Bioengineered Skin Intended for Skin Disease Modeling
Abstract
:1. General Considerations
1.1. Skin
1.2. Bioengineered Skin
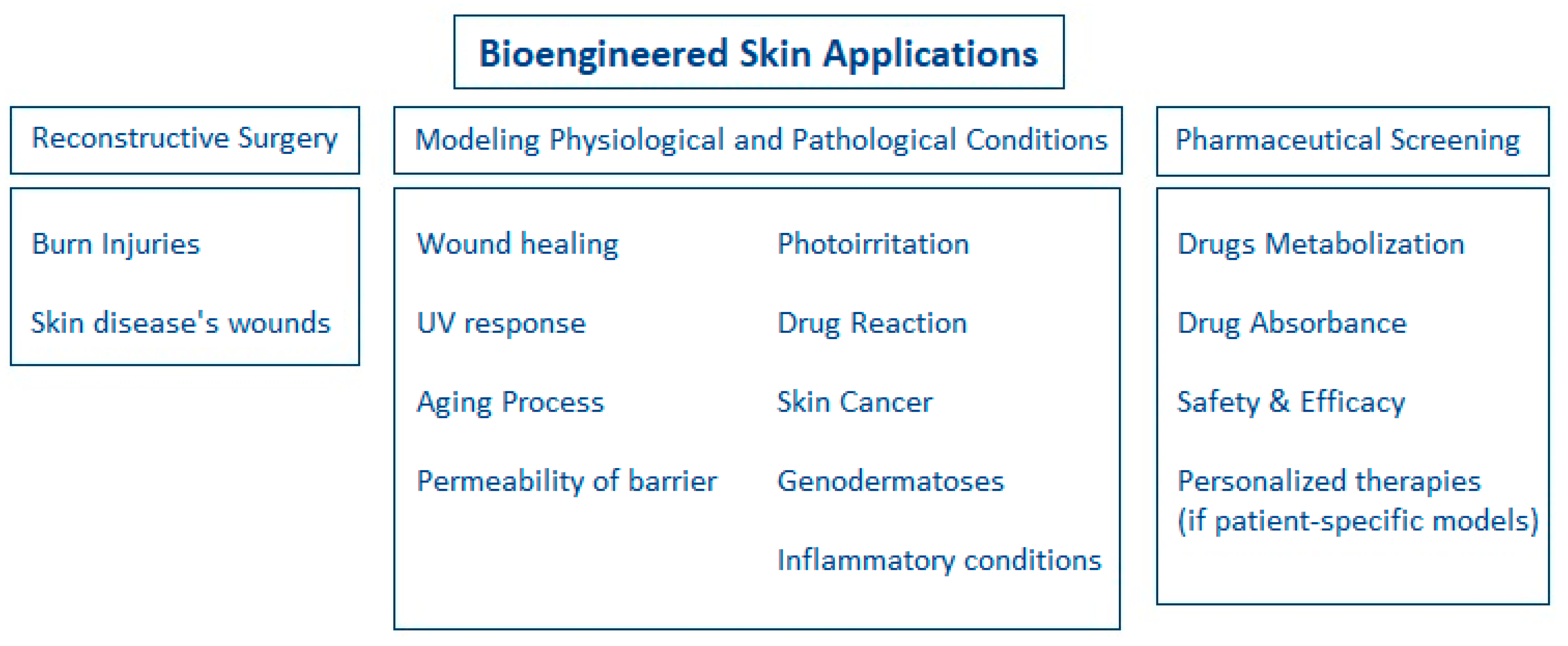
1.3. Applications of Bioengineered Skin
2. Skin Disease Models and Their Fabrication
2.1. Skin Disease Models
2.1.1. Monolayer Models
2.1.2. Reconstructed Human Epidermis (RHS)
2.1.3. De-Epidermalized Dermis (DED)
2.1.4. Collagen Hydrogels
2.1.5. Self-Assembled Models
2.1.6. Skin-on-Chip Models
2.2. Fabrication Methods
2.2.1. Manual Fabrication Methods
2.2.2. Automated Fabrication Methods
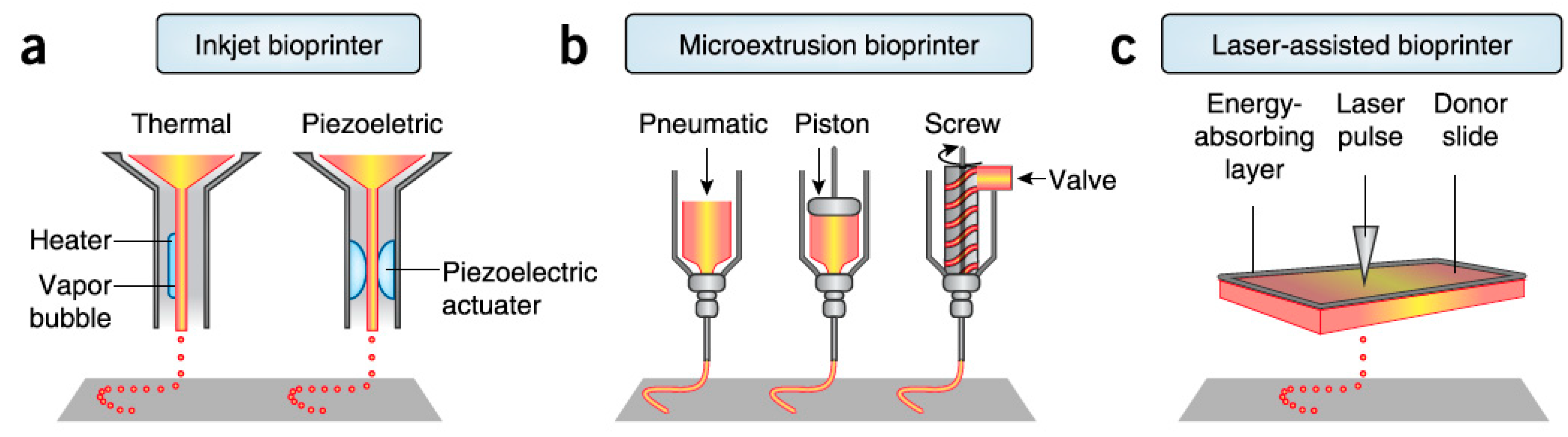
- Laser-assisted bioprinting exploits laser energy for the printing. Small droplets of cells are printed on a substrate that can be either a cell culture plate for generation of 2D construct or a scaffold for formation of 3D construct. Precise deposition of the cells in the 3D construct in a high density is achieved by this method while there are no limitations in biomaterials viscosity. Several dermo-epidermal skin substitutes have been already successful fabricated by this method [2,20]. A downside of laser-assisted bioprinting is the relatively low printing speed [2].
- Inkjet bioprinting is based on the ejection of bio-ink droplets on a substrate. Bio-ink contains cell suspension combined with hydrogels or biopolymers. In thermal inkjet bioprinting the droplets are pushed out due to bubbles generated in the nozzle by a heating element, while in piezoelectric inkjet bioprinting, electric pulses result in the droplets ejection [2,34]. Thermal inkjet printing is considered suitable for biological applications as the printed cells are heated at a temperature of less than 10 °C above ambient temperature and for only 2 microseconds, ensuring cell survival during the printing and a later cell viability of about 90%, while piezoelectric approach operates at frequencies that can harm the cells [34]. Inkjet bioprinting can achieve high resolutions and accuracy in deposition but it is efficient only when bio-inks of low viscosity are printed [2]. It has been used for the printing of keratinocytes on top of a previously extrusion-based printed dermal equivalent and it resulted in a very uniform epidermal layer in which keratinocytes quickly and properly proliferated and differentiated throughout the cultivation period [40].
- Extrusion bioprinting is based on the extrusion of a continuous strand of biopolymers or hydrogels, along with cellular components when desired, through a nozzle when mechanical force is applied. Simultaneous printing of cells, biomaterials and growth factors can be achieved in systems of more than one extruder, contributing to the generation of a more complex skin model. This approach is not considered faster than inkjet and laser-assisted bioprinting, but it is suitable for generation of anatomically relevant structures and sizes [2,37]. It also works with high cell density although shear stresses developed in the nozzle may reduce cell viability. Comparison between 3D extrusion bioprinting and manual deposition of skin components revealed the better long-term maintenance of skin equivalents shape and size in case of 3D printing approach [2]. Due to all these advantages, this method could be employed for the generation of dermis which ideally consists not only of fibroblasts and the dermal matrix, but also of several molecular and cellular components. For example, Byoung Soo Kim et al. used this method to fabricate collagen-based scaffolds, including or not polycaprolactone, to form the dermal component of a skin substitute, the epidermal layer of which was later created by inkjet-printing of keratinocytes, as mentioned above [40]. However, this method shorts on resolution capabilities compared to other bioprinting techniques, which affects the precision in cell spatial arrangement [41].
3. Skin Disease Modeling and Drug Screening
3.1. Modeling of Physiological Conditions
3.2. Modeling of Pathological Conditions
3.3. Evaluation of Compounds Safety and Efficacy
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Hoehn, K.; Marieb, E.N. Study Guide Human Anatomy & Physiology, 9th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001. [Google Scholar] [CrossRef] [PubMed]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, A. Anatomy and Function of the Skin. Nanosci. Dermatol. 2016, 1–14. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; Conti, C.J.; Zapatero-Solana, E.; Larcher, F.; Río, M.D. Current Applications for Bioengineered Skin. In Translating Regenerative Medicine to the Clinic; Academic Press: Cambridge, MA, USA, 2016; pp. 107–120. [Google Scholar]
- Böttcher-Haberzeth, S.; Biedermann, T.; Reichmann, E. Tissue engineering of skin. Burns 2010, 36, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 2002, 8, 787–798. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Lee, J.W.; Cho, D.-W. Extrusion Bioprinting. In Essentials of 3D Biofabrication and Translation; Academic Press: Cambridge, MA, USA, 2015; pp. 123–152. [Google Scholar]
- Bi, H.; Jin, Y. Current progress of skin tissue engineering: Seed cells, bioscaffolds, and construction strategies. Burns Trauma 2013, 1, 63–72. [Google Scholar] [CrossRef] [Green Version]
- El-Serafia, A.T.; El-Serafib, I.T.; Elmasry, M.; Steinvalld, I.; Sjöberg, F. Skin regeneration in three dimensions, current status, challenges and opportunities. Differentiation 2017, 96, 26–29. [Google Scholar] [CrossRef]
- Kastelein, K. Psoriasis-Overview. 2011. Available online: https://www.dermaharmony.com/Psoriasis (accessed on 19 March 2019).
- Guthrie, K.; Bruce, A.; Sangha, N.; Rivera, E.; Basu, J. Potency evaluation of tissue engineered and regenerative medicine products. Trends Biotechnol. 2013, 31, 505–514. [Google Scholar] [CrossRef]
- Lu, G.; Huang, S. Bioengineered skin substitutes: Key elements and novel design for biomedical applications. Int. Wound J. 2013, 10, 365–371. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W. Bioengineering skin using mechanisms of regeneration and repair. Biomaterials 2007, 28, 5100–5113. [Google Scholar] [CrossRef]
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbühl, J.; Schiestl, C.; Meuli, M.; Reichmann, E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part C Methods 2012, 18, 464–474. [Google Scholar] [CrossRef]
- Blozik, E.; Scherer, M. Skin replacement therapies for diabetic foot ulcers: Systematic review and meta-analysis. Diabetes Care 2008, 31, 693–694. [Google Scholar] [CrossRef]
- Fraunhofer Group, Beacon Projects. Available online: https://www.lifesciences.fraunhofer.de/en/leuchtturmprojekte/geschaeftsfeld_2/hautfabrik.html (accessed on 19 March 2019).
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Semlin, L.; Schäfer-Korting, M.; Borelli, C.; Korting, H.C. In vitro models for human skin disease. Drug Discov. Today 2011, 16, 132–139. [Google Scholar] [CrossRef]
- Garcia, M.; Escamez, M.J.; Carretero, M.; Mirones, I.; Martinez-Santamaria, L.; Navarro, M.; Jorcano, J.L.; Meana, A.; Rio, M.D.; Larcher, F. Modeling normal and pathological processes through skin tissue engineering. Mol. Carcinog. 2007, 46, 741–745. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Cañizo, J.F.D.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2016, 9, 015006. [Google Scholar] [CrossRef]
- Danilenko, D.M. Review paper: Preclinical models of psoriasis. Vet. Pathol. 2008, 45, 563–575. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; García, M.; Murillas, R.; Retamosa, L.; Illera, N.; Duarte, B.; Holguín, A.; Puig, S.; Hernández, M.I.; Meana, A.; et al. Development of a bioengineered skin-humanized mouse model for psoriasis: Dissecting epidermal-lymphocyte interacting pathways. Am. J. Pathol. 2010, 177, 3112–3124. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, M.; Panduri, S.; Donetti, E.; Prignano, F. Relevance of in vitro 3-D skin models in dissecting cytokine contribution to psoriasis pathogenesis. Histol. Histopathol. 2017, 32, 893–898. [Google Scholar] [CrossRef]
- Roy, B.; Simard, M.; Lorthois, I.; Bélanger, A.; Maheux, M.; Duque-Fernandez, A.; Rioux, G.; Simard, P.; Deslauriers, M.; Masson, L.-C.; et al. In vitro models for psoriasis. Skin Tissue Models 2018, 103–128. [Google Scholar] [CrossRef]
- Jean, J.; Lapointe, M.; Soucy, J.; Pouliot, R. Development of an in vitro psoriatic skin model by tissue engineering. J. Dermatol. Sci. 2009, 53, 19–25. [Google Scholar] [CrossRef]
- Capallere, C.; Plaza, C.; Meyrignac, C.; Arcioni, M.; Brulas, M.; Busuttil, V.; Garcia, I.; Bauza, E.; Botto, J.-M. Property characterization of reconstructed human epidermis equivalents, and performance as a skin irritation model. Toxicol. Vitro 2018, 53, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Asbill, C.; Kim, N.; El-Kattan, A.; Creek, K.; Wertz, P.; Michniak, B. Evaluation of a human bio-engineered skin equivalent for drug permeation studies. Pharm. Res. 2000, 17, 1092–1097. [Google Scholar] [CrossRef]
- Medalie, D.A.; Eming, S.A.; Tompkins, R.G.; Yarmush, M.L.; Krueger, G.G.; Morgan, J.R. Evaluation of human skin reconstituted from composite grafts of cultured keratinocytes and human acellular dermis transplanted to athymic mice. J. Investig. Dermatol. 1996, 107, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Araghi, B.H.; Beydaghi, V.; Geraili, A.; Moradi, F.; Jafari, P.; Janmaleki, M.; Valente, K.P.; Akbari, M.; Sanati-Nezhad, A. Skin Diseases Modeling using Combined Tissue Engineering and Microfluidic Technologies. Adv. Healthc. Mater. 2016, 5, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Engineered Polymeric Hydrogels for 3D Tissue Models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef]
- Brown, R.A.; Wiseman, M.; Chuo, C.-B.; Cheema, U.; Nazhat, S.N. Ultrarapid Engineering of Biomimetic Materials and Tissues: Fabrication of Nano-and Microstructures by Plastic Compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Patents Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Walles, H.; Pickert, D. The Skin Factory. 2011. Available online: https://www.soci.org/Chemistry-and-Industry/CnI-Data/2011/18/The-skin-factory.aspx (accessed on 19 March 2019).
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.; Tran, T.N.; Yoo, S.-S.; Dai, G.; Karande, P. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-dimensional electrospun silk-fibroin nanofiber for skin tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Schmid, F.F.; Schwarz, T.; Klos, M.; Schuberthan, W.; Walles, H.; Hansmann, J.; Groeber, F.K. Applicability of a Dual-Arm Robotic System for Automated Downstream Analysis of Epidermal Models. Appl. In Vitro Toxicol. 2016, 2, 118–124. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J.S.; Gao, G.; Cho, D.W. Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 2017, 9, 025034. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.G.; Pentoney, S.L. Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discov. Today Technol. 2017, 23, 37–44. [Google Scholar] [CrossRef]
- Fox, S.C.; Biedermann, T.; Schmid Daners, M.; Reichmann, E.; Meboldt, M. Introducing the SkinCreator—make bioengineered skin available for everyone—A Skintegrity Zurich project, eCM Meeting Abstracts 2017, Collection 2. In Proceedings of the eCM XVII, Personalised Therapies for Regenerative Medicine TERMIS-EU 2017 Conference, Davos, Switzerland, 26–30 June 2017. [Google Scholar]
- Fox, S.C.; Biedermann, T.; Polak, J.; Hu, J.; Schmid Daners, M.; Reichmann, E.; Meboldt, M. A simplified fabrication technique for cellularized high-collagen dermal equivalents. Biomed. Mater. 2019. [Google Scholar] [CrossRef]
- Hu, J. Patch Forming of Bioengineered Skin. Master Thesis, ETH Zurich, Zurich, Switzerland, August 2017. [Google Scholar]
- Sarkiri, M. Injection Molding of Bioengineered Skin. 2018. Available online: https://doi.org/10.3929/ethz-b-000286374 (accessed on 19 March 2019).
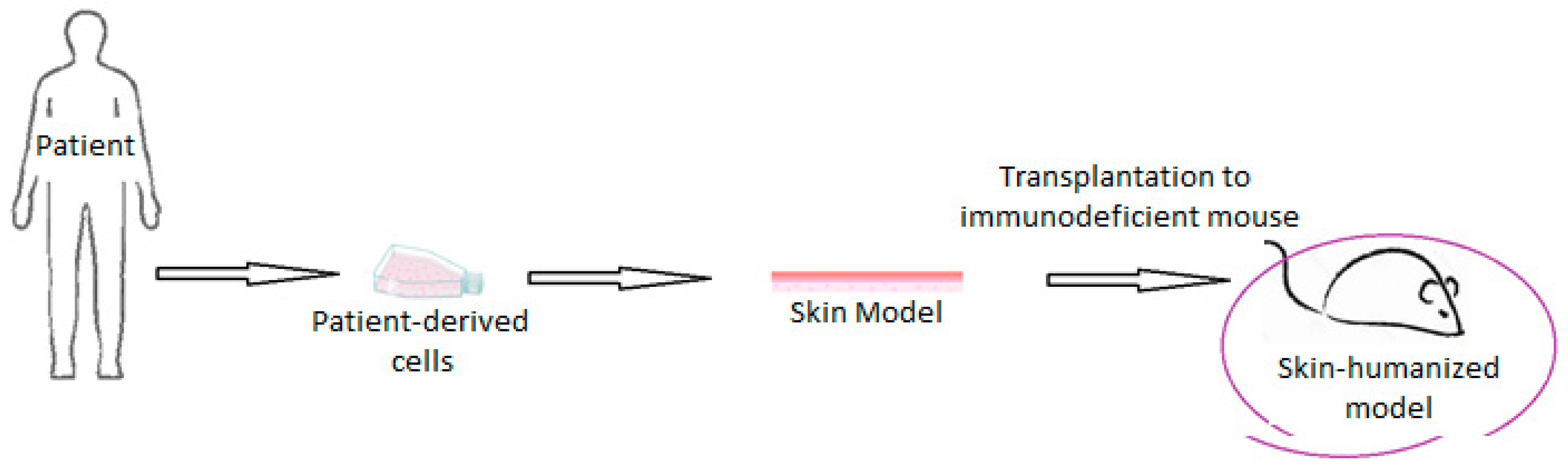
- Carretero, M.; Guerrero-Aspizua, S.; Del Rio, M.D. Applicability of bioengineered human skin: From preclinical skin humanized mouse models to clinical regenerative therapies. Bioeng. Bugs 2011, 2, 203–207. [Google Scholar] [CrossRef]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering-in vivo and in vitro applications. Clin. Plast. Surg. 2012, 39, 33–58. [Google Scholar] [CrossRef]
- Eungdamrong, N.J.; Higgins, C.; Guo, Z.; Lee, W.-H.; Gillette, B.; Sia, S.; Christiano, A.M. Challenges and promises in modeling dermatologic disorders with bioengineered skin. Exp. Biol. Med. 2014, 239, 1215–1224. [Google Scholar] [CrossRef]
- Goebel, C.; Kosemund-Meyen, K.; Gargano, E.M.; Politano, V.; von Bölcshazy, G.; Zupko, K.; Jaiswal, N.; Zhang, J.; Martin, S.; Neumann, D.; et al. Non-animal skin sensitization safety assessments for cosmetic ingredients—What is possible today? Curr. Opin. Toxicol. 2017. [Google Scholar] [CrossRef]
- Roguet, R.; Cohen, C.; Dossou, K.; Rougier, A. Episkin, a reconstituted human epidermis for assessing in vitro the irritancy of topically applied compounds. Toxicol. In Vitro 1994, 8, 283–291. [Google Scholar] [CrossRef]
- Damour, O.; Augustin, C.; Black, A.F. Applications of reconstructed skin models in pharmaco-toxicological trials. Med. Biol. Eng. Comput. 1998, 36, 825–832. [Google Scholar] [PubMed]
- Augustin, C.; Collombel, C.; Damour, O. Use of dermal equivalent and skin equivalent models for identifying phototoxic compounds in vitro. Photodermatol. Photoimmunol. Photomed. 1997, 13, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Schmook, F.P.; Meingassner, J.G.; Billich, A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int. J. Pharm. 2001, 215, 51–56. [Google Scholar]
- Monteiro-Riviere, N.A.; Inman, A.O.; Snider, T.H.; Blank, J.A.; Hobson, D.W. Comparison of an in vitro skin model to normal human skin for dermatological research. Microsc. Res. Tech. 1997, 37, 172–179. [Google Scholar] [CrossRef]
- Westcott, L. L’Oréal to Start Printing 3-D Skin. 2016. Available online: http://www.newsweek.com/loreal-start-printing-3-d-skin-bioengineering-company-334204 (accessed on 19 March 2019).
- Pham, C.; Greenwood, J.; Cleland, H.; Woodruff, P.; Maddern, G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007, 33, 946–957. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Shao, Y.; Mianecki, L.E.; Liao, E.; Perry, D.; Quan, T. Dermal fibroblast expression of stromal cell-derived factor-1 (SDF-1) promotes epidermal keratinocyte proliferation in normal and diseased skin. Protein Cell 2015, 6, 890–903. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Mitsuhashi, Y.; Matsuzaki, Y.; Kaneko, T.; Nakano, H.; Takeda, H.; Moritsugu, R.; Hanada, K. Altered keratinocyte-fibroblast interactions in psoriasis revealed by a combined cell-culture. Hirosaki Med. J. 2004, 56, 1–8. [Google Scholar]
- Saiag, P.; Coulomb, B.; Lebreton, C.; Bell, E.; Dubertret, L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science 1985, 230, 669–672. [Google Scholar]
- Wojas-Pelc, A.; Ciszek, M.; Kurnyta, M.; Marcinkiewicz, J. Cytokine network in psoriasis. Cross-talk between keratinocytes and cells of the skin immune system. Cent. Eur. J. Immunol. 2007, 31, 111–116. [Google Scholar]
- Ayata, R.E.; Bouhout, S.; Auger, M.; Pouliot, R. Study of in vitro capillary-like structures in psoriatic skin substitutes. BioRes. Open Access 2014, 3, 197–205. [Google Scholar] [CrossRef] [PubMed]




| Skin Model | Cells | Matrix | Advantages | Disadvantages |
|---|---|---|---|---|
| Monolayer models | Keratinocytes or fibroblasts | - | Differentiated epidermis | 2D environment, no cellular interactions |
| Reconstructed human epidermis | Keratinocytes | Polycarbonate | Differentiated epidermis, 3D environment | No cellular interactions |
| De-epidermalized dermis | Fibroblasts or fully acellular | Natural ECM | 3D environment, dermo-epidermal equivalent after keratinocytes seeding | Keratinocytes absence, limited availability |
| Collagen hydrogels | Fibroblasts (embedded in collagen hydrogels), keratinocytes (seeded on top of hydrogel) | Collagen I (can be combined with GAGs, chitosan or other collagen types) | 3D environment, dermo-epidermal equivalent, availability, easy production | No native ECM, contraction of hydrogels |
| Self-assembled models | Fibroblasts (embedded in collagen hydrogels), keratinocytes (seeded on top of hydrogel) | Natural ECM | 3D environment, dermo-epidermal equivalent, fully autologous skin model | Slow and tedious process |
| Skin-on-chip models | Fibroblasts, keratinocytes, endothelial cells, other organs’ cell types | Porous membranes, scaffolds, or other | 3D environment, interactions between different cell types or organs | Complex systems, no native ECM |
| Fabrication Method | Potentials | Limitations |
|---|---|---|
| Manual fabrication | Incorporation of several cells/molecules, personalization opportunities, fast adaptation to research needs | Slow and tedious process, non-standardized method |
| Fabrication by robots | Incorporation of several cells/molecules, personalization opportunities, standardized production | Slow process, high-complexity and decreased adaptability |
| 3D bioprinting | Incorporation of several cells/molecules, personalization opportunities, standardized production, faster process | High-complexity and decreased adaptability, expensive |
| Automated injection molding | Personalization opportunities, standardized production, non-complex process, faster than manual or robotic production | Still slow process, validated only for dermis fabrication yet |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkiri, M.; Fox, S.C.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bioengineered Skin Intended for Skin Disease Modeling. Int. J. Mol. Sci. 2019, 20, 1407. https://doi.org/10.3390/ijms20061407
Sarkiri M, Fox SC, Fratila-Apachitei LE, Zadpoor AA. Bioengineered Skin Intended for Skin Disease Modeling. International Journal of Molecular Sciences. 2019; 20(6):1407. https://doi.org/10.3390/ijms20061407
Chicago/Turabian StyleSarkiri, Maria, Stephan C. Fox, Lidy E. Fratila-Apachitei, and Amir A. Zadpoor. 2019. "Bioengineered Skin Intended for Skin Disease Modeling" International Journal of Molecular Sciences 20, no. 6: 1407. https://doi.org/10.3390/ijms20061407
APA StyleSarkiri, M., Fox, S. C., Fratila-Apachitei, L. E., & Zadpoor, A. A. (2019). Bioengineered Skin Intended for Skin Disease Modeling. International Journal of Molecular Sciences, 20(6), 1407. https://doi.org/10.3390/ijms20061407







