FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy?
Abstract
1. Introduction
2. Results
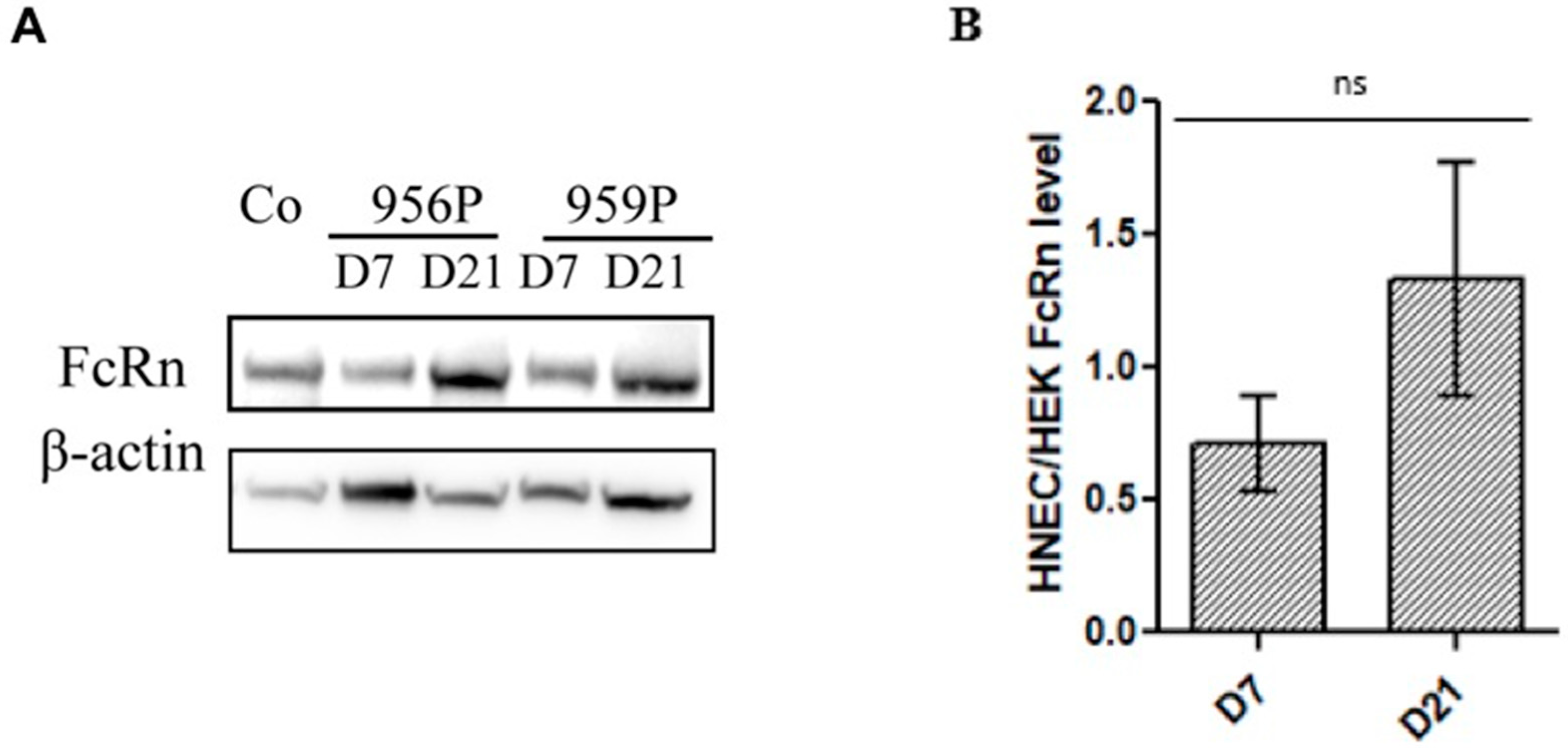
2.1. Expression of FcRn in Human Nasal Epithelial Cells (HNECs)
2.2. Localization of FcRn in HNEC
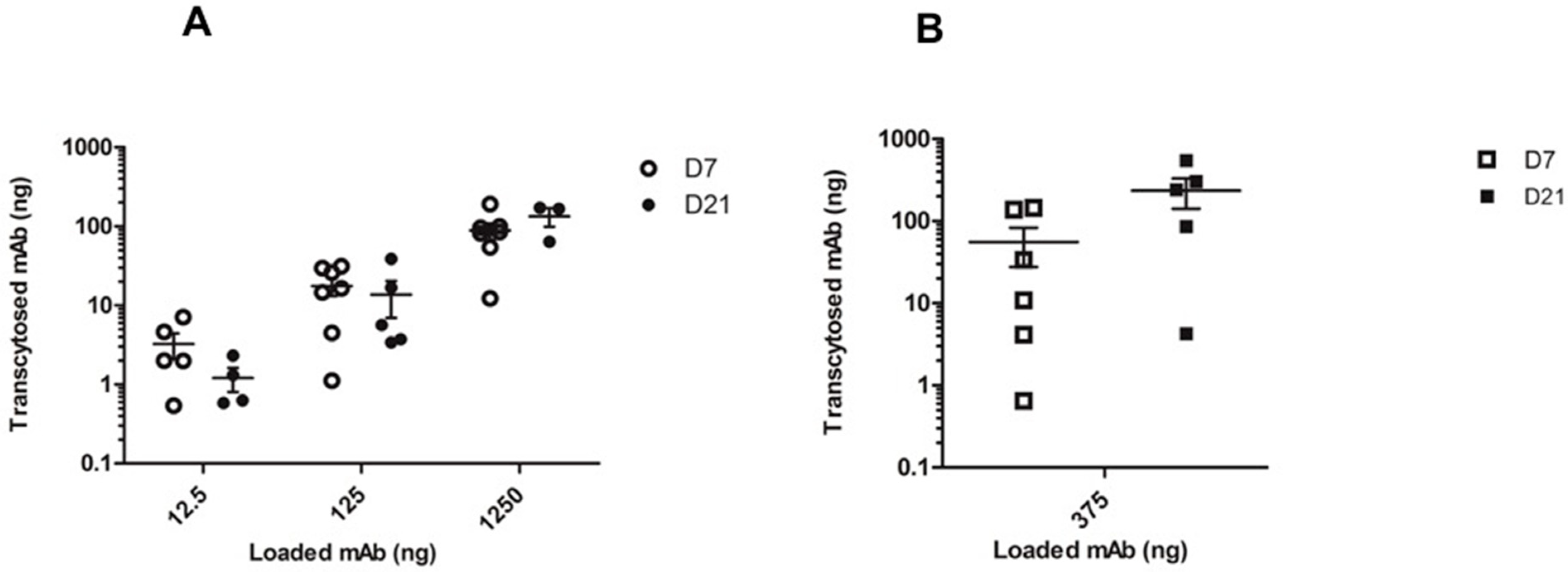
2.3. In Vitro mAb Transcytosis in HNEC ALI Cultures
3. Discussion
4. Methods
4.1. Primary Cultures of Human Nasal Epithelial Cells (HNEC)
4.2. Quantitative Real-Time PCR (qRT-PCR)
4.3. Western Blot
4.4. Immunohistochemical Analysis
4.5. Transcytosis Assay
4.6. Statistical Analysis
4.7. Ethics Approval and Consent to Participate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FcRn | neonatal Fc receptor |
| HNEC | Human Nasal Epithelial Cells |
| NP | Nasal polyps |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| mAbs | Monoclonal antibodies |
| ALI | air–liquid interface |
| TEER | Transepithelial electrical resistance |
| β2m | β2-microglobulin |
| PBS++ | PBS supplemented with 0.49mM MgCl2 and 0.9mM CaCl2 |
References
- Brambell, F.W.; Hemmings, W.A.; Morris, I.G. A Theoretical Model of Gamma-Globulin Catabolism. Nature 1964, 203, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E.; Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nature 1989, 337, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Latvala, S.; Jacobsen, B.; Otteneder, M.B.; Herrmann, A.; Kronenberg, S. Distribution of FcRn Across Species and Tissues. J. Histochem. Cytochem. 2017, 65, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Popov, S.; Borvak, J.; Radu, C.; Matesoi, D.; Medesan, C.; Ober, R.J.; Ward, E.S. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat. Biotechnol. 1997, 15, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Junghans, R.P.; Anderson, C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.A.; Haynes, L.D.; Kim, J.; Bronson, C.L.; Chaudhury, C.; Mohanty, S.; Waldmann, T.A.; Robinson, J.M.; Anderson, C.L. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc. Natl. Acad. Sci. USA 2006, 103, 5084–5089. [Google Scholar] [CrossRef]
- Raghavan, M.; Bonagura, V.R.; Morrison, S.L.; Bjorkman, P.J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995, 34, 14649–14657. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, C.; Brooks, C.L.; Carter, D.C.; Robinson, J.M.; Anderson, C.L. Albumin binding to FcRn: Distinct from the FcRn-IgG interaction. Biochemistry 2006, 45, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Datta-Mannan, A.; Witcher, D.R.; Tang, Y.; Watkins, J.; Wroblewski, V.J. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J. Biol. Chem. 2007, 282, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Waldmann, T.A. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J. Clin. Investig. 1972, 51, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Oganesyan, V.; Damschroder, M.M.; Cook, K.E.; Li, Q.; Gao, C.; Wu, H.; Dall’Acqua, W.F. Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem. 2014, 289, 7812–7824. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.L.; Chaudhury, C.; Kim, J.; Bronson, C.L.; Wani, M.A.; Mohanty, S. Perspective--FcRn transports albumin: Relevance to immunology and medicine. Trends Immunol. 2006, 27, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Christianson, G.J.; Sproule, T.J.; Brown, A.C.; Akilesh, S.; Jung, N.; Petkova, S.; Avanessian, L.; Choi, E.Y.; Shaffer, D.J.; et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 2003, 170, 3528–3533. [Google Scholar] [CrossRef]
- Ward, E.S.; Zhou, J.; Ghetie, V.; Ober, R.J. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int. Immunol. 2003, 15, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef] [PubMed]
- Nissim, A.; Chernajovsky, Y. Historical development of monoclonal antibody therapeutics. Handb. Exp. Pharmacol. 2008. [Google Scholar] [CrossRef]
- Reichert, J.M.; Rosensweig, C.J.; Faden, L.B.; Dewitz, M.C. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 2005, 23, 1073–1078. [Google Scholar] [CrossRef]
- Aue, G.; Lindorfer, M.A.; Beum, P.V.; Pawluczkowycz, A.W.; Vire, B.; Hughes, T.; Taylor, R.P.; Wiestner, A. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica 2010, 95, 329–332. [Google Scholar] [CrossRef]
- Kagan, L.; Mager, D.E. Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab. Dispos. 2013, 41, 248–255. [Google Scholar] [CrossRef]
- Spiekermann, G.M.; Finn, P.W.; Ward, E.S.; Dumont, J.; Dickinson, B.L.; Blumberg, R.S.; Lencer, W.I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002, 196, 303–310. [Google Scholar] [CrossRef]
- Guilleminault, L.; Azzopardi, N.; Arnoult, C.; Sobilo, J.; Herve, V.; Montharu, J.; Guillon, A.; Andres, C.; Herault, O.; Le Pape, A.; et al. Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J. Control. Release 2014, 196, 344–354. [Google Scholar] [CrossRef]
- Bitonti, A.J.; Dumont, J.A.; Low, S.C.; Peters, R.T.; Kropp, K.E.; Palombella, V.J.; Stattel, J.M.; Lu, Y.; Tan, C.A.; Song, J.J.; et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 9763–9768. [Google Scholar] [CrossRef]
- Dumont, J.A.; Bitonti, A.J.; Clark, D.; Evans, S.; Pickford, M.; Newman, S.P. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J. Aerosol Med. 2005, 18, 294–303. [Google Scholar] [CrossRef]
- Heidl, S.; Ellinger, I.; Niederberger, V.; Waltl, E.E.; Fuchs, R. Localization of the human neonatal Fc receptor (FcRn) in human nasal epithelium. Protoplasma 2016, 253, 1557–1564. [Google Scholar] [CrossRef]
- Samson, G.; Garcia de la Calera, A.; Dupuis-Girod, S.; Faure, F.; Decullier, E.; Paintaud, G.; Vignault, C.; Scoazec, J.Y.; Pivot, C.; Plauchu, H.; et al. Ex vivo study of bevacizumab transport through porcine nasal mucosa. Eur. J. Pharm. Biopharm. 2012, 80, 465–469. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 23, 1–298. [Google Scholar]
- Settipane, G.A. Epidemiology of nasal polyps. Allergy Asthma Proc. 1996, 17, 231–236. [Google Scholar] [CrossRef]
- Bachert, C.; Gevaert, P.; Hellings, P. Biotherapeutics in Chronic Rhinosinusitis with and without Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2017. [Google Scholar] [CrossRef]
- Willson, T.J.; Naclerio, R.M.; Lee, S.E. Monoclonal Antibodies for the Treatment of Nasal Polyps. Immunol. Allergy Clin. North Am. 2017, 37, 357–367. [Google Scholar] [CrossRef]
- Chiarella, S.E.; Sy, H.; Peters, A.T. Monoclonal antibody therapy in sinonasal disease. Am. J. Rhinol. Allergy 2017, 31, 93–95. [Google Scholar] [CrossRef]
- Lazard, D.S.; Moore, A.; Hupertan, V.; Martin, C.; Escabasse, V.; Dreyfus, P.; Burgel, P.R.; Amselem, S.; Escudier, E.; Coste, A. Muco-ciliary differentiation of nasal epithelial cells is decreased after wound healing in vitro. Allergy 2009, 64, 1136–1143. [Google Scholar] [CrossRef]
- Botterel, F.; Cordonnier, C.; Barbier, V.; Wingerstmann, L.; Liance, M.; Coste, A.; Escudier, E.; Bretagne, S. Aspergillus fumigatus causes in vitro electrophysiological and morphological modifications in human nasal epithelial cells. Histol. Histopathol. 2002, 17, 1095–1101. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Cho, H.J.; Bian, S.; Roh, H.J.; Lee, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J. Pharm. Sci. 2007, 96, 341–350. [Google Scholar] [CrossRef]
- Prince, O.A.; Krunkosky, T.M.; Sheppard, E.S.; Krause, D.C. Modelling persistent Mycoplasma pneumoniae infection of human airway epithelium. Cell. Microbiol. 2018, 20. [Google Scholar] [CrossRef]
- Haraya, K.; Tachibana, T.; Igawa, T. Improvement of pharmacokinetic properties of therapeutic antibodies by antibody engineering. Drug Metab. Pharmacokinet. 2018. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Christianson, G.J.; Proetzel, G.; Sproule, T.J. Human FcRn Transgenic Mice for Pharmacokinetic Evaluation of Therapeutic Antibodies. Methods Mol. Biol. 2016, 1438, 103–114. [Google Scholar] [CrossRef]
- Papon, J.F.; Coste, A.; Gendron, M.C.; Cordonnier, C.; Wingerstmann, L.; Peynegre, R.; Escudier, E. HLA-DR and ICAM-1 expression and modulation in epithelial cells from nasal polyps. Laryngoscope 2002, 112, 2067–2075. [Google Scholar] [CrossRef]
- Secondo, L.E.; Liu, N.J.; Lewinski, N.A. Methodological considerations when conducting in vitro, air-liquid interface exposures to engineered nanoparticle aerosols. Crit. Rev. Toxicol. 2017, 47, 225–262. [Google Scholar] [CrossRef]
- Li, X. In vitro toxicity testing of cigarette smoke based on the air-liquid interface exposure: A review. Toxicol. In Vitro 2016, 36, 105–113. [Google Scholar] [CrossRef]
- Israel, E.J.; Taylor, S.; Wu, Z.; Mizoguchi, E.; Blumberg, R.S.; Bhan, A.; Simister, N.E. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology 1997, 92, 69–74. [Google Scholar] [CrossRef]
- Li, Z.; Palaniyandi, S.; Zeng, R.; Tuo, W.; Roopenian, D.C.; Zhu, X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 4388–4393. [Google Scholar] [CrossRef]
- Foss, S.; Grevys, A.; Sand, K.M.; Bern, M.; Blundell, P.; Michaelsen, T.E.; Pleass, R.J.; Sandlie, I.; Andersen, J.T. Enhanced FcRn-dependent transepithelial delivery of IgG by Fc-engineering and polymerization. J. Control. Release 2016, 223, 42–52. [Google Scholar] [CrossRef]
- Vllasaliu, D.; Alexander, C.; Garnett, M.; Eaton, M.; Stolnik, S. Fc-mediated transport of nanoparticles across airway epithelial cell layers. J. Control. Release 2012, 158, 479–486. [Google Scholar] [CrossRef]
- Tian, Z.; Sutton, B.J.; Zhang, X. Distribution of rat neonatal Fc receptor in the principal organs of neonatal and pubertal rats. J. Recept. Signal Transduct. Res. 2013. [Google Scholar] [CrossRef]
- Ternant, D.; Arnoult, C.; Pugniere, M.; Dhommee, C.; Drocourt, D.; Perouzel, E.; Passot, C.; Baroukh, N.; Mulleman, D.; Tiraby, G.; et al. IgG1 Allotypes Influence the Pharmacokinetics of Therapeutic Monoclonal Antibodies through FcRn Binding. J. Immunol. 2016, 196, 607–613. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef]
- Claypool, S.M.; Dickinson, B.L.; Wagner, J.S.; Johansen, F.E.; Venu, N.; Borawski, J.A.; Lencer, W.I.; Blumberg, R.S. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol. Biol. Cell 2004, 15, 1746–1759. [Google Scholar] [CrossRef]
- Dickinson, B.L.; Badizadegan, K.; Wu, Z.; Ahouse, J.C.; Zhu, X.; Simister, N.E.; Blumberg, R.S.; Lencer, W.I. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J. Clin. Investig. 1999, 104, 903–911. [Google Scholar] [CrossRef]
- Ulrichts, P.; Guglietta, A.; Dreier, T.; van Bragt, T.; Hanssens, V.; Hofman, E.; Vankerckhoven, B.; Verheesen, P.; Ongenae, N.; Lykhopiy, V.; et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J. Clin. Investig. 2018, 128, 4372–4386. [Google Scholar] [CrossRef]
- England, R.J.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef]
- Coste, A.; Brugel, L.; Maitre, B.; Boussat, S.; Papon, J.F.; Wingerstmann, L.; Peynegre, R.; Escudier, E. Inflammatory cells as well as epithelial cells in nasal polyps express vascular endothelial growth factor. Eur. Respir. J. 2000, 15, 367–372. [Google Scholar] [CrossRef]
- Forero, A.; Fenstermacher, K.; Wohlgemuth, N.; Nishida, A.; Carter, V.; Smith, E.A.; Peng, X.; Hayes, M.; Francis, D.; Treanor, J.; et al. Evaluation of the innate immune responses to influenza and live-attenuated influenza vaccine infection in primary differentiated human nasal epithelial cells. Vaccine 2017, 35, 6112–6121. [Google Scholar] [CrossRef]
- Ramanathan, M., Jr.; Lee, W.K.; Dubin, M.G.; Lin, S.; Spannhake, E.W.; Lane, A.P. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am. J. Rhinol. 2007, 21, 110–116. [Google Scholar] [CrossRef]
- Cao, H.; Ouyang, H.; Grasemann, H.; Bartlett, C.; Du, K.; Duan, R.; Shi, F.; Estrada, M.; Seigel, K.E.; Coates, A.L.; et al. Transducing Airway Basal Cells with a Helper-Dependent Adenoviral Vector for Lung Gene Therapy. Hum. Gene Ther. 2018, 29, 643–652. [Google Scholar] [CrossRef]
- Ternant, D.; Mulleman, D.; Degenne, D.; Willot, S.; Guillaumin, J.M.; Watier, H.; Goupille, P.; Paintaud, G. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther. Drug Monit. 2006, 28, 169–174. [Google Scholar] [CrossRef]
- Kern, J.C.; Cancilla, M.; Dooney, D.; Kwasnjuk, K.; Zhang, R.; Beaumont, M.; Figueroa, I.; Hsieh, S.; Liang, L.; Tomazela, D.; et al. Discovery of Pyrophosphate Diesters as Tunable, Soluble, and Bioorthogonal Linkers for Site-Specific Antibody-Drug Conjugates. J. Am. Chem. Soc. 2016, 138, 1430–1445. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bequignon, E.; Dhommée, C.; Angely, C.; Thomas, L.; Bottier, M.; Escudier, E.; Isabey, D.; Coste, A.; Louis, B.; Papon, J.-F.; et al. FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? Int. J. Mol. Sci. 2019, 20, 1379. https://doi.org/10.3390/ijms20061379
Bequignon E, Dhommée C, Angely C, Thomas L, Bottier M, Escudier E, Isabey D, Coste A, Louis B, Papon J-F, et al. FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? International Journal of Molecular Sciences. 2019; 20(6):1379. https://doi.org/10.3390/ijms20061379
Chicago/Turabian StyleBequignon, Emilie, Christine Dhommée, Christelle Angely, Lucie Thomas, Mathieu Bottier, Estelle Escudier, Daniel Isabey, André Coste, Bruno Louis, Jean-François Papon, and et al. 2019. "FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy?" International Journal of Molecular Sciences 20, no. 6: 1379. https://doi.org/10.3390/ijms20061379
APA StyleBequignon, E., Dhommée, C., Angely, C., Thomas, L., Bottier, M., Escudier, E., Isabey, D., Coste, A., Louis, B., Papon, J.-F., & Gouilleux-Gruart, V. (2019). FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? International Journal of Molecular Sciences, 20(6), 1379. https://doi.org/10.3390/ijms20061379





