A.; Kronmal, R.A.; Nasir, K.; Blumenthal, R.S.; Detrano, R.C.; Bild, D.E.; Guerci, A.D.; Liu, K.; et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2013, 61, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Falk, E.; Li, D.; Nasir, K.; Blaha, M.J.; Sandfort, V.; Rodriguez, C.J.; Ouyang, P.; Budoff, M. Statin trials, cardiovascular events, and coronary artery calcification: Implications for a trial-based approach to statin therapy in MESA. JACC Cardiovasc. Imaging 2018, 11, 221–230. [Google Scholar] [CrossRef]
- Gibson, A.O.; Blaha, M.J.; Arnan, M.K.; Sacco, R.L.; Szklo, M.; Herrington, D.M.; Yeboah, J. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc. Imaging 2014, 7, 1108–1115. [Google Scholar] [CrossRef]
- Nasir, K.; Bittencourt, M.S.; Blaha, M.J.; Blankstein, R.; Agatson, A.S.; Rivera, J.J.; Miedema, M.D.; Sibley, C.T.; Shaw, L.J.; Blumenthal, R.S.; et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2015, 66, 1657–1668. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Nance, R.M.; Drozd, D.R.; Ning, H.; Delaney, J.A.; Heckbert, S.R.; Budoff, M.J.; Mathews, W.C.; Kitahata, M.M.; Saag, M.S.; et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: A study by the centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017, 2, 155–162. [Google Scholar] [CrossRef]
- Raggi, P.; De Francesco, D.; Manicardi, M.; Zona, S.; Bellasi, A.; Stentarelli, C.; Carli, F.; Beghetto, B.; Mussini, C.; Malagoli, A.; et al. Prediction of hard cardiovascular events in HIV patients. J. Antimicrob. Chemother. 2016, 71, 3515–3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triant, V.A. Cardiovascular disease and HIV infection. Curr. HIV/AIDS Rep. 2013, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Marnane, M.; Merwick, A.; Sheehan, O.C.; Hannon, N.; Foran, P.; Grant, T.; Dolan, E.; Moroney, J.; Murphy, S.; O’Rourke, K.; et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann. Neurol. 2012, 71, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.L.; Abdelbaky, A.; Truong, Q.A.; Corsini, E.; MacNabb, M.H.; Lavender, Z.R.; Lawler, M.A.; Grinspoon, S.K.; Brady, T.J.; Nasir, K.; et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc. Imaging 2013, 6, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Lo, J.; Zanni, M.V.; Marmarelis, E.; Ihenachor, E.J.; MacNabb, M.; Wai, B.; Hoffmann, U.; Abbara, S.; Grinspoon, S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2014, 66, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Zona, S.; Scaglioni, R.; Stentarelli, C.; Ligabue, G.; Besutti, G.; Menozzi, M.; Santoro, A.; Malagoli, A.; Bellasi, A.; et al. Epicardial adipose tissue and coronary artery calcium predict incident myocardial infarction and death in HIV-infected patients. J. Cardiovasc. Comput. Tomogr. 2015, 9, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Zanni, M.V.; Toribio, M.; Robbins, G.K.; Burdo, T.H.; Lu, M.T.; Ishai, A.E.; Feldpausch, M.N.; Martin, A.; Melbourne, K.; Triant, V.A.; et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016, 1, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.; Granåsen, G.; Wiklund, U.; Schmermund, A.; Guerci, A.; Erbel, R.; Raggi, P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015, 184, 581–586. [Google Scholar] [CrossRef]
- Ishiwata, Y.; Kaneta, T.; Nawata, S.; Hino-Shishikura, A.; Yoshida, K.; Inoue, T. Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by (18)F-sodium fluoride PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1529–1537. [Google Scholar] [CrossRef]
- Li, X.; Heber, D.; Cal-Gonzalez, J.; Karanikas, G.; Mayerhoefer, M.E.; Rasul, S.; Beitzke, D.; Zhang, X.; Agis, H.; Mitterhauser, M.; et al. Association between osteogenesis and inflammation during the progression of calcified plaque evaluated by (18)F-Fluoride and (18)F-FDG. J. Nucl. Med. 2017, 58, 968–974. [Google Scholar] [CrossRef]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Callister, T.Q.; Cooil, B.; Raya, S.P.; Lippolis, N.J.; Russo, D.J.; Raggi, P. Coronary artery disease: Improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998, 208, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Senior, P.; Shahbaz, S.; Kaul, P.; Hung, R.; Coulden, R.; Yeung, R.; Abele, J. (18)F-sodium fluoride imaging of coronary atherosclerosis in ambulatory patients with diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
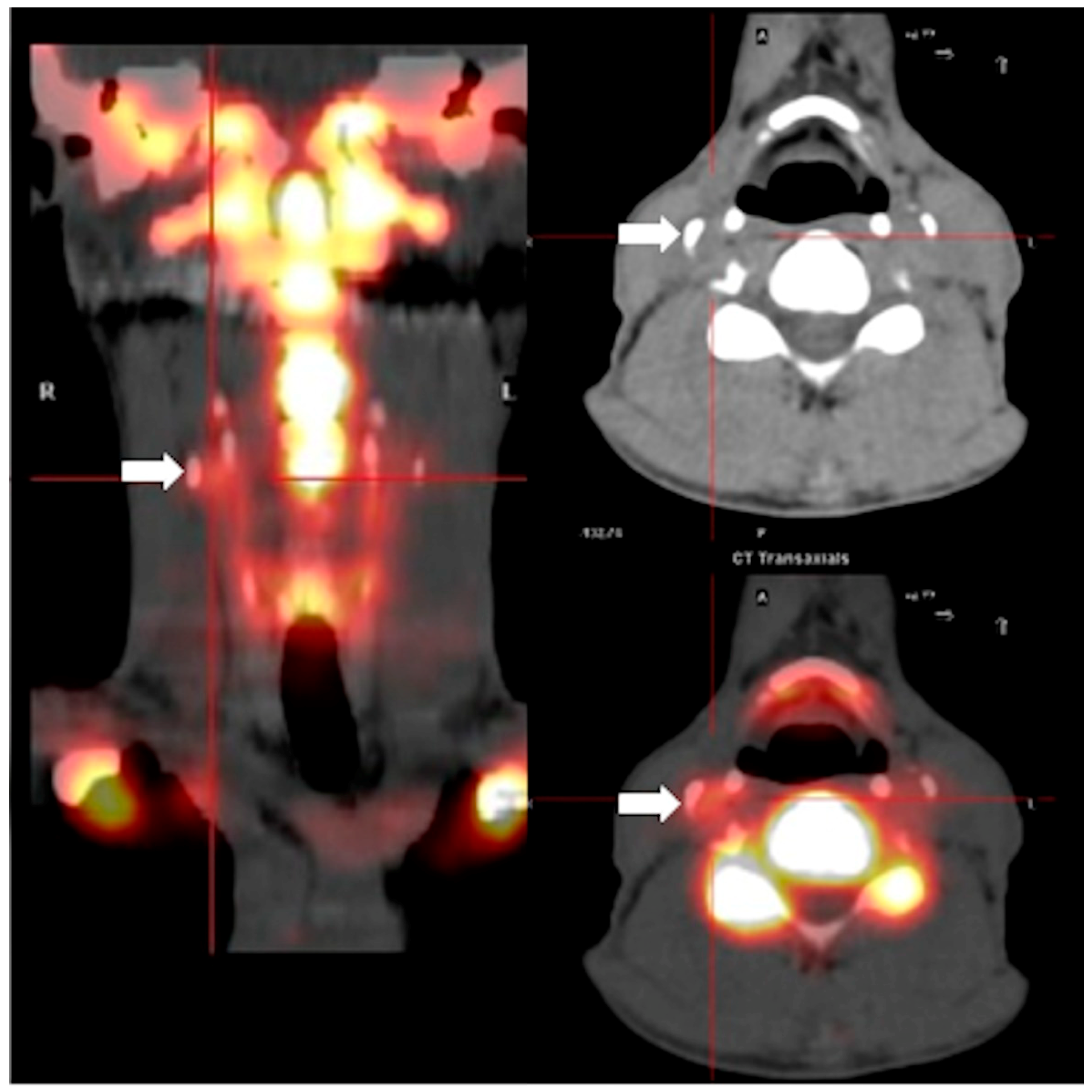
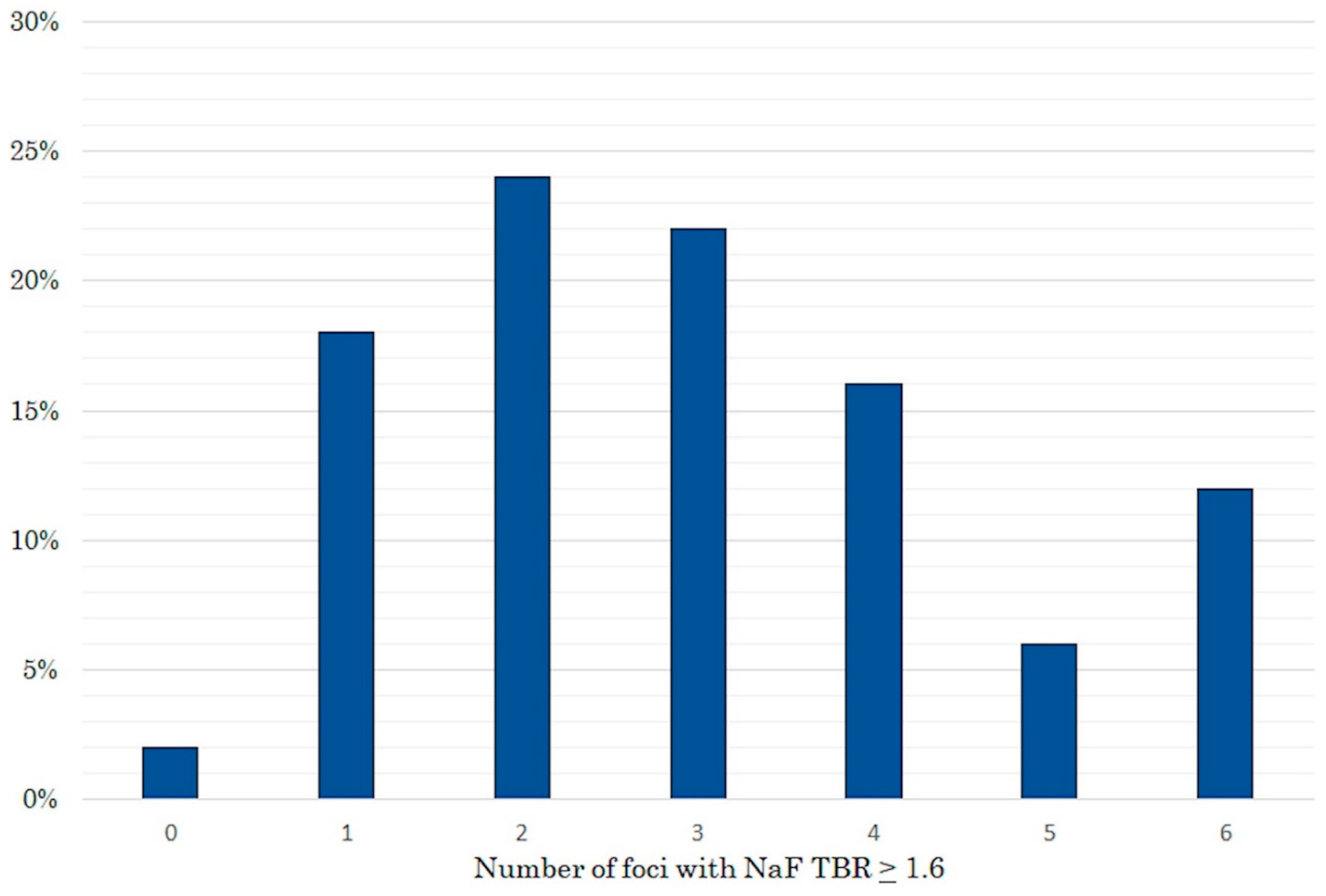
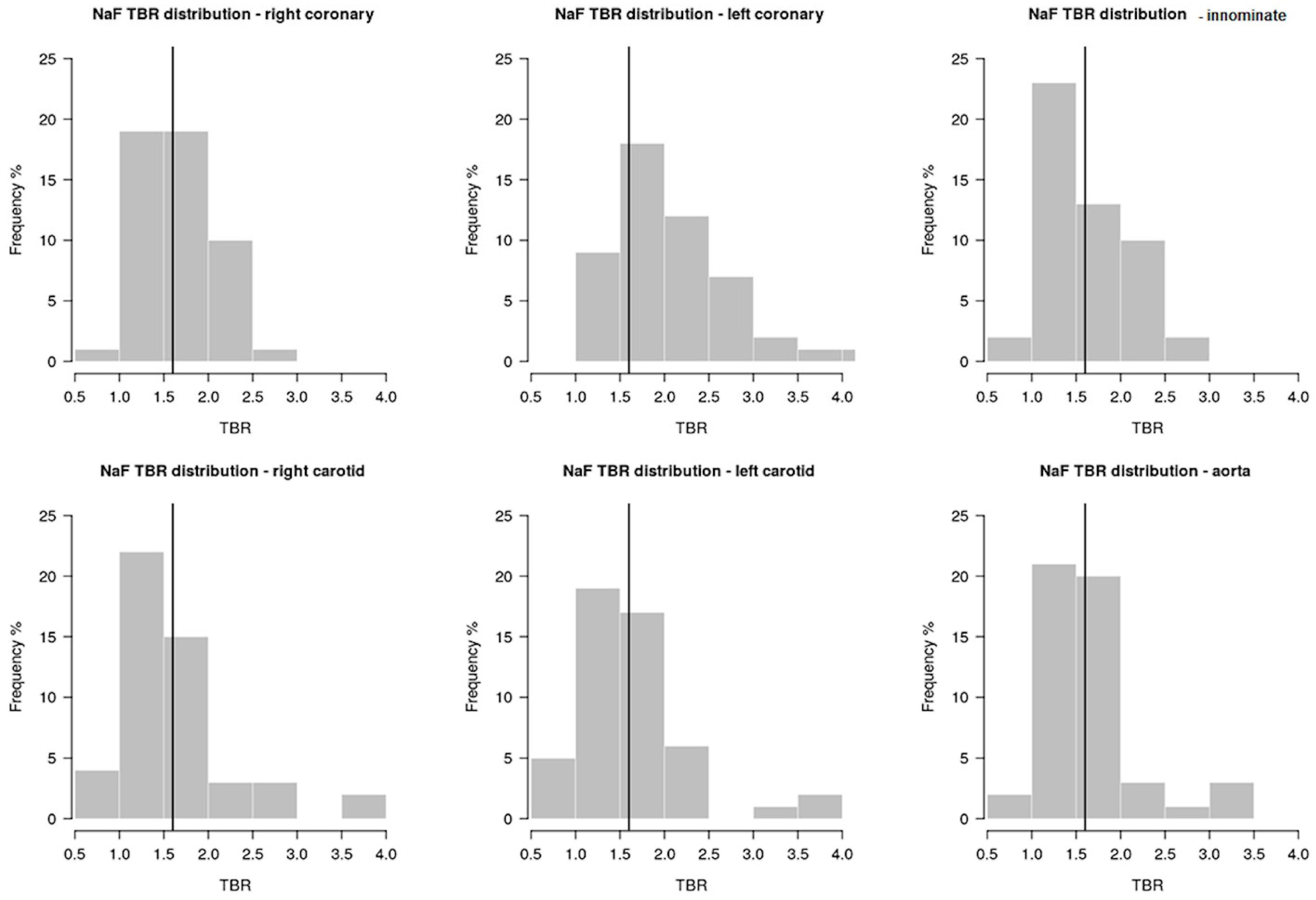
| Variables | Total | No Progression | Progression | p-Value |
|---|---|---|---|---|
| 50 | 19(38%) | 31(62%) | ||
| Demographic and anthropometric variables | ||||
| Age, mean (±SD) | 57.1 (7.82) | 52.53 (4.64) | 59.9 (8.1) | <0.001 |
| Men (%) | 42 (84%) | 13 (68.42%) | 29 (93.55%) | 0.05 |
| Pack year smoking, median (IQR) | 15 (0–33.75) | 16.88 (6.5–30.2) | 13.2 (0–34.5) | 0.88 |
| BMI, kg/m2, mean (±SD) | 26.01 (4.14) | 25.66 (4.2) | 26.21 (4.16) | 0.67 |
| Waist circumference, cm, mean (±SD) | 96.78 (11.3) | 96.81 (11.65) | 96.76 (11.31) | 0.99 |
| HIV variables | ||||
| Nadir CD4 c/microL, median (IQR) | 200 (107.5–324) | 249.5 (142.75–430.5) | 170 (59–286) | 0.02 |
| HIV duration, months, median (IQR) | 277.5 (191.25–354) | 267 (113–340) | 287 (239.5–358.5) | 0.19 |
| CD4/CD8 ratio, mean (±SD) | 0.97 (0.41) | 1.01 (0.37) | 0.94 (0.44) | 0.60 |
| Undetectable HIV viral load (%) | 50 (100%) | 19 (100%) | 31 (100%) | 1.0 |
| Cumulative exposure to INSTIs, months, median (IQR) | 36 (14–73.5) | 28.5 (5.5–47.25) [12] | 39 (23–98) | 0.12 |
| Cumulative exposure to NNRTIs, months, median (IQR) | 63.5 (29.25–123.75) | 39.5 (24–77.5) [10] | 79.5 (29.75–126.5) | 0.23 |
| Cumulative exposure to NRTIs, months, median (IQR) | 169 (103.5–235) | 128 (55–196) | 187.5 (136–251.75) | 0.03 |
| Cumulative exposure to PIs, months, median (IQR) | 123 (51–172) | 94.5 (50.75–136.5) | 129 (51–188) | 0.19 |
| Laboratory variables | ||||
| CRP, mean (±SD) | 0.24 (0.29) | 0.22 (0.15) | 0.25 (0.35) | 0.57 |
| Triglycerides, mg/dL, mean (±SD) | 160.2 (160.51) | 206.53 (231.96) | 133.03 (92.68) | 0.16 |
| LDL cholesterol, mg/dL, mean (±SD) | 103.26 (33.04) | 124.71 (31.33) | 90.69 (27.39) | <0.001 |
| HDL cholesterol, mg/dL, mean (±SD) | 46.13 (12.07) | 45.59 (11.25) | 46.45 (12.72) | 0.82 |
| Total cholesterol, mg/dL, mean (±SD) | 173.15 (39.83) | 198.88 (29.03) | 158.07 (37.83) | <0.001 |
| Glucose, mg/dL, mean (±SD) | 103.26 (31.15) | 95.06 (16.17) | 108.34 (36.94) | 0.30 |
| HOMA, mean (±SD) | 3.52 (4.24) | 3.62 (3.06) | 3.46 (4.97) | 0.22 |
| CKD-Epi, mL/min/1.73m2, mean (±SD) | 79.77 (22.49) | 85.73 (21.72) | 76.33 (22.78) | 0.28 |
| Comorbidities | ||||
| CKD (%) | 19 (38%) | 5 (26.32%) | 14 (45.16%) | 0.30 |
| COPD (%) | 4 (8%) | 0 (0%) | 4 (12.9%) | 0.27 |
| Osteoporosis (%) | 15 (30%) | 4 (21.05%) | 11 (35.48%) | 0.44 |
| Dyslipidemia (%) | 49 (98%) | 19 (100%) | 30 (96.77%) | 1 |
| Cardiovascular variables | ||||
| Statin use (%) | 7 (14%) | 2 (10.53%) | 5 (16.13%) | 0.89 |
| Systolic blood pressure, mmHg, mean (±SD) | 127.25 (15.29) | 121.94 (11.34) | 130.43 (16.59) | 0.08 |
| Diastolic blood pressure, mmHg, mean (±SD) | 81.58 (8.82) | 81.33 (8.51) | 81.73 (9.15) | 0.88 |
| ASCVD, mean (±SD) | 10.31 (9.9) | 7.39 (7.05) | 13.59 (11.99) | 0.21 |
| Calcium score, median (IQR), | 104 (38.5–348) | 4 (2–6) | 206 (104–490) | 0.05 |
| Hypertension (%) | 33 (66%) | 8 (42.11%) | 25 (80.65%) | 0.01 |
| Type 2 diabetes mellitus (%) | 14 (28%) | 5 (26.32%) | 9 (29.03%) | 1 |
| CVD (%) | 7 (14%) | 1 (5.26%) | 6 (19.35%) | 0.33 |
| Nuclear medicine variables | ||||
| Aortic arch TBR | 1.65 (0.52) | 1.53 (0.51) | 1.73 (0.52) | 0.16 |
| Innominate artery TBR | 1.66 (0.49) | 1.67 (0.57) | 1.65 (0.45) | 0.87 |
| Right carotid artery TBR | 1.69 (0.8) | 1.59 (0.72) | 1.75 (0.85) | 0.42 |
| Left carotid artery TBR | 1.64 (0.66) | 1.51 (0.69) | 1.72 (0.63) | 0.14 |
| RCA TBR | 1.65 (0.41) | 1.6 (0.39) | 1.67 (0.43) | 0.55 |
| LCA TBR | 2.08 (0.66) | 1.93 (0.43) | 2.17 (0.76) | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raggi, P.; Prandini, N.; Ligabue, G.; Braglia, G.; Esposito, F.; Milic, J.; Malagoli, A.; Scaglioni, R.; Besutti, G.; Beghetto, B.; et al. Molecular Imaging of Vascular Calcification with 18F-Sodium-Fluoride in Patients Infected with Human Immunodeficiency Virus. Int. J. Mol. Sci. 2019, 20, 1183. https://doi.org/10.3390/ijms20051183
Raggi P, Prandini N, Ligabue G, Braglia G, Esposito F, Milic J, Malagoli A, Scaglioni R, Besutti G, Beghetto B, et al. Molecular Imaging of Vascular Calcification with 18F-Sodium-Fluoride in Patients Infected with Human Immunodeficiency Virus. International Journal of Molecular Sciences. 2019; 20(5):1183. https://doi.org/10.3390/ijms20051183
Chicago/Turabian StyleRaggi, Paolo, Napoleone Prandini, Guido Ligabue, Giovanni Braglia, Francesco Esposito, Jovana Milic, Andrea Malagoli, Riccardo Scaglioni, Giulia Besutti, Barbara Beghetto, and et al. 2019. "Molecular Imaging of Vascular Calcification with 18F-Sodium-Fluoride in Patients Infected with Human Immunodeficiency Virus" International Journal of Molecular Sciences 20, no. 5: 1183. https://doi.org/10.3390/ijms20051183
APA StyleRaggi, P., Prandini, N., Ligabue, G., Braglia, G., Esposito, F., Milic, J., Malagoli, A., Scaglioni, R., Besutti, G., Beghetto, B., Nardini, G., Roncaglia, E., Mussini, C., & Guaraldi, G. (2019). Molecular Imaging of Vascular Calcification with 18F-Sodium-Fluoride in Patients Infected with Human Immunodeficiency Virus. International Journal of Molecular Sciences, 20(5), 1183. https://doi.org/10.3390/ijms20051183





