Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming
Abstract
:1. Introduction
2. Classification of Protease Inhibitors
3. Mechanisms of Inhibition of Protease Inhibitors
4. Plant Serine Protease Inhibitors (SPIs)
5. SPI as Protein Defense in Plants
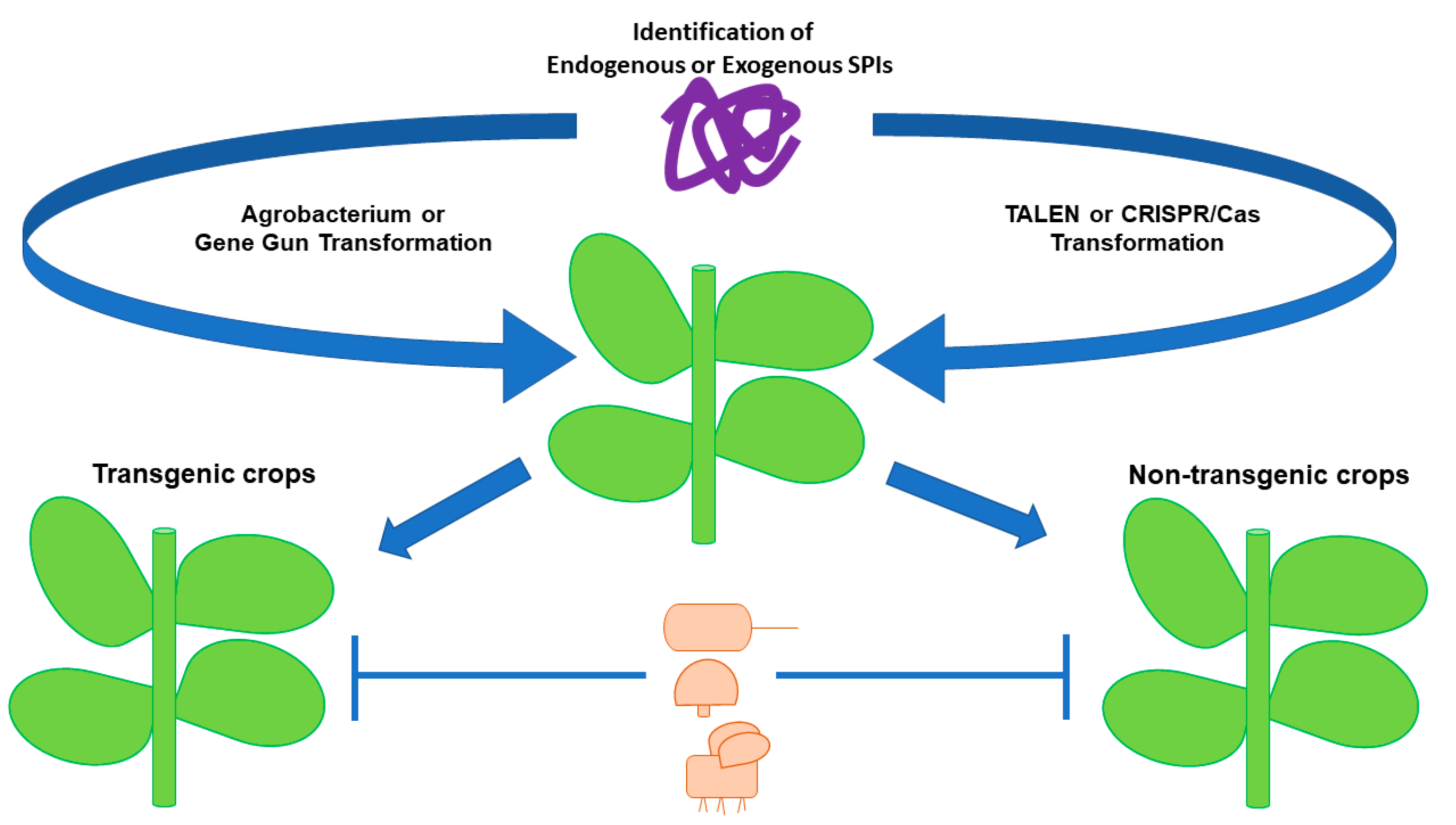
6. Plant SPIs: Biotechnology Application in Agriculture
7. Challenges and Perspectives in Pathogen Resistance
8. Plant SPIs: Biotechnology Application in Molecular Farming
9. Challenges and Perspectives in Preventing Proteolysis in Plant Protein Factories
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santamaría, M.E.; Diaz-Mendoza, M.; Diaz, I.; Martinez, M. Plant protein peptidase inhibitors: An evolutionary overview based on comparative genomics. BMC Genom. 2014, 15, 812. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Emonet, C.; Foata, F.; Affolter, M.; Delley, M.; Fisseha, M.; Blum-Sperisen, S.; Kochhar, S.; Arigoni, F. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 2006, 281, 17246–17252. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Tolle, D.P.; Barrett, A.J. Evolutionary families of peptidase inhibitors. Biochem. J. 2004, 378, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, R.F.; Song, Z.W.; Chi, C.W. Structural features and molecular evolution of Bowman-Birk protease inhibitors and their potential application. Acta Biochim. Biophys. Sin. (Shanghai) 2005, 37, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bitoun, E.; Chavanas, S.; Irvine, A.D.; Lonie, L.; Bodemer, C.; Paradisi, M.; Hamel-Teillac, D.; Ansai, S.; Mitsuhashi, Y.; Taïeb, A.; et al. Netherton syndrome: Disease expression and spectrum of SPINK5 mutations in 21 families. J. Investig. Dermatol. 2002, 118, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lomas, D.A.; Lourbakos, A.; Cumming, S.A.; Belorgey, D. Hypersensitive mousetraps, alpha1-antitrypsin deficiency and dementia. Biochem. Soc. Trans. 2002, 30, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.C. Protease inhibitors in the treatment of hereditary angioedema. Transfus. Apher. Sci. 2003, 29, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A. Protease inhibitor in plants: Genes for improving defenses against insects and pathogens. Ann. Rev. Phytopathol. 1990, 28, 425–449. [Google Scholar] [CrossRef]
- Lawrence, P.K.; Koundal, K.R. Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 2002, 5, 93–109. [Google Scholar] [CrossRef]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A. A cut above the rest: The regulatory function of plant proteases. Planta 2004, 220, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Valueva, T.A.; Mosolov, V.V. Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochemistry (Moscow) 2004, 69, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoorn, R.A.; Jones, J.D. The plant proteolytic machinery and its role in defence. Curr. Opin. Plant Biol. 2004, 7, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Mosolov, V.V.; Valueva, T.A. Proteinase Inhibitors and Their Function in Plants: A Review. Appl. Biochem. Microbiol. 2005, 41, 227–246. [Google Scholar] [CrossRef]
- Salas, C.E.; Gomes, M.T.; Hernandez, M.; Lopes, M.T. Plant cysteine proteinases: Evaluation of the pharmacological activity. Phytochemistry 2008, 69, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Cambra, I.; Martinez, M.; Pozancos, C.; González-Melendi, P.; Grbic, V.; Castañera, P.; Ortego, F.; Diaz, I. Gene pyramiding of peptidase inhibitors enhances plant resistance to the spider mite Tetranychus urticae. PLoS ONE 2012, 7, e43011. [Google Scholar] [CrossRef] [PubMed]
- Horger, A.C.; van der Hoorn, R.A. The structural basis of specific protease-inhibitor interactions at the plant-pathogen interface. Curr. Opin. Struct. Biol. 2013, 23, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Volpicella, M.; Leoni, C.; Costanza, A.; De Leo, F.; Gallerani, R.; Ceci, L.R. Cystatins, serpins and other families of protease inhibitors in plants. Curr. Protein Pept. Sci. 2011, 12, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Kato, I. Protein inhibitors of proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. Plant Protease Inhibitors: Plant Protease Inhibitors: Significance in Nutrition, Plant Protection, Cancer Prevention, and Genetic Engineering; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–170. [Google Scholar]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a Kunitz-type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C.; Lesk, A.M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986, 5, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D. Peptidase inhibitors in the MEROPS database. Biochimie 2010, 92, 1463–1483. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Bateman, K.S.; James, M.N. Plant protein proteinase inhibitors: Structure and mechanism of inhibition. Curr. Protein Pept. Sci. 2011, 12, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Mishra, M.; Suresh, C.G.; Gupta, V.S.; Giri, A.P. Complementation of intramolecular interactions for structural-functional stability of plant serine proteinase inhibitors. Biochim. Biophys. Acta 2013, 1830, 5087–5094. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Qasim, M.A. What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim. Biophys. Acta 2000, 1477, 324–337. [Google Scholar] [CrossRef]
- Farady, C.J.; Craik, C.S. Mechanisms of macromolecular protease inhibitors. ChemBioChem 2010, 11, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M. Seed storage proteins: The enzyme inhibitors. In Methods in Plant Biochemistry; Rogers, L.J., Ed.; Academic Press: New York, NY, USA, 1991; Volume 5, pp. 259–305. [Google Scholar]
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed]
- De Leo, F.; Volpicella, M.; Licciulli, F.; Liuni, S.; Gallerani, R.; Ceci, L.R. PLANT-PIs: A database for plant protease inhibitors and their genes. Nucleic Acids Res. 2002, 30, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Holz, F.M.; van der Hoorn, R.A. Juggling jobs: Roles and mechanisms of multifunctional protease inhibitors in plants. New Phytol. 2016, 210, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Antao, C.M.; Malcata, F.X. Plant serine proteases: Biochemical, physiological and molecular features. Plant Physiol. Biochem. 2005, 43, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.H.; Hejgaard, J. Serpins in plants and green algae. Funct. Integr. Genom. 2008, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.; Pandey, P.K.; Singh, D.; Khan, M.Y. Serine protease inhibitors in plants: Nature’s arsenal crafted for insect predators. Phytochem. Rev. 2013, 12, 1–34. [Google Scholar] [CrossRef]
- Silverstein, K.A.; Moskal, W.A., Jr.; Wu, H.C.; Underwood, B.A.; Graham, M.A.; Town, C.D.; VandenBosch, K.A. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 2007, 51, 262–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.; Cambra, I.; González-Melendi, P.; Santamaría, M.E.; Díaz, I. C1A cysteine-proteases and their inhibitors in plants. Physiol. Plant. 2012, 145, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.; van Doorn, W.G. Delay of Iris flower senescence by protease inhibitors. New Phytol. 2005, 165, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Tanpure, R.S.; Singh, R.K.; Gupta, V.S.; Giri, A.P. Resistance through inhibition: Ectopic expression of serine protease inhibitor offers stress tolerance via delayed senescence in yeast cell. Biochem. Biophys. Res. Commun. 2014, 452, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.; Santamaria, M.E. Biotechnological approaches to combat phytophagous arthropods. In Arthropod-Plant Interactions: Novel Insights and Approaches for IPM; Smagghe, G., Diaz, I., Eds.; Springer: Amsterdam, The Netherlands, 2012; Volume 14, pp. 159–176. [Google Scholar]
- Arnaiz, A.; Talavera-Mateo, L.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I.; Santamaria, M.E. Arabidopsis Kunitz Trypsin Inhibitors in Defense Against Spider Mites. Front. Plant Sci. 2018, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Fluhr, R.; Lampl, N.; Roberts, T.H. Serpin protease inhibitors in plant biology. Physiol. Plant. 2012, 145, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.H.; Ahn, J.W.; Lampl, N.; Fluhr, R. Plants and the study of serpin biology. Methods Enzymol. 2011, 499, 347–366. [Google Scholar] [PubMed]
- Savelkoul, F.H.; van der Poel, A.F.; Tamminga, S. The presence and inactivation of trypsin inhibitors, tannins, lectins and amylase inhibitors in legume seeds during germination. A review. Plant Foods Hum. Nutr. 1992, 42, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. Serpin1 and WSCP differentially regulate the activity of the cysteine protease RD21 during plant development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Boex-Fontvieille, E.; Rustgi, S.; Reinbothe, S.; Reinbothe, C. A Kunitz-type protease inhibitor regulates programmed cell death during flower development in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 6119–6135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.C.; Hwang, I.; Cheong, H.; Nah, J.W.; Hahm, K.S.; Park, Y. Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Roy-Downing, W.L.; Mauxion, F.; Fauvarque, M.O.; Reviron, M.P.; de Vienne, D.; Vartanian, N.; Giraudat, J. A Brassica napus transcript encoding a protein related to the Künitz protease inhibitor family accumulates upon water stress in leaves, not in seeds. Plant J. 1992, 2, 685–693. [Google Scholar]
- Srinivasan, T.; Kumar, K.R.; Kirti, P.B. Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol. 2009, 50, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Dramé, K.N.; Passaquet, C.; Repellin, A.; Zuily-Fodil, Y. Cloning, characterization and differential expression of a Bowman-Birk inhibitor during progressive water deficit and subsequent recovery in peanut (Arachis hypogaea) leaves. J. Plant Physiol. 2013, 170, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Khadeeva, N.V.; Kochieva, E.Z.; Tcherednitchenko, M.Y.; Yakovleva, E.Y.; Sydoruk, K.V.; Bogush, V.G.; Dunaevsky, Y.E.; Belozersky, M.A. Use of buckwheat seed protease inhibitor gene for improvement of tobacco and potato plant resistance to biotic stress. Biochemistry (Mosc) 2009, 74, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, F.; Tsuda, K. Understanding the plant immune system. Mol. Plant Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Schwessinger, B.; Zipfel, C. News from the frontline: Recent insights into PAMP-triggered immunity in plants. Curr. Opin. Plant Biol. 2008, 11, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, E.E.; Chernyshova, M.P.; Ignatov, V.V. The extracellular proteases of the phytopathogenic bacterium Xanthomonas campestris. Mikrobiologiia 2003, 72, 498–502. [Google Scholar] [PubMed]
- Cheng, Z.; Li, J.F.; Niu, Y.; Zhang, X.C.; Woody, O.Z.; Xiong, Y.; Djonović, S.; Millet, Y.; Bush, J.; McConkey, B.J.; et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature 2015, 521, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.; Fazili, K.M. Plant protease inhibitors: A defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2007, 2, 68–85. [Google Scholar]
- Gatehouse, A.M.; Norton, E.; Davison, G.M.; Babbé, S.M.; Newell, C.A.; Gatehouse, J.A. Digestive proteolytic activity in larvae of tomato moth, Lacanobia oleracea; effects of plant protease inhibitors in vitro and in vivo. J. Insect Physiol. 1999, 45, 545–558. [Google Scholar] [CrossRef]
- van der Hoorn, R.A.L. Plant Proteases: From Phenotypes to Molecular Mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant-pathogen interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Baldwin, I.T. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Boex-Fontvieille, E.; Rustgi, S.; Von Wettstein, D.; Pollmann, S.; Reinbothe, S.; Reinbothe, C. Jasmonic acid protects etiolated seedlings of Arabidopsis thaliana against herbivorous arthropods. Plant Signal. Behav. 2016, 11, e1214349. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Ryan, C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 1990, 87, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Turra, D.; Bellin, D.; Lorito, M.; Gebhardt, C. Genotype-dependent expression of specific members of potato protease inhibitor gene families in different tissues and in response to wounding and nematode infection. J. Plant Physiol. 2009, 166, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.J.; Chen, J.; Liu, M.; Pan, N.; Okamoto, H.; Lin, Z.; Li, C.; Li, D.; Wang, J.; Zhu, G.; et al. Molecular cloning and functional analysis of a novel type of Bowman-Birk inhibitor gene family in rice. Plant Physiol. 2003, 133, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y. Protein proteinase inhibitors in legume seeds—Overview. Arch. Latinoam. Nutr. 1996, 44, 26–30. [Google Scholar]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.; van Koningsveld, G.A.; Voragen, A.G. Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. J. Agric. Food Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Christeller, J.; Laing, W. Plant serine proteinase inhibitors. Protein Pept. Lett. 2005, 12, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.C.; Aoki, K.; Xiang, Y.; Campbell, L.R.; Hull, R.J.; Xoconostle-Cázares, B.; Monzer, J.; Lee, J.Y.; Ullman, D.E.; Lucas, W.J. Characterization of cucurbita maxima phloem serpin-1 (CmPS-1). A developmentally regulated elastase inhibitor. J. Biol. Chem. 2000, 275, 35122–35128. [Google Scholar] [CrossRef] [PubMed]
- La Cour Petersen, M.; Hejgaard, J.; Thompson, G.A.; Schulz, A. Cucurbit phloem serpins are graft-transmissible and appear to be resistant to turnover in the sieve element-companion cell complex. J. Exp. Bot. 2005, 56, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Gorman, M.J.; Jiang, H.; Kanost, M.R. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J. Biol. Chem. 2003, 278, 46556–46564. [Google Scholar] [CrossRef] [PubMed]
- Hilder, V.A.; Gatehouse, M.R.; Boulter, D. Transgene plants conferring insect tolerance: Protease inhibitor approach. Transgenic Plants 1993, 1, 317–338. [Google Scholar]
- Baldwin, I.T.; Schultz, J.C. Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 1983, 221, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Lingling, L.; Lei, J.; Song, M.; Li, L.; Cao, B. Study on transformation of cowpea trypsin inhibitor gene into cauli flower (Brassica oleracea L. var. botrytis). Afr. J. Biotechnol. 2005, 4, 45–49. [Google Scholar]
- Pujol, M.; Hernandez, C.A.; Armas, R.; Coll, Y.; Alfonso-Rubi, J.; Perez, M.; Ayra, C.; González, A. Inhibition of Heliothis virescens larvae growth in transgenic tobacco plants expressing cowpea trypsin inhibitor. Biotechnol. Appl. 2005, 22, 27–130. [Google Scholar]
- Quilis, J.; Meynard, D.; Vila, L.; Aviles, F.X.; Guiderdoni, E.; Segundo, B.S. A potato carboxypeptidase inhibitor gene provides pathogen resistance in transgenic rice. Plant Biotechnol. J. 2007, 5, 537–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quilis, J.; López-García, B.; Meynard, D.; Guiderdoni, E.; San Segundo, B. Inducible expression of a fusion gene encoding two proteinase inhibitors leads to insect and pathogen resistance in transgenic rice. Plant Biotechnol. J. 2014, 12, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Down, R.E.; Ford, L.; Mosson, H.J.; Fitches, E.; Gatehouse, J.A.; Gatehouse, A.M. Protease activity in the larval stage of the parasitoid wasp, Eulophus pennicornis (Nees) (Hymenoptera: Eulophidae); effects of protease inhibitors. Parasitology 1999, 119, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, H.; Cherqui, A.; Campan, E.D.; Rahbé, Y.; Duport, G.; Jouanin, L.; Kaiser, L.; Giordanengo, P. Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J. Insect Physiol. 2005, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.M.; Hussey, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.C.; Sharma, K.K.; Seetharama, N.; Ortiz, R. Prospects for using transgenic resistance to insects in crop improvement. Electron. J. Biotechnol. 2000, 3, 21–22. [Google Scholar] [CrossRef]
- Major, I.T.; Constabel, C.P. Functional analysis of the Kunitz trypsin inhibitor family in poplar reveals biochemical diversity and multiplicity in defense against herbivores. Plant Physiol. 2008, 146, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Botelho-Junior, S.; Machado, O.L.; Fernandes, K.V.; Lemos, F.J.; Perdizio, V.A.; Oliveira, A.E.; Monteiro, L.R.; Filho, M.L.; Jacinto, T. Defense response in non-genomic model species: Methyl jasmonate exposure reveals the passion fruit leaves’ ability to assemble a cocktail of functionally diversified Kunitz-type trypsin inhibitors and recruit two of them against papain. Planta 2014, 240, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Migliolo, L.; de Oliveira, A.S.; Santos, E.A.; Franco, O.L.; de Sales, M.P. Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine- and cysteine-proteinases. J. Mol. Graph. Model. 2010, 29, 148–156. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.S.; de Oliveira, C.F.; Parra, J.R.; Marangoni, S.; Macedo, M.L. Short and long-term antinutritional effect of the trypsin inhibitor ApTI for biological control of sugarcane borer. J. Insect Physiol. 2014, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valueva, T.A.; Revina, T.A.; Gvozdeva, E.L.; Gerasimova, N.G.; Il’inskaia, L.I.; Ozeretskovakaia, O.L. Effect of elicitors on accumulation of protease inhibitors in injured potato tubers. Prikl. Biokhim. Mikrobiol. 2001, 37, 601–606. [Google Scholar] [PubMed]
- Laluk, K.; Mengiste, T. The Arabidopsis extracellular unusual serine proteinase inhibitor functions in resistance to necrotrophic fungi and insect herbivory. Plant J. 2011, 68, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Smigocki, A.C.; Ivic-Haymes, S.; Li, H.; Savić, J. Pest protection conferred by a Beta vulgaris serine proteinase inhibitor gene. PLoS ONE 2013, 8, e57303. [Google Scholar] [CrossRef] [PubMed]
- Hilder, V.A.; Gatehouse, A.M.R.; Sheerman, S.E.; Barker, R.F.; Boulter, D. A novel mechanism of insect resistance engineered into tobacco. Nature 1987, 330, 160–163. [Google Scholar] [CrossRef]
- Altpeter, F.; Diaz, I.; McAuslane, H.; Gaddour, K.; Carbonero, P.; Vasil, I.K. Increased insect resistance in transgenic wheat stably expressing trypsin inhibitor CMe. Mol. Breed. 1999, 5, 53–63. [Google Scholar] [CrossRef]
- Lee, I.; Lee, S.H.; Koo, C.; Jin, C.H.; Lim, C.O.; Mun, H.; Han, S.Y.; Cho, J. Soybean Kunitz trypsin inhibitor (SKTI) confers resistance to the brown planthopper Nilaparvata lugens Stal in transgenic rice. Mol. Breed. 1999, 5, 1–9. [Google Scholar] [CrossRef]
- Vila, L.; Quilis, J.; Meynard, D.; Breitler, J.C.; Marfa, V.; Murillo, I.; Vassal, J.M.; Messeguer, J.; Guiderdoni, E.; San Segundo, B. Expression of the maize proteinase inhibitor (mpi) gene in rice plants enhances resistance against the striped stem borer (Chilo suppressalis): Effects on larval growth and insect gut proteinases. Plant Biotechnol. J. 2005, 3, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Dunse, K.M.; Stevens, J.A.; Lay, F.T.; Gaspar, Y.M.; Heath, R.L.; Anderson, M.A. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. USA 2010, 107, 15011–15015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartl, M.; Giri, A.P.; Kaur, H.; Baldwin, I.T. Serine protease inhibitors specifically defend Solanum nigrum against generalist herbivores but do not influence plant growth and development. Plant Cell 2010, 22, 4158–4175. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, J.A. Prospects for using proteinase inhibitors to protect transgenic plants against attack by herbivorous insects. Curr. Protein Pept. Sci. 2011, 12, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ding, L.W.; Ge, Z.J.; Wang, Z.Y.; Hu, B.L.; Yang, X.B.; Sun, Q.Y.; Xu, Z.F. The Characterization of SaPIN2b, a Plant Trichome-Localized Proteinase Inhibitor from Solanum americanum. Int. J. Mol. Sci. 2012, 13, 15162–15176. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Rubí, J.; Ortego, F.; Castañera, P.; Carbonero, P.; Díaz, I. Transgenic expression of trypsin inhibitor CMe from barley in indica and japonica rice, confers resistance to the rice weevil Sitophilus oryzae. Transgenic Res. 2003, 12, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.; Pérez-Hedo, M.; Urbaneja, A.; Rambla, J.L.; Granell, A.; Gaddour, K.; Beltrán, J.P.; Cañas, L.A. Expression of two barley proteinase inhibitors in tomato promotes endogenous defensive response and enhances resistance to Tuta absoluta. BMC Plant Biol. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Stuiver, M.H.; Custers, J.H. Engineering disease resistance in plants. Nature 2001, 411, 865–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunaevsky, Y.E.; Gladysheva, I.P.; Pavlukova, E.B.; Beliakova, G.A.; Gladyshev, D.P.; Papisova, A.I.; Larionova, N.I.; Belozersky, M.A. The anionic protease inhibitor BWI-1 from buckwheat seeds. Kinetic properties and possible biological role. Physiol. Plant. 1997, 101, 483–488. [Google Scholar] [CrossRef]
- Valueva, T.A.; Revina, T.A.; Kladnitskaya, G.V.; Mosolov, V.V. Kunitz-type proteinase inhibitors from intact and Phytophthora-infected potato tubers. FEBS Lett. 1998, 426, 131–134. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. A Bowman-Birk-type trypsin-chymotrypsin inhibitor from broad beans. Biochem. Biophys. Res. Commun. 2001, 289, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, A.I.; Longstaff, C.; Jones, B.L. Kinetics of the inhibition of fusarium serine proteinases by barley (Hordeum vulgare L.) inhibitors. J. Agric. Food Chem. 2007, 55, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Pariani, S.; Contreras, M.; Rossi, F.R.; Sander, V.; Corigliano, M.G.; Simón, F.; Busi, M.V.; Gomez-Casati, D.F.; Pieckenstain, F.L.; Duschak, V.G.; et al. Characterization of a novel Kazal-type serine proteinase inhibitor of Arabidopsis thaliana. Biochimie 2016, 123, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Windwarder, M.; Gattinger, P.; Mach, L.; Strasser, R.; Altmann, F.; Steinkellner, H. Proteolytic and N-glycan processing of human α1-antitrypsin expressed in Nicotiana benthamiana. Plant Physiol. 2014, 166, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.; Khalf, M.; Sainsbury, F.; D’Aoust, M.A.; Michaud, D. A protease activity-depleted environment for heterologous proteins migrating towards the leaf cell apoplast. Plant Biotechnol. J. 2012, 10, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Khalf, M.; Goulet, M.C.; D’Aoust, M.A.; Sainsbury, F.; Michaud, D. Protection of recombinant mammalian antibodies from development-dependent proteolysis in leaves of Nicotiana benthamiana. PLoS ONE 2013, 8, e70203. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Holz, F.; Madeira, L.; Zahid, M.A.; Songer, M.; Kourelis, J.; Fesenko, M.; Ninck, S.; Kaschani, F.; Kaiser, M.; van der Hoorn, R.A.L. Three unrelated protease inhibitors enhance accumulation of pharmaceutical recombinant proteins in Nicotiana benthamiana. Plant Biotechnol. J. 2018, 16, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Komarnytsky, S.; Borisjuk, N.; Yakoby, N.; Garvey, A.; Raskin, I. Cosecretion of protease inhibitor stabilizes antibodies produced by plant roots. Plant Physiol. 2006, 141, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Kim, H.M.; Lee, H.J.; Shin, Y.J.; Kwon, T.H.; Lee, N.J.; Jang, Y.S.; Yang, M.S. Reduced protease activity in transformed rice cell suspension cultures expressing a proteinase inhibitor. Protein Expr. Purif. 2007, 53, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Boulter, D. Insect pest control by copying nature using genetically engineered crops. Phytochemistry 1993, 34, 1453–1466. [Google Scholar] [CrossRef]
- Urwin, P.E.; McPherson, M.J.; Atkinson, H.J. Enhanced transgenic plant resistance to nematodes by dual proteinase inhibitor constructs. Planta 1998, 204, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Alfageme, F.; Maharramov, J.; Carrillo, L.; Vandenabeele, S.; Vercammen, D.; Van Breusegem, F.; Smagghe, G. Potential use of a serpin from Arabidopsis for pest control. PLoS ONE 2011, 6, e20278. [Google Scholar] [CrossRef]
- Ferry, N.; Edwards, M.G.; Gatehouse, J.; Capell, T.; Christou, P.; Gatehouse, A.M.R. Transgenic plants for insect pest control: A forward looking scientific perspective. Transgenic Res. 2006, 15, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.A.; Bakker, P.L.; Peters, J.; Bosch, D.; Stiekema, W.J. Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 8041–8045. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, C.; Jean, C.; Fournier, M.; Yelle, S.; Michaud, D. Adult Colorado potato beetles, Leptinotarsa decemlineata compensate for nutritional stress on oryzacystatin I-transgenic potato plants by hypertrophic behavior and over-production of insensitive proteases. Arch. Insect Biochem. Physiol. 2000, 44, 69–81. [Google Scholar] [CrossRef]
- Harsulkar, A.M.; Giri, A.P.; Patankar, A.G.; Gupta, V.S.; Sainani, M.N.; Ranjekar, P.K.; Deshpande, V.V. Successive use of non-host plant proteinase inhibitors required for effective inhibition of Helicoverpa armigera gut proteinases and larval growth. Plant Physiol. 1999, 121, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Trujillo, M.; Simpson, J.; Herrera-Estrella, L.R. Transgenic plants in modern agriculture. J. New Seeds 2002, 4, 1–23. [Google Scholar] [CrossRef]
- Ahmad, P.; Ashraf, M.; Younis, M.; Hu, X.; Kumar, A.; Akram, N.A.; Al-Qurainy, F. Role of transgenic plants in agriculture and biopharming. Biotechnol. Adv. 2012, 30, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Nejat, N.; Rookes, J.; Mantri, N.L.; Cahill, D.M. Plant-pathogen interactions: Toward development of next-generation disease-resistant plants. Crit. Rev. Biotechnol. 2017, 37, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Cafferkey, R.; Bowdish, K. Production of antibodies in transgenic plants. Nature 1989, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.E.; Witcher, D.R.; Maddock, S.; Meyer, T.; Baszczynski, C.; Bailey, M.; Flynn, P.; Register, J.; Marshall, L.; Bond, D.; et al. Commercial production of avidin from transgenic maize: Characterization of transformant, production, processing, extraction and purification. Mol. Breed. 1997, 3, 291–306. [Google Scholar] [CrossRef]
- Hood, E.E.; Howard, J.A. Commercial plant-produced recombinant avidin. In Commercial Plant-Produced Recombinant Protein Products; Howard, J.A., Hood, E.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 68, pp. 15–25. [Google Scholar]
- Webster, D.E.; Thomas, M.C. Post-translational modification of plant-made foreign proteins; glycosylation and beyond. Biotechnol. Adv. 2012, 30, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as Factories for Human Pharmaceuticals: Applications and Challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschofen, M.; Knopp, D.; Hood, E.; Stoger, E. Plant molecular farming: Much more than medicines. Annu. Rev. Anal. Chem. (Palo Alto Calif) 2016, 9, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Twyman, R.M.; Schillberg, S.; Fischer, R. Optimizing the yield of recombinant pharmaceutical proteins in plants. Curr. Pharm. Des. 2013, 19, 5486–5494. [Google Scholar] [CrossRef] [PubMed]
- Stoger, E.; Fischer, R.; Moloney, M.; Ma, J.K. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 2014, 65, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Ahvari, H.; Schillberg, S.; Schiermeyer, A. Tackling unwanted proteolysis in plant production hosts used for molecular farming. Front. Plant Sci. 2016, 7, 267. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol. 2006, 24, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Schlüter, U.; van Wyk, S.; Kunert, K.J.; Vorster, B.J. Proteolysis of recombinant proteins in bioengineered plant cells. Bioengineered 2014, 5, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Kibido, T.; du Plessis, M.; van der Vyver, C.; Beyene, G.; Vorster, B.J.; Kunert, K.J.; Schlüter, U. Use of transgenic oryzacystatin-I-expressing plants enhances recombinant protein production. Appl. Biochem. Biotechnol. 2012, 168, 1608–1620. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.; Boulaflous, A.; Benchabane, M.; Gomord, V.; Michaud, D. Protein modifications in the plant secretory pathway: Current status and practical implications in molecular pharming. Vaccine 2005, 23, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Rivard, D.; Anguenot, R.; Brunelle, F.; Le, V.Q.; Vézina, L.P.; Trépanier, S.; Michaud, D. An in-built proteinase inhibitor system for the protection of recombinant proteins recovered from transgenic plants. Plant Biotechnol. J. 2006, 4, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchabane, M.; Goulet, C.; Rivard, D.; Faye, L.; Gomord, V.; Michaud, D. Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol. J. 2008, 6, 633–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, M.B.; Tang, Y.; McLeod, D.A.; Janda, K.D.; Hiatt, A. Evaluation of immunoglobulins from plant cells. Biotechnol. Prog. 1991, 7, 455–461. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, C.; De Rycke, R.; Beeckman, T.; De Neve, M.; Van Montagu, M.; Engler, G.; Depicker, A. Accumulation pattern of IgG antibodies and Fab fragments in transgenic Arabidopsis thaliana plants. Plant Cell Physiol. 1998, 39, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Foyer, C.H.; Kunert, K.J. Two new cysteine proteinases with specific expression patterns in mature and senescent tobacco (Nicotiana tabacum L.) leaves. J. Exp. Bot. 2006, 57, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.; Benchabane, M.; Anguenot, R.; Brunelle, F.; Khalf, M.; Michaud, D. A companion protease inhibitor for the protection of cytosol-targeted recombinant proteins in plants. Plant Biotechnol. J. 2010, 8, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Vyver, C.; Schneidereit, J.; Driscoll, S.; Turner, J.; Kunert, K.; Foyer, C.H. Oryzacystatin I expression in transformed tobacco produces a conditional growth phenotype and enhances chilling tolerance. Plant Biotechnol. J. 2003, 1, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagny, S.; Denmat-Ouisse, L.A.; Gomord, V.; Faye, L. Fusion with HDEL protects cell wall invertase from early degradation when N-glycosylation is inhibited. Plant Cell Physiol. 2003, 44, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pimpl, P.; Taylor, J.P.; Snowden, C.; Hillmer, S.; Robinson, D.G.; Denecke, J. Golgi-mediated vacuolar sorting of the endoplasmic reticulum chaperone BiP may play an active role in quality control within the secretory pathway. Plant Cell 2006, 18, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Laguia-Becher, M.; Martín, V.; Kraemer, M.; Corigliano, M.; Yacono, M.L.; Goldman, A.; Clemente, M. Effect of codon optimization and subcellular targeting on Toxoplasma gondii antigen SAG1 expression in tobacco leaves to use in subcutaneous and oral immunization in mice. BMC Biotechnol. 2010, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Hehle, V.K.; Paul, M.J.; Drake, P.M.; Ma, J.K.; van Dolleweerd, C.J. Antibody degradation in tobacco plants: A predominantly apoplastic process. BMC Biotechnol. 2011, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, A.; Frigerio, L.; Lord, J.M.; Roberts, L.M.; Ceriotti, A. Endoplasmic reticulum-associated degradation of ricin A chain has unique and plant-specific features. Plant Physiol. 2005, 137, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Agarwal, S.; Sanyal, I.; Jain, G.K.; Amla, D.V. Differential subcellular targeting of recombinant human α₁-proteinase inhibitor influences yield, biological activity and in planta stability of the protein in transgenic tomato plants. Plant Sci. 2012, 196, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Pillay, P.; Kunert, K.J.; van Wyk, S.G.; Makgopa, M.E.; Cullis, C.A.; Vorster, B.J. Agroinfiltration contributes to VP1 recombinant protein degradation. Bioengineered 2016, 7, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Stoger, E.; Sack, M.; Nicholson, L.; Fischer, R.; Christou, P. Recent progress in plantibody technology. Curr. Pharm. Des. 2005, 11, 2439–2457. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, M.; Alves, G.; Vertommen, D.; Ma, J.; Boutry, M.; Navarre, C. Identification of peptidases in Nicotiana tabacum leaf intercellular fluid. Proteomics 2008, 8, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.; Chandrasekar, B.; Mandal, M.K.; Kaschani, F.; Kaiser, M.; Both, L.; van der Hoorn, R.A.L.; Schiermeyer, A.; Abranches, R. Low Protease Content in Medicago truncatula Cell Cultures Facilitates Recombinant Protein Production. Biotechnol. J. 2018, 13, e1800050. [Google Scholar] [CrossRef] [PubMed]


| SPI Name | Origen | Role and Function | Biotechnology Application | References |
|---|---|---|---|---|
| A. thaliana Kunitz trypsin inhibitors (AtKTI4, AtKTI5) | Arabidopsis thaliana | Inhibitory activity against serine and cysteine protease; effect on mite performance (fecundity and mortality) | Protection against spider mite | [42] |
| AtSerpin1 | Arabidopsis thaliana | Inhibition of digestive protease activity; inhibition of larval growth; inhibition of RD21 activity | Protection against insect disease | [47,103] |
| Kunitz type protease inhibitor (AtWSCP) | Arabidopsis thaliana | Inhibition of cysteine RD21 activity; controlling cell death | Protection against herbivore attack | [45,47] |
| Potato type 1 inhibitors | Solanum tuberosum | Differential expression pattern after wounding and nematode infection | Protection against nematodes | [68] |
| Bowman-Birk-type inhibitor | Oryza sativa | Arrest fungal invasion; inhibition of fungal growth | Protection against fungal disease | [69] |
| Phloem serpin-1 (CmPS-1) | Cucurbita maxima | Inhibition of elastase activity; increase of the aphid mortality | Protection against insect disease | [73,74] |
| Cowpea trypsin inhibitor gene (CPTI) | Vigna unguiculata | Inhibition of larval growth | Protection against insect disease | [78,79,93] |
| Potato carboxypeptidase inhibitor (PCI) | Solanum tuberosum | Antifungal activity; inhibition of larval growth | Protection against fungal and insect disease | [80,81] |
| Maize proteinase inhibitor (mPI) | Zea mays | Inhibition of digestive serine proteinases; inhibition of larval and fungal growth | Protection against fungal and insect disease | [81,96] |
| Soybean Kunitz inhibitor (SKTI) | Glycine max | Inhibition of digestive proteases present in insects and parasites | Protection against parasitic and insect disease | [83,86,95] |
| Soybean Bowman-Birk inhibitor (SbBBI) | Glycine max | Inhibition of digestive protease activity; inhibition of aphid growth | Protection against aphid parasitoids | [83] |
| Poplar Kunitz trypsin inhibitor | Populus trichocarpa x Populus deltoides | Inhibition of midgut protease present in lepidopteran pests | Protection against insect disease | [86] |
| Passion fruit Kunitz type inhibitors (PfKI) | Passiflora edulis Sims | Inhibition of midgut proteases present in lepidopteran and coleopteran pests and Aedes aegypti | Protection against insect disease and Control of vectors of neglected tropical diseases | [87] |
| Kunitz trypsin inhibitor (ApKTI) | Adenanthera pavonina | Inhibitory activity against trypsin and papain proteases; inhibition of midgut proteases and larval growth | Protection against insect disease | [88,89] |
| Unusual serine protease inhibitor (UPI) | Arabidopsis thaliana | Chymotrypsin inhibitory activity; effect on the fungal and larval growth | Protection against fungal and insect disease | [91] |
| Serine proteinase inhibitor (BvSTI) | Beta vulgaris | Trypsin inhibitor activity; effect on larval weights | Protection against lepidopteran insect disease | [92] |
| Serine protease inhibitor CMe (BTI-CMe) | Barley (Hordeum vulgare) | Inhibition of midgut protease activity; effect on larval growth and survival of insects | Protection against insect disease | [94,101,102] |
| Potato type I (StPin1A) inhibitor/Potato type II (NaPI) inhibitor | Solanum tuberosum Nicotiana alata | Protease inhibitory activity; effect on larval growth | Protection against Helicoverpa spp. | [97] |
| PI-I and PI-II-class inhibitors | Solanum nigrum | Serine protease inhibitory activity | Protection against insect disease | [98] |
| Potato Type II Proteinase Inhibitors (SaPIN2b) | Solanum americanum | Inhibition of midgut protease activity | Protection against insect disease | [97,100] |
| Serine protease inhibitor (BWI-1a) | Fagopyrum sculentum | Inhibition of spore germination, mycelial growth, bacterial growth and survival of insects | Protection against insect, fungal and bacterial disease | [104,59] |
| Serine protease inhibitors (PSPI-21, PSPI-22) | Solanum tuberosum | Trypsin and chymotrypsin inhibitory activity; inhibition of mycelial growth | Protection against fungal disease | [105] |
| Bowman-Birk-type inhibitor | Vicia faba | Trypsin and chymotrypsin inhibitory activity; inhibition of mycelial growth | Protection against fungal disease | [106] |
| Chymotrypsin/subtilisin inhibitor 2, amylase/subtilisin inhibitor, Bowman-Birk trypsin inhibitor | Hordeum vulgare | Inhibition of subtilisin and trypsin proteases of Fusarium culmorum | Protection against fungal disease | [107] |
| Kazal type inhibitor (AtKPI-1) | Arabidopsis thaliana | Inhibition of conidial germination | Protection against fungal disease | [108] |
| Tomato cathepsin D inhibitor (CDI) | Solanum tuberosum | Improvement of the stability of proteins in leaf crude extracts | Achieves high yields of recombinant proteins in the extraction/recovery process | [109,110,111,112] |
| Bowman–Birk type protease inhibitor (BBI) | Glycine max | Reduction of the degradation of immunoglobulins in the secretion pathway | Achieves high yields of therapeutic proteins in transgenic plants | [113] |
| Chymotrypsin and trypsin inhibitor | Nicotiana alata | Reduction of the extracellular protease activity | Achieves high yields of recombinant proteins in cell suspension culture | [114] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. Int. J. Mol. Sci. 2019, 20, 1345. https://doi.org/10.3390/ijms20061345
Clemente M, Corigliano MG, Pariani SA, Sánchez-López EF, Sander VA, Ramos-Duarte VA. Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. International Journal of Molecular Sciences. 2019; 20(6):1345. https://doi.org/10.3390/ijms20061345
Chicago/Turabian StyleClemente, Marina, Mariana G. Corigliano, Sebastián A. Pariani, Edwin F. Sánchez-López, Valeria A. Sander, and Víctor A. Ramos-Duarte. 2019. "Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming" International Journal of Molecular Sciences 20, no. 6: 1345. https://doi.org/10.3390/ijms20061345
APA StyleClemente, M., Corigliano, M. G., Pariani, S. A., Sánchez-López, E. F., Sander, V. A., & Ramos-Duarte, V. A. (2019). Plant Serine Protease Inhibitors: Biotechnology Application in Agriculture and Molecular Farming. International Journal of Molecular Sciences, 20(6), 1345. https://doi.org/10.3390/ijms20061345






