The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence
Abstract
:1. Introduction
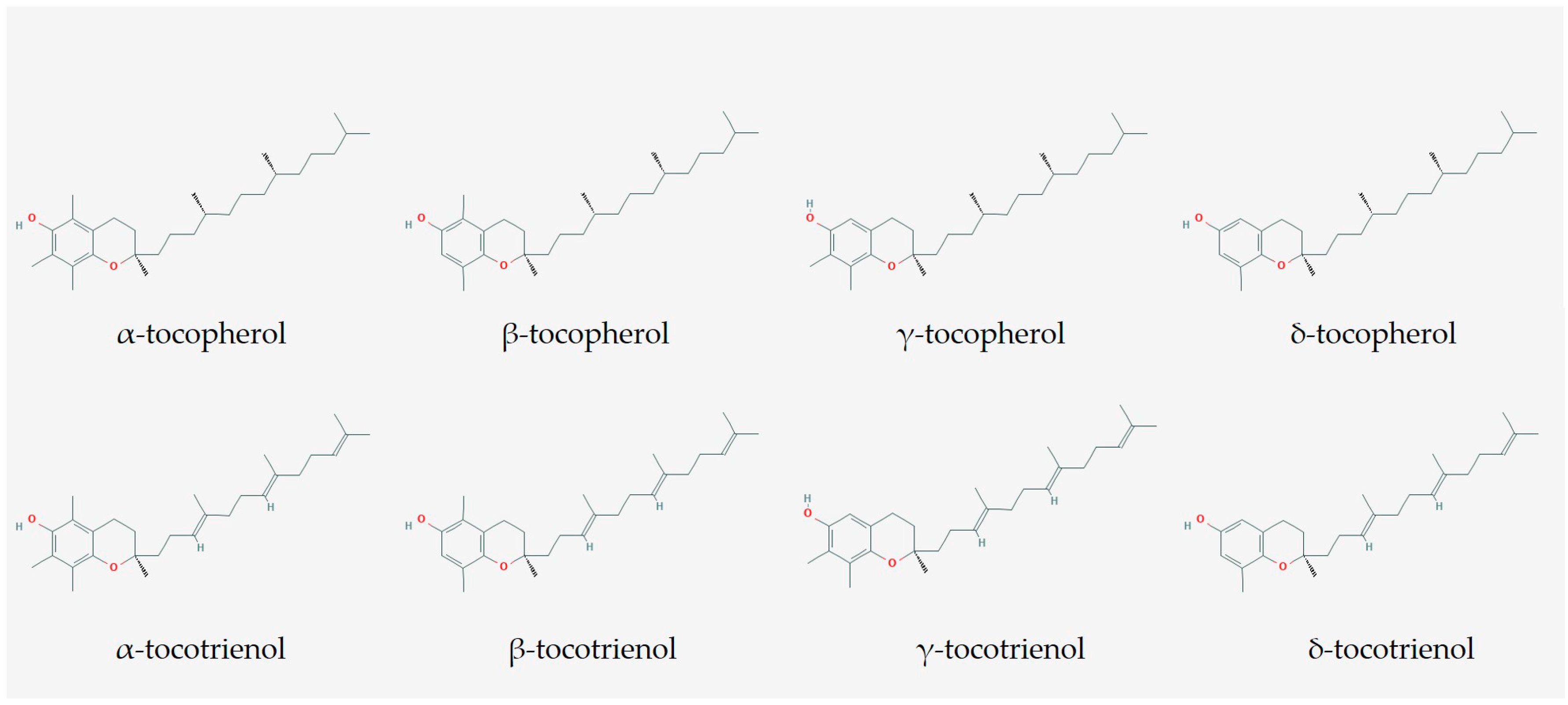
2. The Composition of T3 Used
3. Effects of T3 on Bone Growth
4. Effects of T3 on Bone Loss Due to Androgen Deficiency (Late-Onset or Drug-Induced Hypogonadism)
5. Effects of T3 on Bone Loss Due to Metabolic Syndrome
6. Effects of T3 on Bone Loss Due to Cigarette-Smoking
7. Effects of T3 on Bone Loss Due to Alcohol
8. Effects of T3 on Bone Loss Due to Glucocorticoid
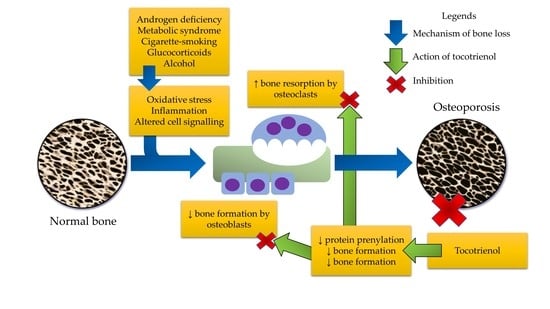
9. Mechanism of Action of T3 in Protecting Bone Health
9.1. Oxidative Stress
9.2. Inflammation
9.3. Mevalonate Pathway
10. Perspectives on the Use of T3
11. Limitations
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edwards, M.H.; Dennison, E.M.; Aihie Sayer, A.; Fielding, R.; Cooper, C. Osteoporosis and sarcopenia in older age. Bone 2015, 80, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M. Gender differences in osteoporosis and fractures. Clin. Orthop. Relat. Res. 2011, 469, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the united states, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, B.D.; Moores, T.S.; Ahmad, S.; Cattell, A.; Roberts, P.J. Cause of death and factors associated with early in-hospital mortality after hip fracture. Bone Jt. J. 2015, 97-B, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, E.H.; Arad, M.; Fleissig, Y.; Adunsky, A. Gender differences in functional outcome of elderly hip fracture patients. Geriatr. Gerontol. Int. 2014, 14, 845–850. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, C.H.; Farley, J.F. Patterns of pharmacological treatment for osteoporosis among patients qualified for pharmacotherapy according to the national osteoporosis foundation guidelines. Ann. Pharmacother. 2015, 49, 995–1003. [Google Scholar] [CrossRef]
- Willson, T.; Nelson, S.D.; Newbold, J.; Nelson, R.E.; LaFleur, J. The clinical epidemiology of male osteoporosis: A review of the recent literature. Clin. Epidemiol. 2015, 7, 65–76. [Google Scholar]
- Amelio, P.; Isaia, G.C. Male osteoporosis in the elderly. Int. J. Endocrinol. 2015, 2015, 8. [Google Scholar]
- NIH Osteoporosis and Related Bone Diseases-National Resource Center. Osteoporosis in Men. Available online: https://www.bones.nih.gov/health-info/bone/osteoporosis/men (accessed on 12 March 2019).
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Almeida, M. Basic biology of skeletal aging: Role of stress response pathways. J. Gerontol. 2013, 68, 1197–1208. [Google Scholar]
- Giusti, A.; Bianchi, G. Treatment of primary osteoporosis in men. Clin. Interv. Aging 2015, 10, 105–115. [Google Scholar] [PubMed]
- Bliuc, D.; Alarkawi, D.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: The dubbo osteoporosis epidemiology study. J. Bone Miner Res. 2015, 30, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Vitamin e as an antiosteoporotic agent via receptor activator of nuclear factor kappa-b ligand signaling disruption: Current evidence and other potential research areas. Evid. Based Complement Altern. Med. 2012, 2012, 747020. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Mo, H.; Soelaiman, I.-N. A review of the possible mechanisms of action of tocotrienol—A potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin e of the 21st century: It’s potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Fairus, S.; Nor, R.M.; Cheng, H.M.; Sundram, K. Alpha-tocotrienol is the most abundant tocotrienol isomer circulated in plasma and lipoproteins after postprandial tocotrienol-rich vitamin e supplementation. Nutr. J. 2012, 11, 5. [Google Scholar] [CrossRef]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin e analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef]
- Muhammad, N.; Luke, D.A.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Two different isomers of vitamin e prevent bone loss in postmenopausal osteoporosis rat model. Evid. Based Complement Altern. Med. 2012, 2012, 161527. [Google Scholar] [CrossRef]
- Muhammad, N.; Razali, S.; Shuid, A.N.; Mohamed, N.; Soelaiman, I.N. Membandingkan kesan antara fraksi-kaya tokotrienol, kalsium dan estrogen terhadap metabolisme tulang tikus terovariektomi. Sains Malays. 2013, 42, 1591–1597. [Google Scholar]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Mohd Ali, H.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.-N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid. Based Complement Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015, 125, 42–48. [Google Scholar] [CrossRef]
- Chin, K.Y.; Abdul-Majeed, S.; Mohamed, N.; Ima-Nirwana, S. The effects of tocotrienol and lovastatin co-supplementation on bone dynamic histomorphometry and bone morphogenetic protein-2 expression in rats with estrogen deficiency. Nutrients 2017, 9, 143. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima Nirwana, S. The effects of annatto-derived tocotrienol supplementation in osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Effect of tocotrienol from bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Dev. Ther. 2018, 12, 555–564. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. Effects of tocotrienol from bixa orellana (annatto) on bone histomorphometry in a male osteoporosis model induced by buserelin. Biomed. Pharmacother. 2018, 103, 453–462. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Exploring the potential of tocotrienol from bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone 2018, 116, 8–21. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Jamil, N.A.; Ima-Nirwana, S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed. Pharmacother. 2018, 98, 191–200. [Google Scholar] [CrossRef]
- Ima Nirwana, S.; Kiftiah, A.; Zainal, A.; Norazlina, M.; Gapor, M.; Khalid, B.A.K. Palm vitamin e prevents osteoporosis in orchidectomised growing male rats. Nat. Prod. Sci. 2000, 6, 155–160. [Google Scholar]
- Ima-Nirwana, S.; Fakhrurazi, H. Palm vitamin e protects bone against dexamethasone-induced osteoporosis in male rats. Med. J. 2002, 57, 133–141. [Google Scholar]
- Ima Nirwana, S.; Suhaniza, S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J. Med. Food 2004, 7, 45–51. [Google Scholar] [CrossRef]
- Zakaria, S.; Mat-Husain, S.Z.; Ying-Hwey, K.; Xin-Kai, K.; Mohd-Badawi, A.; Abd-Ghani, N.A.; Aziz, M.A.; Mohamed, N. Vitamin e improved bone strength and bone minerals in male rats given alcohol. Iran J. Basic Med. Sci. 2017, 20, 1360–1367. [Google Scholar]
- Wong, S.; Chin, K.-Y.; Suhaimi, F.; Ahmad, F.; Ima-Nirwana, S. The effects of vitamin e from elaeis guineensis (oil palm) in a rat model of bone loss due to metabolic syndrome. Int. J. Environ. Res. Public Health 2018, 15, 1828. [Google Scholar] [CrossRef]
- Chin, K.Y.; Abdul-Majeed, S.; Fozi, N.F.; Ima-Nirwana, S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients 2014, 6, 4974–4983. [Google Scholar] [CrossRef]
- Chin, K.Y.; Gengatharan, D.; Mohd Nasru, F.S.; Khairussam, R.A.; Ern, S.L.; Aminuddin, S.A.; Ima-Nirwana, S. The effects of annatto tocotrienol on bone biomechanical strength and bone calcium content in an animal model of osteoporosis due to testosterone deficiency. Nutrients 2016, 8, 808. [Google Scholar] [CrossRef]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Ahmad Nazrun, S.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague–dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef]
- Norazlina, M.; Hermizi, H.; Faizah, O.; Ima-Nirwana, S. Vitamin e reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch. Med. Sci. 2010, 6, 505–512. [Google Scholar] [CrossRef]
- Mehat, M.; Shuid, A.; Mohamed, N.; Muhammad, N.; Soelaiman, I. Beneficial effects of vitamin e isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J. Bone Miner Metab. 2010, 28, 503–509. [Google Scholar] [CrossRef]
- Shuid, A.; Mehat, Z.; Mohamed, N.; Muhammad, N.; Soelaiman, I. Vitamin e exhibits bone anabolic actions in normal male rats. J. Bone Miner Metab. 2010, 28, 149–156. [Google Scholar] [CrossRef]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm tocotrienol exerted better antioxidant activities in bone than α-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef]
- Gordon, C.M.; Zemel, B.S.; Wren, T.A.; Leonard, M.B.; Bachrach, L.K.; Rauch, F.; Gilsanz, V.; Rosen, C.J.; Winer, K.K. The determinants of peak bone mass. J. Pediatr. 2017, 180, 261–269. [Google Scholar] [CrossRef]
- Tennant, K.G.; Leonard, S.W.; Wong, C.P.; Iwaniec, U.T.; Turner, R.T.; Traber, M.G. High-dietary alpha-tocopherol or mixed tocotrienols have no effect on bone mass, density, or turnover in male rats during skeletal maturation. J. Med. Food 2017, 20, 700–708. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef]
- Abd Manan, N.; Mohamed, N.; Shuid, A.N. Effects of low-dose versus high-dose gamma-tocotrienol on the bone cells exposed to the hydrogen peroxide-induced oxidative stress and apoptosis. Evid. Based Complement Altern. Med. 2012, 2012, 680834. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Sun, J.; Jiang, Y.; Zhang, H.; Zhang, P.; Fei, B.; Xu, Y. Iron-induced oxidative stress stimulates osteoclast differentiation via nf-kappab signaling pathway in mouse model. Metabolism 2018, 83, 167–176. [Google Scholar] [CrossRef]
- Li, D.Y.; Yu, J.C.; Xiao, L.; Miao, W.; Ji, K.; Wang, S.C.; Geng, Y.X. Autophagy attenuates the oxidative stress-induced apoptosis of mc3t3-e1 osteoblasts. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5548–5556. [Google Scholar]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.Y.; Ima-Nirwana, S. Sex steroids and bone health status in men. Int. J. Endocrinol. 2012, 2012, 208719. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, M. Effects of androgen and progestin on the proliferation and differentiation of osteoblasts. Exp. Ther. Med. 2018, 16, 4722–4728. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Ambrogini, E.; Bartell, S.M.; Manolagas, S.C. Oxidative stress stimulates apoptosis and activates nf-kappab in osteoblastic cells via a pkcbeta/p66shc signaling cascade: Counter regulation by estrogens or androgens. Mol. Endocrinol. 2010, 24, 2030–2037. [Google Scholar] [CrossRef]
- Proell, V.; Xu, H.; Schuler, C.; Weber, K.; Hofbauer, L.C.; Erben, R.G. Orchiectomy upregulates free soluble rankl in bone marrow of aged rats. Bone 2009, 45, 677–681. [Google Scholar] [CrossRef]
- Sinnesael, M.; Boonen, S.; Claessens, F.; Gielen, E.; Vanderschueren, D. Testosterone and the male skeleton: A dual mode of action. J. Osteoporos. 2011, 2011, 240328. [Google Scholar] [CrossRef]
- Herrmann, B.L.; Saller, B.; Janssen, O.E.; Gocke, P.; Bockisch, A.; Sperling, H.; Mann, K.; Broecker, M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the cyp19 gene. J. Clin. Endocrinol. Metab. 2002, 87, 5476–5484. [Google Scholar] [CrossRef]
- Lanfranco, F.; Zirilli, L.; Baldi, M.; Pignatti, E.; Corneli, G.; Ghigo, E.; Aimaretti, G.; Carani, C.; Rochira, V. A novel mutation in the human aromatase gene: Insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone 2008, 43, 628–635. [Google Scholar] [CrossRef]
- Morishima, A.; Grumbach, M.M.; Simpson, E.R.; Fisher, C.; Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995, 80, 3689–3698. [Google Scholar]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Dudek, P.; Kozakowski, J.; Zgliczyński, W. Late-onset hypogonadism. Prz Menopauzal. 2017, 16, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, H.S.; Kim, Y.S.; Kim, M.J.; Kim, K.M.; Joo, N.S.; Haam, J.H. The association of testosterone, sex hormone-binding globulin, and insulin-like growth factor-1 with bone parameters in korean men aged 50 years or older. J. Bone Miner Metab. 2017, 35, 659–665. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Soelaiman, I.-N.; Naina Mohamed, I.; Shahar, S.; Teng, N.I.M.F.; Suhana Mohd Ramli, E.; Ahmad, F.; Aminuddin, A.; Zurinah Wan Ngah, W. Testosterone is associated with age-related changes in bone health status, muscle strength and body composition in men. Aging Male 2012, 15, 240–245. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. Effect of androgen deprivation therapy (adt) on bone health status in men with prostate cancer. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 276–284. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Soelaiman, I.N. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66. [Google Scholar] [CrossRef]
- Brogden, R.N.; Buckley, M.M.; Ward, A. Buserelin. A review of its pharmacodynamic and pharmacokinetic properties, and clinical profile. Drugs 1990, 39, 399–437. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Zulkepli, M.A.A.C.; Theseira, K.M.; Zulkifli, N.; Shahrom, N.Q.N.A.M.R.; Jamil, N.A.; Soelaiman, I.-N.; Chin, K.-Y. Establishing an animal model of secondary osteoporosis by using a gonadotropin-releasing hormone agonist. Int. J. Med. Sci. 2018, 15, 300–308. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.; Ahmad, F.; Ima-Nirwana, S. The relationship between metabolic syndrome and osteoporosis: A review. Nutrients 2016, 8, 347. [Google Scholar] [CrossRef]
- Abbasi, M.; Farzam, S.A.; Mamaghani, Z.; Yazdi, Z. Relationship between metabolic syndrome and its components with bone densitometry in postmenopausal women. Diabetes Metab. Syndr. 2017, 11, S73–S76. [Google Scholar] [CrossRef]
- Alhazidou, E.; Pergialiotis, V.; Panagopoulos, P.; Chrelias, C.; Hatziagelaki, E.; Papantoniou, N.; Trakakis, E. The impact of the metabolic syndrome on bone mass density: A prospective case control study. Horm. Mol. Biol. Clin. Investig. 2017, 33. [Google Scholar] [CrossRef]
- Loke, S.S.; Chang, H.W.; Li, W.C. Association between metabolic syndrome and bone mineral density in a taiwanese elderly population. J. Bone Miner Metab. 2018, 36, 200–208. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Caudarella, R.; Vescini, F.; Rizzoli, E.; Francucci, C.M. Salt intake, hypertension, and osteoporosis. J. Endocrinol. Investig. 2009, 32, 15–20. [Google Scholar]
- Liu, J.; Mao, J.; Jiang, Y.; Xia, L.; Mao, L.; Wu, Y.; Ma, P.; Fang, B. Ages induce apoptosis in rat osteoblast cells by activating the caspase-3 signaling pathway under a high-glucose environment in vitro. Appl. Biochem. Biotechnol. 2016, 178, 1015–1027. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yang, L.; Zhou, J.; Tang, Y.; Zheng, L.; Qin, P. Runx2 alleviates high glucose-suppressed osteogenic differentiation via pi3k/akt/gsk3beta/beta-catenin pathway. Cell Biol Int. 2017, 41, 822–832. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Yang, B.; Shi, Y.X.; Zhang, W.L.; Liu, F.; Zhao, W.; Yang, M.W. High glucose downregulates the effects of autophagy on osteoclastogenesis via the ampk/mtor/ulk1 pathway. Biochem. Biophys. Res. Commun. 2018, 503, 428–435. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Brissette, L.; Falstrault, L.; Ouellet, P.; Moreau, R. Influence of oxidized low-density lipoproteins (ldl) on the viability of osteoblastic cells. Free Radic. Biol. Med. 2008, 44, 506–517. [Google Scholar] [CrossRef]
- Maziere, C.; Savitsky, V.; Galmiche, A.; Gomila, C.; Massy, Z.; Maziere, J.C. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim. Biophys. Acta 2010, 1802, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.; Luan, L.; Ren, C. Oxidized low-density lipoprotein promotes osteoclast differentiation from cd68 positive mononuclear cells by regulating hmgb1 release. Biochem. Biophys. Res Commun. 2018, 495, 1356–1362. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Effects of metabolic syndrome on bone mineral density, histomorphometry and remodelling markers in male rats. PLoS ONE 2018, 13, e0192416. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Zhao, P.; Liu, B.; Yuan, Z.-C. Effect of cigarette smoking on risk of hip fracture in men: A meta-analysis of 14 prospective cohort studies. PLoS ONE 2016, 11, e0168990. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, A.R.; Kim, J.H.; Kim, K.M.; Koo, B.K.; Shin, C.S.; Kim, S.W. Amount of smoking, pulmonary function, and bone mineral density in middle-aged korean men: Knhanes 2008–2011. J. Bone Miner Metab. 2018, 36, 95–102. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, S.J.; Kim, H.J.; Lee, S.J.; Park, Y.J.; Lee, J.; You, H.K. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 2012, 90, 109–115. [Google Scholar] [CrossRef]
- Liang, D.; Wang, K.J.; Tang, Z.Q.; Liu, R.H.; Zeng, F.; Cheng, M.Y.; Lian, Q.S.; Wu, H.K. Effects of nicotine on the metabolism and gene expression profile of spraguedawley rat primary osteoblasts. Mol. Med. Rep. 2018, 17, 8269–8281. [Google Scholar]
- Kallala, R.; Barrow, J.; Graham, S.M.; Kanakaris, N.; Giannoudis, P.V. The in vitro and in vivo effects of nicotine on bone, bone cells and fracture repair. Expert Opin. Drug Saf. 2013, 12, 209–233. [Google Scholar] [CrossRef]
- Hapidin, H.; Othman, F.; Soelaiman, I.N.; Shuid, A.N.; Luke, D.A.; Mohamed, N. Negative effects of nicotine on bone-resorbing cytokines and bone histomorphometric parameters in male rats. J. Bone Miner Metab. 2007, 25, 93–98. [Google Scholar] [CrossRef]
- Hapidin, H.; Othman, F.; Soelaiman, I.N.; Shuid, A.N.; Mohamed, N. Effects of nicotine administration and nicotine cessation on bone histomorphometry and bone biomarkers in sprague-dawley male rats. Calcif. Tissue Int. 2011, 88, 41–47. [Google Scholar] [CrossRef]
- Abukhadir, S.S.A.; Mohamed, N.; Makpol, S.; Muhammad, N. Effects of palm vitamin e on bone-formation-related gene expression in nicotine-treated rats. Evid. Based Complement Altern. Med. 2012, 2012, 656025. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, S.; Kim, K.; Lee, G.; Park, S.M. Association between alcohol consumption and bone mineral density in elderly korean men and women. Arch. Osteoporos 2018, 13, 46. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Liu, Y.; Chen, H.; Shi, S.; Liu, Y. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol. Life Sci. 2017, 74, 4443–4453. [Google Scholar] [CrossRef]
- Maneesh, M.; Dutta, S.; Chakrabarti, A.; Vasudevan, D.M. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J. Physiol. Pharmacol. 2006, 50, 291–296. [Google Scholar]
- Perrien, D.S.; Brown, E.C.; Fletcher, T.W.; Irby, D.J.; Aronson, J.; Gao, G.G.; Skinner, R.A.; Hogue, W.R.; Feige, U.; Suva, L.J.; et al. Interleukin-1 and tumor necrosis factor antagonists attenuate ethanol-induced inhibition of bone formation in a rat model of distraction osteogenesis. J. Pharmacol. Exp. Ther. 2002, 303, 904–908. [Google Scholar] [CrossRef]
- Liu, Y.; Kou, X.; Chen, C.; Yu, W.; Su, Y.; Kim, Y.; Shi, S.; Liu, Y. Chronic high dose alcohol induces osteopenia via activation of mtor signaling in bone marrow mesenchymal stem cells. Stem. Cells 2016, 34, 2157–2168. [Google Scholar] [CrossRef]
- Gonzalez-Reimers, E.; Martin-Gonzalez, C.; de la Vega-Prieto, M.J.; Pelazas-Gonzalez, R.; Fernandez-Rodriguez, C.; Lopez-Prieto, J.; Alvisa-Negrin, J.; Santolaria-Fernandez, F. Serum sclerostin in alcoholics: A pilot study. Alcohol. Alcohol. 2013, 48, 278–282. [Google Scholar] [CrossRef]
- Whittier, X.; Saag, K.G. Glucocorticoid-induced osteoporosis. Rheum. Dis. Clin. N. Am. 2016, 42, 177–189, x. [Google Scholar] [CrossRef]
- Mak, W.; Shao, X.; Dunstan, C.R.; Seibel, M.J.; Zhou, H. Biphasic glucocorticoid-dependent regulation of wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Gifre, L.; Ruiz-Gaspa, S.; Monegal, A.; Nomdedeu, B.; Filella, X.; Guanabens, N.; Peris, P. Effect of glucocorticoid treatment on wnt signalling antagonists (sclerostin and dkk-1) and their relationship with bone turnover. Bone 2013, 57, 272–276. [Google Scholar] [CrossRef]
- Smith, E.; Frenkel, B. Glucocorticoids inhibit the transcriptional activity of lef/tcf in differentiating osteoblasts in a glycogen synthase kinase-3β-dependent and -independent manner. J. Biol. Chem. 2005, 280, 2388–2394. [Google Scholar] [CrossRef]
- Koromila, T.; Baniwal, S.K.; Song, Y.S.; Martin, A.; Xiong, J.; Frenkel, B. Glucocorticoids antagonize runx2 during osteoblast differentiation in cultures of st2 pluripotent mesenchymal cells. J. Cell Biochem. 2014, 115, 27–33. [Google Scholar] [CrossRef]
- Piemontese, M.; Xiong, J.; Fujiwara, Y.; Thostenson, J.D.; O’Brien, C.A. Cortical bone loss caused by glucocorticoid excess requires rankl production by osteocytes and is associated with reduced opg expression in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E587–E593. [Google Scholar] [CrossRef]
- Mac, A.M.; White, R.H.; Chipps, B.E. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann. Int. Med. 1986, 104, 648–651. [Google Scholar]
- Gennari, C. Differential effect of glucocorticoids on calcium absorption and bone mass. Rheumatology 1993, 32, 11–14. [Google Scholar] [CrossRef]
- Prummel, M.F.; Wiersinga, W.M.; Oosting, H.; Endert, E. The effect of long-term prednisone treatment on growth hormone and insulin-like growth factor-i. J. Endocrinol. Investig. 1996, 19, 620–623. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Karademirci, M.; Kutlu, R.; Kilinc, I. Relationship between smoking and total antioxidant status, total oxidant status, oxidative stress index, Vit C, Vit E. Clin. Respir J. 2018, 12, 2006–2012. [Google Scholar] [CrossRef]
- Budzynski, J.; Ziolkowski, M.; Klopocka, M.; Czarnecki, D. Oxidoreductive homeostasis in alcohol-dependent male patients and the risk of alcohol drinking relapse in a 6-month follow-up. Alcohol 2016, 50, 57–64. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Bonaccorsi, G.; Piva, I.; Greco, P.; Cervellati, C. Oxidative stress as a possible pathogenic cofactor of post-menopausal osteoporosis: Existing evidence in support of the axis oestrogen deficiency-redox imbalance-bone loss. Indian J. Med. Res. 2018, 147, 341–351. [Google Scholar]
- Makary, S.; Abdo, M.; Fekry, E. Oxidative stress burden inhibits spermatogenesis in adult male rats: Testosterone protective effect. Can. J. Physiol. Pharmacol. 2018, 96, 372–381. [Google Scholar] [CrossRef]
- Son, S.W.; Lee, J.S.; Kim, H.G.; Kim, D.W.; Ahn, Y.C.; Son, C.G. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem. 2016, 136, 106–117. [Google Scholar] [CrossRef]
- Eleawa, S.M.; Sakr, H.F.; Hussein, A.M.; Assiri, A.S.; Bayoumy, N.M.; Alkhateeb, M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol. 2013, 209, 136–147. [Google Scholar] [CrossRef]
- Skogastierna, C.; Hotzen, M.; Rane, A.; Ekstrom, L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur. J. Prev. Cardiol. 2014, 21, 1049–1054. [Google Scholar] [CrossRef]
- Contini, M.D.; Millen, N.; Gonzalez, M.; Benmelej, A.; Fabro, A.; Mahieu, S. Orchiectomy attenuates oxidative stress induced by aluminum in rats. Toxicol. Ind. Health 2016, 32, 1515–1526. [Google Scholar] [CrossRef]
- Tothova, L.; Celec, P.; Ostatnikova, D.; Okuliarova, M.; Zeman, M.; Hodosy, J. Effect of exogenous testosterone on oxidative status of the testes in adult male rats. Andrologia 2013, 45, 417–423. [Google Scholar] [CrossRef]
- Nizar, A.; Ahmad Nazrun, S.; Norazlina, M.; Norliza, M.; Ima Nirwana, S. Low dose of tocotrienols protects osteoblasts against oxidative stress. Clin. Ter. 2011, 162, 533–538. [Google Scholar]
- Takano, H.; Momota, Y.; Kani, K.; Aota, K.; Yamamura, Y.; Yamanoi, T.; Azuma, M. Gamma-tocotrienol prevents 5-fu-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-fu-induced activation of nrf2. Int. J. Oncol. 2015, 46, 1453–1460. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. Nf-κb signaling in inflammation. Signal. Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The nf-kappab family of transcription factors and its regulation. Cold Spring Harb. Perspect Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Otero, J.E.; Dai, S.; Foglia, D.; Alhawagri, M.; Vacher, J.; Pasparakis, M.; Abu-Amer, Y. Defective osteoclastogenesis by ikkbeta-null precursors is a result of receptor activator of NF-κB ligand (rankl)-induced jnk-dependent apoptosis and impaired differentiation. J. Biol. Chem. 2008, 283, 24546–24553. [Google Scholar] [CrossRef]
- Ruocco, M.G.; Maeda, S.; Park, J.M.; Lawrence, T.; Hsu, L.C.; Cao, Y.; Schett, G.; Wagner, E.F.; Karin, M. Iκb kinase ikkβ, but not ikkα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 2005, 201, 1677–1687. [Google Scholar] [CrossRef]
- Yao, Z.; Xing, L.; Boyce, B.F. NF-κB p100 limits tnf-induced bone resorption in mice by a traf3-dependent mechanism. J. Clin. Investig. 2009, 119, 3024–3034. [Google Scholar] [CrossRef]
- Xiu, Y.; Xu, H.; Zhao, C.; Li, J.; Morita, Y.; Yao, Z.; Xing, L.; Boyce, B.F. Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J. Clin. Investig. 2014, 124, 297–310. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Z.; Tang, E.; Fan, Z.; McCauley, L.; Franceschi, R.; Guan, K.; Krebsbach, P.H.; Wang, C.Y. Inhibition of osteoblastic bone formation by NF-κB. Nat. Med. 2009, 15, 682–689. [Google Scholar] [CrossRef]
- Loganathan, R.; Selvaduray, K.R.; Nesaretnam, K.; Radhakrishnan, A.K. Tocotrienols promote apoptosis in human breast cancer cells by inducing poly(adp-ribose) polymerase cleavage and inhibiting NF-κB activity. Cell Prolif. 2013, 46, 203–213. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q. Gamma-tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing c/ebpβand NF-κB in macrophages. J. Nutr. Biochem. 2013, 24, 1146–1152. [Google Scholar] [CrossRef]
- Sun, W.G.; Song, R.P.; Wang, Y.; Ge, S.; Zhang, Y.H.; Wang, H.X.; Liu, J.; Liu, L.X. R-tocotrienol inhibits cell proliferation of human gastric cancer by regulating NF-κB activity. J. Agric. Food Chem. 2018. [Google Scholar]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-κB activation in pancreatic cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. Gamma-tocotrienol inhibits NF-κB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Khalid, B.A.K.; Luke, D.A.; Ima Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharmacol. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef]
- Brooks, R.; Kalia, P.; Ireland, D.; Beeton, C.; Rushton, N. Direct inhibition of osteoclast formation and activity by the vitamin E isomer γ-tocotrienol. Int. J. Vitam Nutr. Res. 2011, 81, 358–367. [Google Scholar] [CrossRef]
- Mo, H.; Yeganehjoo, H.; Shah, A.; Mo, W.K.; Soelaiman, I.N.; Shen, C.-L. Mevalonate-suppressive dietary isoprenoids for bone health. J. Nutr. Biochem. 2012, 23, 1543–1551. [Google Scholar] [CrossRef]
- Wan Hasan, W.N.; Chin, K.Y.; Jolly, J.J.; Abd Ghafar, N.; Soelaiman, I.N. Identifying potential therapeutics for osteoporosis by exploiting the relationship between mevalonate pathway and bone metabolism. Endocr. Metab. Immune Disord Drug Targets 2018, 18, 450–457. [Google Scholar] [CrossRef]
- Takase, H.; Yano, S.; Yamaguchi, T.; Kanazawa, I.; Hayashi, K.; Yamamoto, M.; Yamauchi, M.; Sugimoto, T. Parathyroid hormone upregulates BMP-2 mrna expression through mevalonate kinase and rho kinase inhibition in osteoblastic mc3t3-e1 cells. Horm. Metab Res. 2009, 41, 861–865. [Google Scholar] [CrossRef]
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small gtpases. Small GTPases 2011, 2, 117–130. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I. A review on the use of statins and tocotrienols, individually or in combination for the treatment of osteoporosis. Curr. Drug Targets 2013, 14, 1579–1590. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos Int. 2017, 28, 47–57. [Google Scholar] [CrossRef]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols regulate cholesterol production in mammalian cells by post-transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme a reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar]
- Yeganehjoo, H.; DeBose-Boyd, R.; McFarlin, B.K.; Mo, H. Synergistic impact of d-delta-tocotrienol and geranylgeraniol on the growth and hmg coa reductase of human du145 prostate carcinoma cells. Nutr. Cancer 2017, 69, 682–691. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, T.Y.; Huang, S.P.; Ho, C.T.; Huang, T.C. Differentiation and apoptosis induction by lovastatin and gamma-tocotrienol in hl-60 cells via ras/erk/NF-κB and ras/akt/NF-κB signaling dependent down-regulation of glyoxalase 1 and hmg-coa reductase. Cell Signal. 2015, 27, 2182–2190. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, H.; Jin, H.; Koo, P.T.; Tsang, D.J.; Yang, C.S. Synergistic actions of atorvastatin with gamma-tocotrienol and celecoxib against human colon cancer ht29 and hct116 cells. Int. J. Cancer 2010, 126, 852–863. [Google Scholar]
- Yamasaki, M.; Nishimura, M.; Sakakibara, Y.; Suiko, M.; Morishita, K.; Nishiyama, K. δ-tocotrienol induces apoptotic cell death via depletion of intracellular squalene in ed40515 cells. Food Funct 2014, 5, 2842–2849. [Google Scholar] [CrossRef]
- Deng, L.; Ding, Y.; Peng, Y.; Wu, Y.; Fan, J.; Li, W.; Yang, R.; Yang, M.; Fu, Q. Γ-tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as hmg-coa reductase inhibitor. Bone 2014, 67, 200–207. [Google Scholar] [CrossRef]
- Shen, C.-L.; Wang, S.; Yang, S.; Tomison, M.D.; Abbasi, M.; Hao, L.; Scott, S.; Khan, M.S.; Romero, A.W.; Felton, C.K.; et al. A 12-week evaluation of annatto tocotrienol supplementation for postmenopausal women: Safety, quality of life, body composition, physical activity, and nutrient intake. BMC Complement. Altern. Med. 2018, 18, 198. [Google Scholar] [CrossRef]
- Shen, C.L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos Int. 2018, 29, 881–891. [Google Scholar] [CrossRef]
- Ima-Nirwana, S.; Nurshazwani, Y.; Nazrun, A.S.; Norliza, M.; Norazlina, M. Subacute and subchronic toxicity studies of palm vitamin E in mice. J. Pharmacol. Toxicol. 2011, 6, 166–173. [Google Scholar] [CrossRef]
- Deng, L.; Peng, Y.; Wu, Y.; Yang, M.; Ding, Y.; Chen, Q.; Fu, Q. Tissue distribution of emulsified γ-tocotrienol and its long-term biological effects after subcutaneous administration. Lipids Health Dis. 2014, 13, 66. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhanced solubility and oral bioavailability of gamma-tocotrienol using a self-emulsifying drug delivery system (sedds). Lipids 2014, 49, 819–829. [Google Scholar] [CrossRef]
- Maniam, G.; Mai, C.-W.; Zulkefeli, M.; Dufès, C.; Tan, D.M.-Y.; Fu, J.-Y. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Tan, B.; Llobrera, J. Annatto Extract Compositions Including Tocotrienols and Tocopherols and Methods of Use. Patent WO2005009135A1, 3 February 2005. [Google Scholar]
- Lane, R.H. Compositions and Methods for Treating and Preventing Bone Diseases Using Tocotrienols. Patent WO2000002553A1, 20 January 2000. [Google Scholar]
- Cheng, H.S.; Ton, S.H.; Tan, J.B.L.; Abdul Kadir, K. The ameliorative effects of a tocotrienol-rich fraction on the age-rage axis and hypertension in high-fat-diet-fed rats with metabolic syndrome. Nutrients 2017, 9, 984. [Google Scholar] [CrossRef]
- Wong, W.Y.; Ward, L.C.; Fong, C.W.; Yap, W.N.; Brown, L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet-induced obese rats. Eur. J. Nutr. 2017, 56, 133–150. [Google Scholar] [CrossRef]
- Chin, K.Y.; Tay, S.S. A review on the relationship between tocotrienol and alzheimer disease. Nutrients 2018, 10, 881. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Swaminathan, M.; Chakravarthi, S.; Radhakrishnan, A. Therapeutic efficacy of vitamin E delta-tocotrienol in collagen-induced rat model of arthritis. BioMed Res. Int. 2014, 2014, 539540. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Tudawe, D.; Chakravarthi, S.; Chiew, G.S.; Haleagrahara, N. Effect of gamma-tocotrienol in counteracting oxidative stress and joint damage in collagen-induced arthritis in rats. Exp. Ther. Med. 2014, 7, 1408–1414. [Google Scholar] [CrossRef]
- Lim, J.J.; Ngah, W.Z.; Mouly, V.; Abdul Karim, N. Reversal of myoblast aging by tocotrienol rich fraction posttreatment. Oxid. Med. Cell Longev. 2013, 2013, 978101. [Google Scholar] [CrossRef]
- Khor, S.C.; Razak, A.M.; Wan Ngah, W.Z.; Mohd Yusof, Y.A.; Abdul Karim, N.; Makpol, S. The tocotrienol-rich fraction is superior to tocopherol in promoting myogenic differentiation in the prevention of replicative senescence of myoblasts. PLoS ONE 2016, 11, e0149265. [Google Scholar] [CrossRef]
- Friedman, S.M.; Mendelson, D.A. Epidemiology of fragility fractures. Clin. Geriatr. Med. 2014, 30, 175–181. [Google Scholar] [CrossRef]

| Reference | Vitamin E Used | Composition of Vitamin E (%) | |||
|---|---|---|---|---|---|
| αTP | αT3 | γT3 | δT3 | ||
| [35] | Palm vitamin E | 24.4 | 21.6 | 27.7 | 11 |
| [36,37] | Palm vitamin E | 24.83 | 20.73 | 26.68 | 13.32 |
| [38] | Palm vitamin E | 22.48 | 23.16 | 36.89 | 12.57 |
| [33,39] | Palm vitamin E | 21.9 | 24.7 | 36.9 | 12 |
| [46] | Palm T3 | 18.43 | 14.62 | 32.45 | 23.93 |
| [29,30,31,32,40,41] | Annatto T3 | 10 | 90 | ||
| [42,43] | Palm T3-enriched fraction | 43 | 31 | 14 | |
| Ref | Induction of Bone Loss | Treatment | Period | Skeletal Properties Affected by T3 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV/TV | Tb.N | Tb.Th | Tb.Sp | SMI | Connectivity Density | Cortical Indices | Ob.N or Ob.S | Oc.N or Oc.S | ES/BS | OS/BS | OV/BV | sLS/BS | dLS/BS | MS | MAR | BFS/MS | BMD | Bone Calcium Content | Biomechanical Strength | ||||
| [29] | Orchidectomy | AnT3 60 mg/kg | 2 months | ↑ | ↑ | ↔ | ↓ | ↔ | ↔ | ↓ | ↑ | ↔ | ↔ | ↔ | |||||||||
| [40] | Orchidectomy | AnT3 60 mg/kg | 2 months | ↑ | ↓ | ↓ | ↑ | ↑ | |||||||||||||||
| [41] | Orchidectomy | AnT3 60 mg/kg | 2 months | ↑ (tibia) | ↔ | ||||||||||||||||||
| [35] | Orchidectomy | PVE 30 mg/kg | 8 months | ↑ | ↑ (lumbar) | ||||||||||||||||||
| [30] | Chemical castration by buserelin | AnT3 60 or 100 mg/kg | 3 months | ↑ | ↑ | ↔ | ↓ | ↔ | ↑ | ↑ thickness | ↑ (femur) | ↑ | |||||||||||
| [31] | Chemical castration by buserelin | AnT3 60 or 100 mg/kg | 3 months | ↑ | ↔ | ↑ | ↔ | ↓ | ↔ | ↔ | ↔ | ↓ | ↑ (60 mg/kg only) | ↔ | ↔ | ↔ | |||||||
| [32] | Metabolic syndrome | AnT3 60 or 100 mg/kg | 4 months | ↑ | ↑ | ↔ | ↓ | ↓ | ↑ | ↔ | ↑ | ↔ | ↔ | ↔ | ↔ | ↑ (60 mg/kg) | ↔ | ↔ | ↑ (60 mg/kg only) | ↔ | ↔ (femoral) | ↑ | |
| [33] | Metabolic syndrome | Palm T3 60 or 100 mg/kg | 4 months | ↑ | ↑ | ↔ | ↓ | ↓ | ↔ | ↔ | ↔ (femoral) | ↑ | |||||||||||
| [39] | Metabolic syndrome | Palm T3 60 or 100 mg/kg | 4 months | ↑ | ↔ | ↔ | ↔ | ↑ | ↔ | ↓ (100 mg/kg) | ↑ | ↔ | ↓ (60 mg/kg) | ↔ | ↔ | ↔ | ↔ | ↔ | |||||
| [37] | Glucocorticoid | γ-T3 60 mg/kg | 2 months | ↔ | ↑ (lumbar) | ||||||||||||||||||
| [36] | Glucocorticoid | PVE 60 mg/kg | 2 months | ↑ | ↑ (femoral) | ||||||||||||||||||
| [42] | Nicotine | Palm T3 enriched fraction, γ-T3 60 mg/kg | 2 months | ↑ | ↔ | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | |||||||||||
| [38] | Alcohol | PVE 60 mg/kg | 2 months | ↑ (tibial) | ↑ | ||||||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Ima-Nirwana, S. The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence. Int. J. Mol. Sci. 2019, 20, 1355. https://doi.org/10.3390/ijms20061355
Chin K-Y, Ima-Nirwana S. The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence. International Journal of Molecular Sciences. 2019; 20(6):1355. https://doi.org/10.3390/ijms20061355
Chicago/Turabian StyleChin, Kok-Yong, and Soelaiman Ima-Nirwana. 2019. "The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence" International Journal of Molecular Sciences 20, no. 6: 1355. https://doi.org/10.3390/ijms20061355
APA StyleChin, K.-Y., & Ima-Nirwana, S. (2019). The Role of Tocotrienol in Preventing Male Osteoporosis—A Review of Current Evidence. International Journal of Molecular Sciences, 20(6), 1355. https://doi.org/10.3390/ijms20061355