Control of Silver Coating on Raman Label Incorporated Gold Nanoparticles Assembled Silica Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
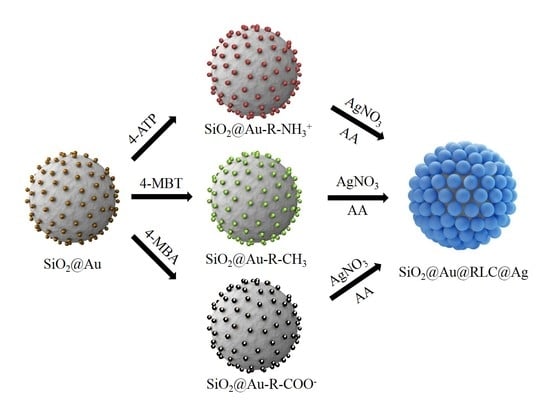
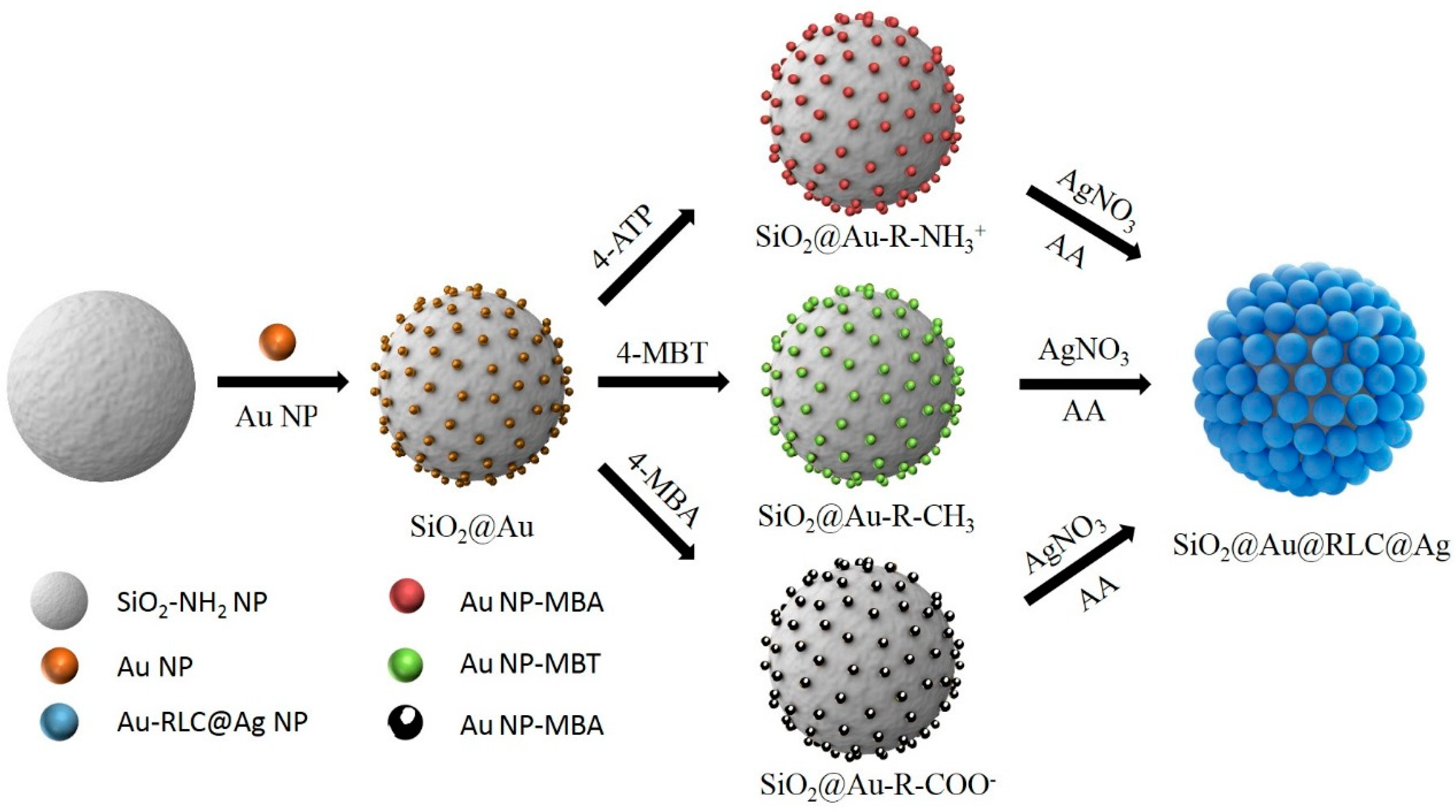
2.1. Preparation of SiO2@Au@RLC@Ag
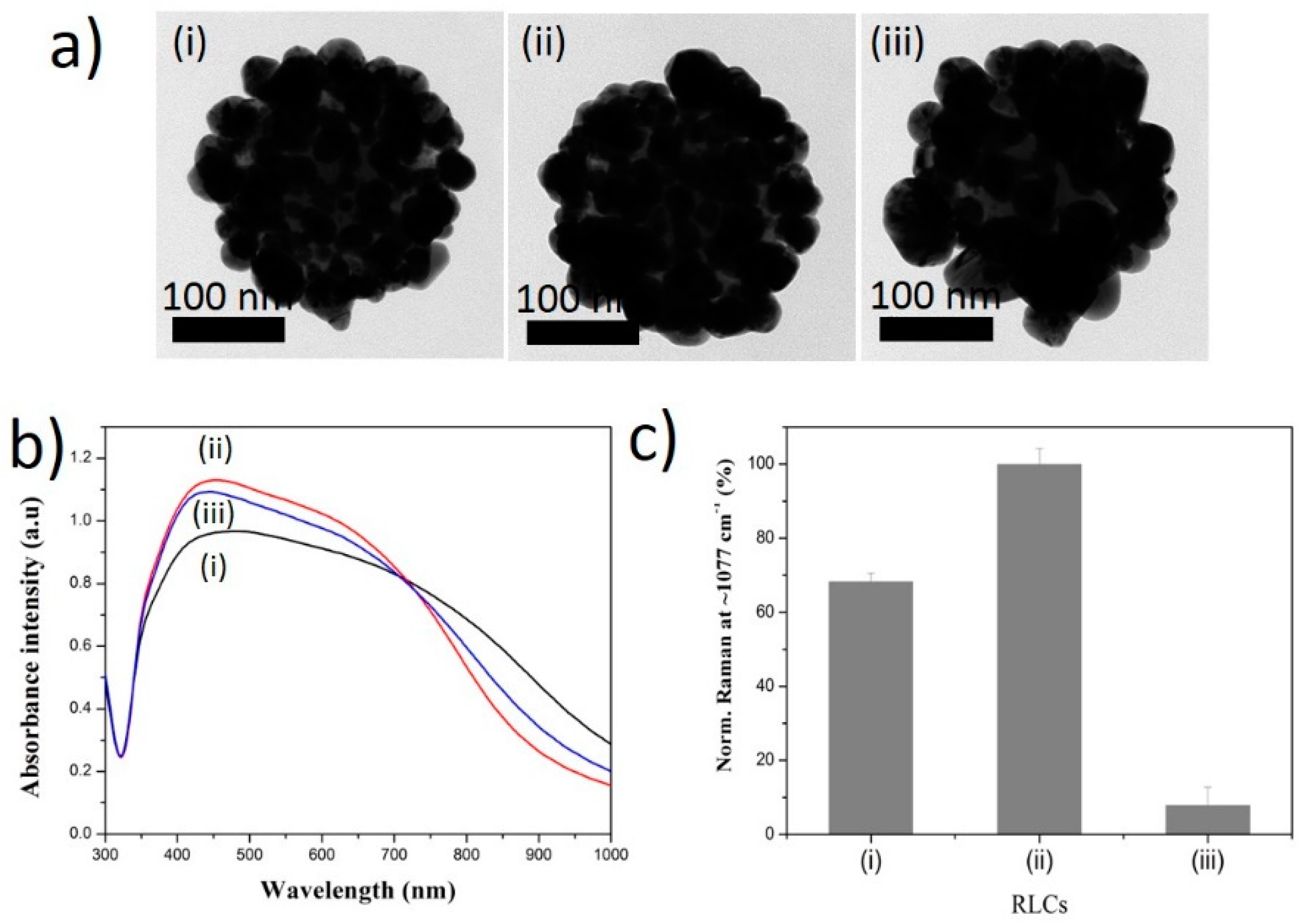
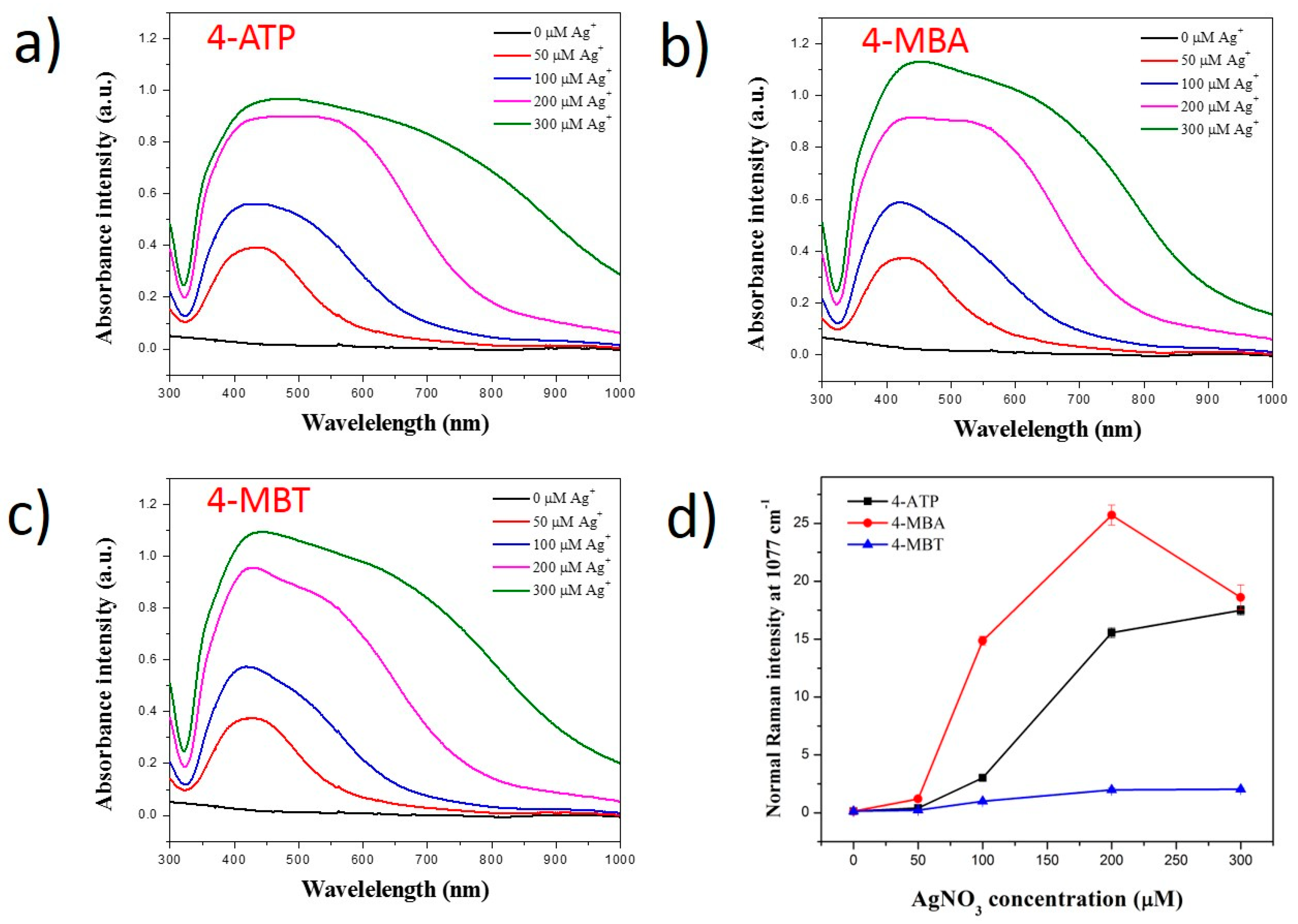
2.2. Effect of Silver Ion Concentration on Ag Shell Coating on SiO2@Au@RLCs
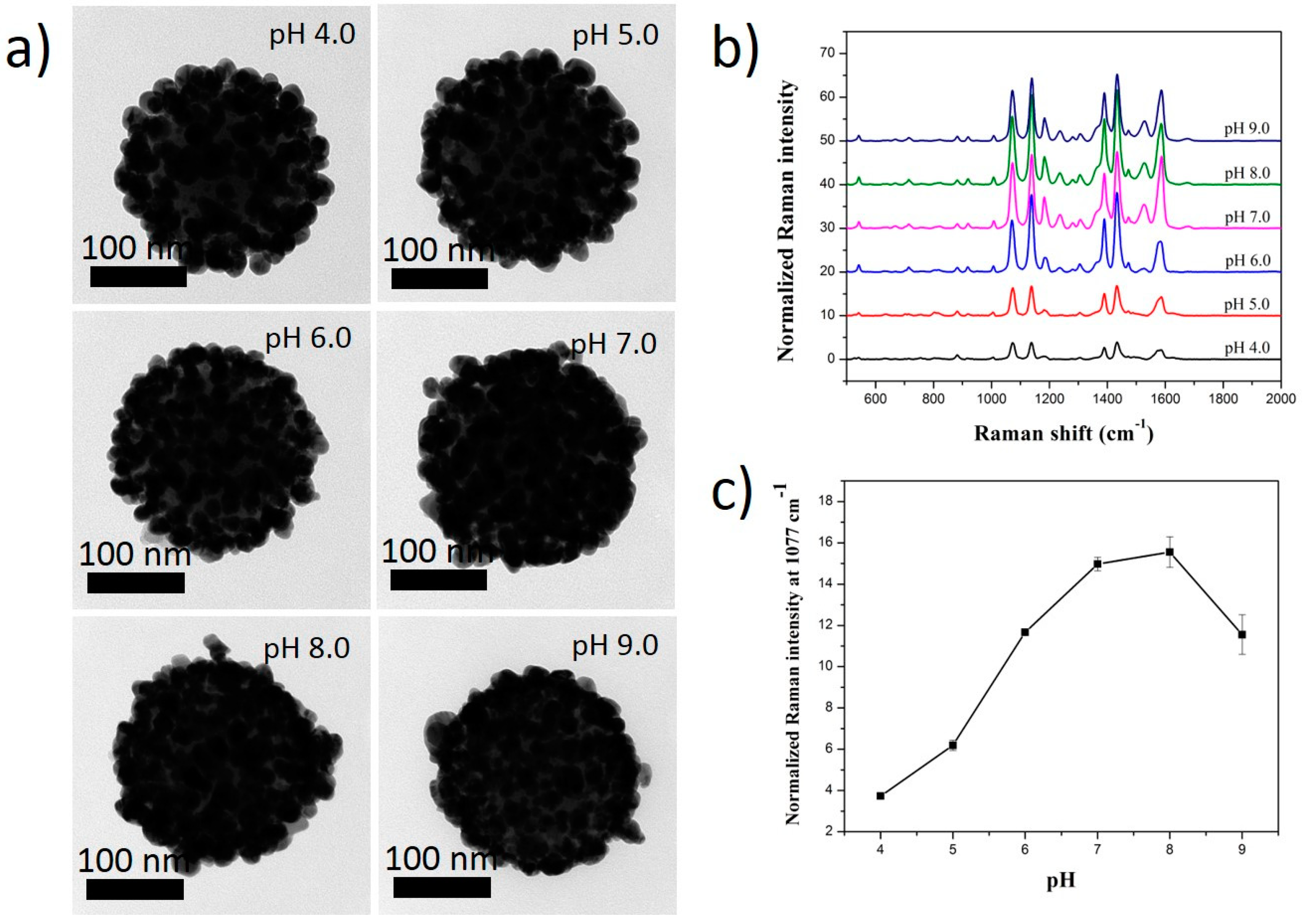
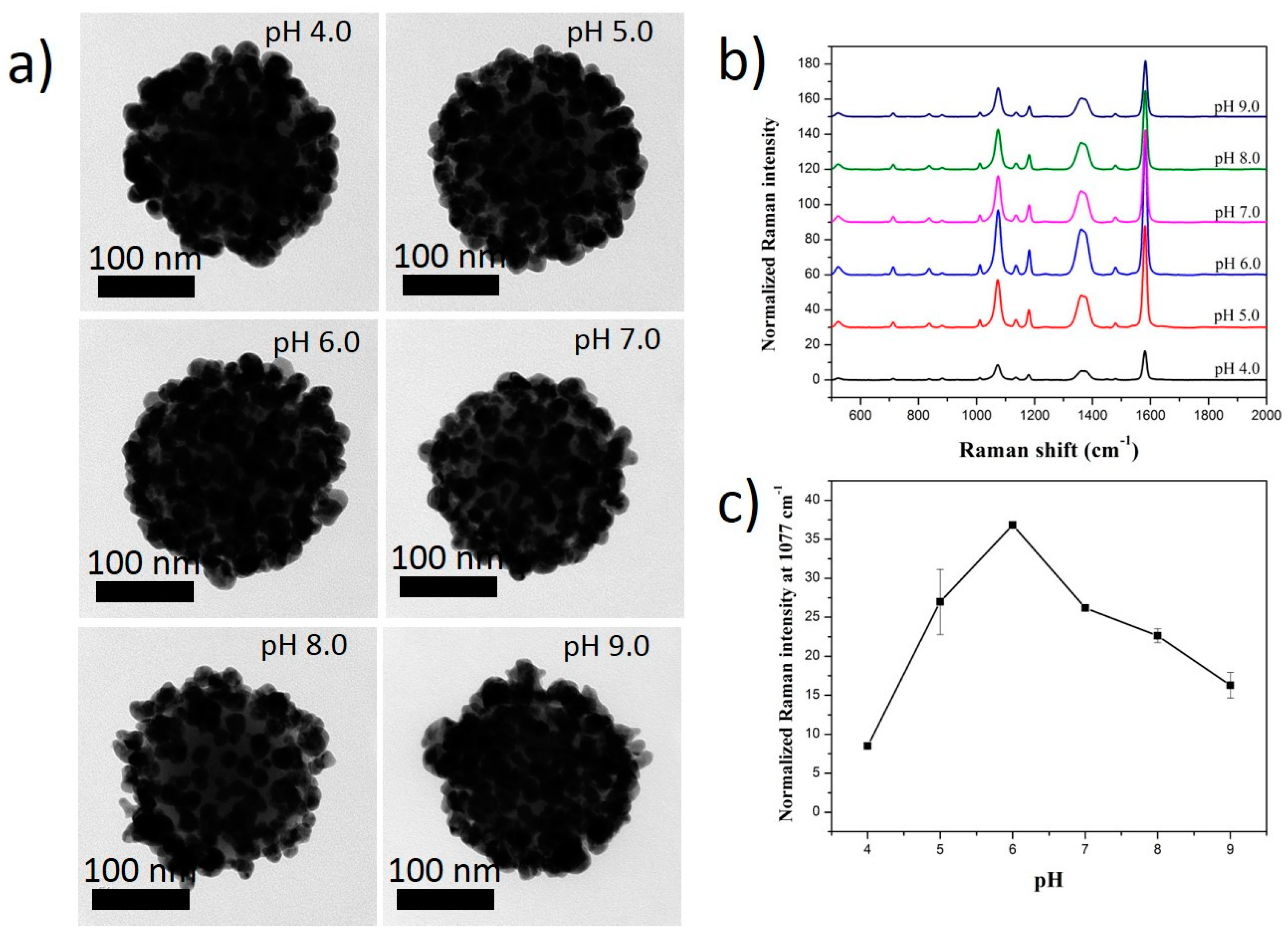
2.3. Effect of pH Solution on the Ag Shell Coating of SiO2@Au@RLC@Ag NPs
3. Experiment
3.1. Materials
3.2. Preparation of SiO2@Au NP Templates
3.3. Incorporating RLC into SiO2@Au
3.4. Preparation of SiO2@Au@RLC@Ag NPs
3.5. SERS Measurement of the SiO2@Au@RLC@Ag NPs
3.6. Transmission Electron Microscopy (TEM) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Schlücker, S. Surface-enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, B.; Chen, L. Sers tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428. [Google Scholar] [CrossRef] [PubMed]
- Culha, M.; Cullum, B.; Lavrik, N.; Klutse, C.K. Surface-enhanced raman scattering as an emerging characterization and detection technique. J. Nanotechnol. 2012, 2012, 15. [Google Scholar] [CrossRef]
- Jun, B.-H.; Kim, G.; Jeong, S.; Noh, M.S.; Pham, X.-H.; Kang, H.; Cho, M.-H.; Kim, J.-H.; Lee, Y.-S.; Jeong, D.H. Silica core-based surface-enhanced raman scattering (sers) tag: Advances in multifunctional sers nanoprobes for bioimaging and targeting of biomarkers#. Bull. Korean Chem. Soc. 2015, 36, 963–978. [Google Scholar]
- Goodacre, R.; Graham, D.; Faulds, K. Recent developments in quantitative sers: Moving towards absolute quantification. TrAC Trends Anal. Chem. 2018, 102, 359–368. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Yang, S.; Zheng, H.; Li, Y. Facile synthesis of mos2 nanosheet-silver nanoparticles composite for surface enhanced raman scattering and electrochemical activity. J. Alloys Compd. 2013, 559, 87–91. [Google Scholar] [CrossRef]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A hierarchically ordered array of silver-nanorod bundles for surface-enhanced raman scattering detection of phenolic pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef]
- Du, Y.; Wei, W.; Zhang, X.; Li, Y. Tuning metamaterials nanostructure of janus gold nanoparticle film for surface-enhanced raman scattering. J. Phys. Chem. C 2018, 122, 7997–8002. [Google Scholar] [CrossRef]
- Kasera, S.; Biedermann, F.; Baumberg, J.J.; Scherman, O.A.; Mahajan, S. Quantitative sers using the sequestration of small molecules inside precise plasmonic nanoconstructs. Nano Lett. 2012, 12, 5924–5928. [Google Scholar] [CrossRef]
- Lim, D.-K.; Jeon, K.-S.; Hwang, J.-H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.-M. Highly uniform and reproducible surface-enhanced raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nano 2011, 6, 452–460. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Luo, Y.; Liang, A.; Wen, G.; Jiang, Z. A sensitive gold nanoplasmonic sers quantitative analysis method for sulfate in serum using fullerene as catalyst. Nanomaterials 2018, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Li, X.; Zhang, X.; Wen, G.; Jiang, Z. A sensitive sers quantitative analysis method for ni2+ by the dimethylglyoxime reaction regulating a graphene oxide nanoribbon catalytic gold nanoreaction. Luminescence 2018, 33, 1033–1039. [Google Scholar] [CrossRef]
- Liang, A.; Wang, H.; Yao, D.; Jiang, Z. A simple and sensitive sers quantitative analysis method for urea using the dimethylglyoxime product as molecular probes in nanosilver sol substrate. Food Chem. 2019, 271, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Q.; Li, S.-X.; Chen, Z.-P.; Chen, Y.; Yu, R.-Q. Quantitative sers analysis based on multiple-internal-standard embedded core-shell nanoparticles and spectral shape deformation quantitative theory. Chemometr. Intell. Lab. Syst. 2018, 177, 47–54. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Jiang, C.; Li, C.; Lin, H.; Huang, J.; Wang, S.; Liu, G.; Yan, X.; Zhong, Q.; et al. Reliable quantitative sers analysis facilitated by core–shell nanoparticles with embedded internal standards. Angew. Chem. Int. Ed. 2015, 54, 7308–7312. [Google Scholar] [CrossRef]
- Kammer, E.; Olschewski, K.; Bocklitz, T.; Rosch, P.; Weber, K.; Cialla, D.; Popp, J. A new calibration concept for a reproducible quantitative detection based on sers measurements in a microfluidic device demonstrated on the model analyte adenine. Phys. Chem. Chem. Phys. 2014, 16, 9056–9063. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, R.; Joshi, P.; Zhang, P. Quantitative surface-enhanced raman measurements with embedded internal reference. Anal. Chim. Acta 2015, 874, 49–53. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Tao, W.; Yu, B.; Du, Y. Quantitative analysis of thymine with surface-enhanced raman spectroscopy and partial least squares (pls) regression. Anal. Bioanal. Chem. 2010, 398, 1827–1832. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.-P.; Zuo, Q.; Shi, C.-X.; Yu, R.-Q. Surface-enhanced raman spectroscopy based on conical holed enhancing substrates. Anal. Chim. Acta 2015, 887, 45–50. [Google Scholar] [CrossRef]
- Lorén, A.; Engelbrektsson, J.; Eliasson, C.; Josefson, M.; Abrahamsson, J.; Johansson, M.; Abrahamsson, K. Internal standard in surface-enhanced raman spectroscopy. Anal. Chem. 2004, 76, 7391–7395. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.-P.; Jin, J.-W.; Yu, R.-Q. Quantitative determination of ametryn in river water using surface-enhanced raman spectroscopy coupled with an advanced chemometric model. Chemometr. Intell. Lab. Syst. 2015, 142, 166–171. [Google Scholar] [CrossRef]
- Xia, T.-H.; Chen, Z.-P.; Chen, Y.; Jin, J.-W.; Yu, R.-Q. Improving the quantitative accuracy of surface-enhanced raman spectroscopy by the combination of microfluidics with a multiplicative effects model. Anal. Methods 2014, 6, 2363–2370. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.-P.; Long, S.-Y.; Yu, R.-Q. Generalized ratiometric indicator based surface-enhanced raman spectroscopy for the detection of cd2+ in environmental water samples. Anal. Chem. 2014, 86, 12236–12242. [Google Scholar] [CrossRef]
- Hammond, P.T. Form and function in multilayer assembly: New applications at the nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning upconversion through energy migration in core–shell nanoparticles. Nat. Mater. 2011, 10, 968. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.-J. Synthesis of m@tio2 (m = au, pd, pt) core–shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Xu, Y.-J. Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Strobbia, P.; Languirand, E.R.; Cullum, B.M. Recent Advances in Plasmonic Nanostructures for Sensing: A Review. Opt. Eng. 2015, 54, 100902. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Pandikumar, A.; Lim, S.-P.; Jayabal, S.; Huang, N.M.; Lim, H.N.; Ramaraj, R. Titania@gold plasmonic nanoarchitectures: An ideal photoanode for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2016, 60, 408–420. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Akita, T.; Xu, Q. A one-pot protocol for synthesis of non-noble metal-based core–shell nanoparticles under ambient conditions: Toward highly active and cost-effective catalysts for hydrolytic dehydrogenation of nh3bh3. Chem. Commun. 2011, 47, 10999–11001. [Google Scholar] [CrossRef]
- Caruso, F.; Spasova, M.; Salgueiriño-Maceira, V.; Liz-Marzán, L.M. Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates. Adv. Mater. 2001, 13, 1090–1094. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhang, Y.-J.; Ding, S.-Y.; Panneerselvam, R.; Tian, Z.-Q. Core–shell nanoparticle-enhanced raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442. [Google Scholar] [CrossRef]
- Raemdonck, K.; Demeester, J.; De Smedt, S. Advanced nanogel engineering for drug delivery. Soft Matter 2009, 5, 707–715. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, C.; Zhang, Z.; Li, G. An intrinsic internal standard substrate of au@ps-b-p4vp for rapid quantification by surface enhanced raman scattering. Analyst 2017, 142, 2936–2944. [Google Scholar] [CrossRef]
- Hahm, E.; Cha, M.G.; Kang, E.J.; Pham, X.-H.; Lee, S.H.; Kim, H.-M.; Kim, D.-E.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Multi-layer ag-embedded silica nanostructure as sers-based chemical sensor with dual-function internal standards. ACS Appl. Mater. Interfaces 2018, 10, 40748–40755. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, H.; Chen, T.; Tay, Y.Y.; Yao, L.; Yan, Q.; Li, S.; Chen, H. Engineering “hot” nanoparticles for surface-enhanced raman scattering by embedding reporter molecules in metal layers. Small 2012, 8, 246–251. [Google Scholar] [CrossRef]
- Gandra, N.; Singamaneni, S. Bilayered raman-intense gold nanostructures with hidden tags (brights) for high-resolution bioimaging. Adv. Mater. 2013, 25, 1022–1027. [Google Scholar] [CrossRef]
- Pham, X.-H.; Lee, M.; Shim, S.; Jeong, S.; Kim, H.-M.; Hahm, E.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Highly sensitive and reliable sers probes based on nanogap control of a au-ag alloy on silica nanoparticles. RSC Adv. 2017, 7, 7015–7021. [Google Scholar] [CrossRef]
- Shim, S.; Pham, X.-H.; Cha, M.G.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Size effect of gold on ag-coated au nanoparticle-embedded silica nanospheres. RSC Adv. 2016, 6, 48644–48650. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kang, E.; Ha, Y.N.; Lee, S.H.; Rho, W.-Y.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Gold-silver bimetallic nanoparticles with a raman labeling chemical assembled on silica nanoparticles as an internal-standard-containing nanoprobe. J. Alloys Compd. 2019, 779, 360–366. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Martin, M.N.; Basham, J.I.; Chando, P.; Eah, S.-K. Charged gold nanoparticles in non-polar solvents: 10-min synthesis and 2d self-assembly. Langmuir 2010, 26, 7410–7417. [Google Scholar] [CrossRef]
- Martin, M.N.; Li, D.; Dass, A.; Eah, S.-K. Ultrafast, 2 min synthesis of monolayer-protected gold nanoclusters (d < 2 nm). Nanoscale 2012, 4, 4091–4094. [Google Scholar]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Genov, D.A.; Sarychev, A.K.; Shalaev, V.M. Metal-dielectric composite filters with controlled spectral windows of transparency. J. Nonlinear Opt. Phys. Mater. 2003, 12, 419–440. [Google Scholar] [CrossRef]
- Biswas, A.; Eilers, H.; Hidden, F.; Aktas, O.C.; Kiran, C.V.S. Large broadband visible to infrared plasmonic absorption from ag nanoparticles with a fractal structure embedded in a teflon af® matrix. Appl. Phys. Lett. 2006, 88, 013103. [Google Scholar] [CrossRef]
- Yang, J.-K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.-J.; Kim, H.-I.; Lee, H.-Y.; Jeong, D.H.; Kim, J.-H.; et al. Single-step and rapid growth of silver nanoshells as sers-active nanostructures for label-free detection of pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef] [PubMed]
- Alqadi, M.K.; Abo Noqtah, O.A.; Alzoubi, F.Y.; Alzouby, J.; Aljarrah, K. Ph effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater. Sci. Poland 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Bryant, M.A.; Crooks, R.M. Determination of surface pka values of surface-confined molecules derivatized with ph-sensitive pendant groups. Langmuir 1993, 9, 385–387. [Google Scholar] [CrossRef]
- Zhang, H.; He, H.-X.; Mu, T.; Liu, Z.-F. Force titration of amino group-terminated self-assembled monolayers of 4-aminothiophenol on gold using chemical force microscopy. Thin Solid Films 1998, 327–329, 778–780. [Google Scholar] [CrossRef]
- Bayram, S.; Zahr, O.K.; Blum, A.S. Short ligands offer long-term water stability and plasmon tunability for silver nanoparticles. RSC Adv. 2015, 5, 6553–6559. [Google Scholar] [CrossRef]
- Koivisto, J.; Chen, X.; Donnini, S.; Lahtinen, T.; Häkkinen, H.; Groenhof, G.; Pettersson, M. Acid–base properties and surface charge distribution of the water-soluble au102(pmba)44 nanocluster. J. Phys. Chem. C 2016, 120, 10041–10050. [Google Scholar] [CrossRef]
- Clark, R.A.; Trout, C.J.; Ritchey, L.E.; Marciniak, A.N.; Weinzierl, M.; Schirra, C.N.; Christopher Kurtz, D. Electrochemical titration of carboxylic acid terminated sams on evaporated gold: Understanding the ferricyanide electrochemistry at the electrode surface. J. Electroanal. Chem. 2013, 689, 284–290. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.-L.; He, H.-X.; Zhu, T.; Liu, Z.-F. Study on the surface dissociation properties of 6-(10-mercaptodecaoxyl)quinoline self-assembled monolayer on gold by chemical force titration. Mate. Sci. Eng. C 1999, 8, 191–194. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Cho, E.C.; Li, Z.; Yu, T.; Zeng, J.; Xie, Z.; Xia, Y. Au@ag core−shell nanocubes with finely tuned and well-controlled sizes, shell thicknesses, and optical properties. ACS Nano 2010, 4, 6725–6734. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, X.-H.; Hahm, E.; Kang, E.; Son, B.S.; Ha, Y.; Kim, H.-M.; Jeong, D.H.; Jun, B.-H. Control of Silver Coating on Raman Label Incorporated Gold Nanoparticles Assembled Silica Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1258. https://doi.org/10.3390/ijms20061258
Pham X-H, Hahm E, Kang E, Son BS, Ha Y, Kim H-M, Jeong DH, Jun B-H. Control of Silver Coating on Raman Label Incorporated Gold Nanoparticles Assembled Silica Nanoparticles. International Journal of Molecular Sciences. 2019; 20(6):1258. https://doi.org/10.3390/ijms20061258
Chicago/Turabian StylePham, Xuan-Hung, Eunil Hahm, Eunji Kang, Byung Sung Son, Yuna Ha, Hyung-Mo Kim, Dae Hong Jeong, and Bong-Hyun Jun. 2019. "Control of Silver Coating on Raman Label Incorporated Gold Nanoparticles Assembled Silica Nanoparticles" International Journal of Molecular Sciences 20, no. 6: 1258. https://doi.org/10.3390/ijms20061258
APA StylePham, X.-H., Hahm, E., Kang, E., Son, B. S., Ha, Y., Kim, H.-M., Jeong, D. H., & Jun, B.-H. (2019). Control of Silver Coating on Raman Label Incorporated Gold Nanoparticles Assembled Silica Nanoparticles. International Journal of Molecular Sciences, 20(6), 1258. https://doi.org/10.3390/ijms20061258