A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine
Abstract
1. Introduction
2. Chemical Composition
2.1. Astragalus Triterpene Saponins
2.2. Astragalus Flavonoids
2.3. Astragalus Polysaccharides (APS)
2.4. Other Constituents
3. Pharmacological Activities
3.1. Effects of AR and Its Main Components on Cardiovascular Diseases
3.2. Effects of AR and its Main Components on Diabetes Mellitus
3.2.1. Effects of AR and Its Main Components on T1DM
3.2.2. Effects of AR and Its Main Components on T2DM
3.3. Effects of AR and Its Main Components on Cancer
3.4. Effects of AR and Its Main Components on Respiratory Diseases
3.5. Effects of AR and Its Main Components on Nervous System Diseases
3.6. Other Pharmacological Activities
4. Pharmacokinetic Studies
4.1. Pharmacokinetic Studies on AR Extracts
4.2. Pharmacokinetic Behaviors of Bioactive Compounds from AR
4.2.1. Drug-Metabolizing Enzymes and Drug Transporters
4.2.2. Formononetin
4.2.3. Calycosin-7-β-d-Glucoside (CG)
4.2.4. Ononin
4.2.5. Astragaloside IV (AS-IV)
5. AR as Personalized Medicine
6. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR | Astragali radix |
| BB | Bioavailability barrier |
| DMEs | Drug-metabolizing enzymes |
| ETs | Efflux transporters |
| HPGPC | High-performance gel-permeation chromatography |
| GC | Chromatography |
| NF-κB | Nuclear factor-kappa B |
| TLR4 | Toll-like receptor 4 |
| PGC-1α | Peroxisome proliferator-activated receptor-γ coactivator 1 α |
| NFATc3 | Nuclear factor of activated T cell scytoplasmic 3 |
| CaMKII | Calmodulin II kinase |
| SERCA2a | Sarcoplasmic reticulum Ca2+-ATPase |
| PPARα | Proliferator-activatedαreceptor |
| α-SMA | α-smooth muscle actin |
| ECM | Extracellular matrix |
| Col-1 | Colgen I |
| TRPM | Transient receptor potential channel |
| TRPC6 | Transient receptor potential channel 6 |
| ROS | Reactive oxygen species |
| CT-1 | Cardiotrophin-1 |
| HO-1 | Heme oxygenase-1 |
| NQO1 | NAD(P)H dehydrogenase (quinone) 1 |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| LPS | Lipopolysaccharide |
| tBHP | Tert-butyl hydroperoxide |
| SOD | Superoxide dismutase |
| CHD | Coronary heart disease |
| NOD | Non-obese diabetic |
| gal-1 | Galectin-1 |
| Glut4 | Glucose transporter 4 |
| AMPK | AMP-activated protein kinase |
| PKB | Protein kinase B |
| PTP1B | Protein tyrosine phosphatase 1B |
| ER | Endoplasmic reticulum |
| ATF6 | Transcription activator 6 |
| MOMP | Mitochondrial extracorporeal membrane permeability |
| PPAR | Peroxisome proliferator-activated receptor |
| MCP-1 | Monocyte chemotactic protein-1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| iNOS | Inducible nitric oxide synthase |
| EMT | Epithelial-mesenchymal transition |
| STZ | Streptozotocin |
| IGF1 | Insulin-like growth factor 1 |
| PI3K | Phosphatidylinositol3-kinase |
| Akt | Protein kinase B |
| GRP78 | Glucose-regulated protein 78 |
| FasL | Fas ligand |
| HDAC5 | Histone deacetylase 5 |
| CAV-1 | Caveolin-1 |
| TGF-β | Transforming growth factor-β |
| ECM | Extracellular matrix |
| LDHA | Lactate dehydrogenase A |
| TIGAR | TP53-induced glycolysis and apoptosis regulator |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signalregulated kinase |
| TNF | Tumor necrosis factor |
| IFN | Interferon |
| KMT2D | Histone-lysine N-methyltransferase 2Dlysine (K) -specific methyltransferase 2D |
| CREBBP | CREB binding protein |
| Foxo3a | Forkhead box O transcription factor 3a |
| BIP | Binding immunoglobulin protein |
| CHOP | Chomologous protein |
| EGFL7 | Epidermal growth factor-like domain 7 |
| HK-II | Hexokinase-II |
| mPTP | Mitochondrial permeability transition pore |
| PD | Parkinson’s disease |
| TH | Tyrosine hydroxylase |
| MPTP | 1-methyl-4-phenyl-1 |
| 6-OHDA | 6-hydroxydopamine |
| IRS1 | Insulin receptor substrate 1 |
| APS | Astragalus Polysaccharides |
| AS-IV | Astragaloside IV |
| ISO | Isoproterenol |
| CG | Calycosin-7-β-glucoside |
| C-3′-G | Calycosin-3′-glucuronide |
| CG-3′-G | CG-3′-glucuronide |
| F-7-G | Formononetin-7-glucuronide |
| D-7-G | Daidzein-7-glucuronide |
| CYPs | Cytochromes P450 |
| SULT | sulfotransferase |
| GST | GlutathioneS-transferase |
| UGT | Uridine 5′-diphospho (UDP)-glucuronosyltransferase |
| NATs | N-acetyltransferases |
| P-gp | P-glycoprotein |
| MRP2 | Multidrug resistance-associated protein 2 |
| BCRP | Breast cancer resistance protein |
| MRPs | Multidrug resistance-related proteins |
| OATs | Organic anion transporters |
| SGLT-1 | Sodium-dependent glucose transporter 1 |
| BSβG | Broad-specific β-glucuronides |
| LPH | Lactase phlorizin hydrolase |
| Bra B | Brachyoside B |
| Cyc B | Cyclogaleginoside B |
| CA | Cycloastragenol |
| iso-CA | Iso-cycloastragenol |
| CA-2H | Dehydrogenated metabolite of CA |
| AUC | Area under the curve |
| T1/2 | Elimination half-life |
| CL | Clearance |
| Vd | Apparent volume of distribution |
| MRT | Mean residence time |
References
- Yu, W.; Zhao, H.; Zong, X.; Ji, X.; Han, X.; Wang, Y.; Zhang, Y.; Ma, K.; Cui, N.; Wang, S. The effects of radix Astragali water abstract on energy metabolism in rat Yang-Deficiency cold syndrome model through PPAR signaling pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 9194362. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Guo, Q.S.; Shi, H.Z.; Shi, G.W.; Yan, S.M.; Wu, M.J.; Wang, S.; Lu, X.; Chen, P.P.; Yan, X.L. Effect of Astragali radix on growth, immunity and related gene expression of Whitmania pigra. Zhongguo Zhong Yao Za Zhi 2018, 43, 3611–3617. [Google Scholar] [PubMed]
- Zhang, L.; Gong, A.G.; Riaz, K.; Deng, J.Y.; Ho, C.M.; Lin, H.Q.; Dong, T.T.; Lee, Y.K.; Tsim, K.W. A novel combination of four flavonoids derived from Astragali radix relieves the symptoms of cyclophosphamide-induced anemic rats. FEBS Open Bio 2017, 7, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, Y.; Xu, Y.; Guo, X.; Li, X.; Zhang, A.L.; May, B.H.; Xue, C.C.; Wen, Z.; Lin, L. Oral huangqi formulae for stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2013, 2013, 705315. [Google Scholar] [PubMed]
- Yu, J.; Ji, H.Y.; Liu, A.J. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.M.; Gao, J.W.; Shi, Z.; Huang, P.; Lu, Y.S.; Yao, M.C.; Huang, M. Herb-drug pharmacokinetic interaction between radix Astragali and pioglitazone in rats. J. Ethnopharmacol. 2012, 144, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.Z.; Hao, J.Q.; Hao, X.L.; Feng, M.L. Preparation of Astragalus membranaceus lectin and evaluation of its biological function. Biomed. Rep. 2018, 9, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhu, J.Z.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Huang, Y.Y.; Zhang, Q.H.; Zhang, K.J.; Zheng, G.Q.; Wang, Y. A preclinical systematic review and meta-analysis of astragaloside IV for myocardial ischemia/reperfusion injury. Front. Physiol. 2018, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Jiang, M.; Zhao, J.; Fan, C.; Wang, Y.; Peng, W. Huangqi (astragalus) decoction ameliorates diabetic nephropathy via IRS1-PI3K-GLUT signaling pathway. Am. J. Transl. Res. 2018, 10, 2491–2501. [Google Scholar]
- Zheng, Y.; Dai, Y.; Liu, W.; Wang, N.; Cai, Y.; Wang, S.; Zhang, F.; Liu, P.; Chen, Q.; Wang, Z. Astragaloside IV enhances taxol chemosensitivity of breast cancer via caveolin-1-targeting oxidant damage. J. Cell. Physiol. 2019, 234, 4277–4290. [Google Scholar] [CrossRef]
- Jin, H.; Luo, Q.; Zheng, Y.; Nurahmat, M.; Wu, J.; Li, B.; Lv, Y.; Wang, G.; Duan, X.; Dong, J. CD4+CD25+Foxp3+ T cells contribute to the antiasthmatic effects of Astragalus membranaceus extract in a rat model of asthma. Int. Immunopharmacol. 2013, 15, 42–49. [Google Scholar] [CrossRef]
- Qu, Y.Z.; Li, M.; Zhao, Y.L.; Zhao, Z.W.; Wei, X.Y.; Liu, J.P.; Gao, L.; Gao, G.D. Astragaloside IV attenuates cerebral ischemia-reperfusion-induced increase in permeability of the blood-brain barrier in rats. Eur. J. Pharmacol. 2009, 606, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.J. Protective capability of Astragalus (Huangqi) on auditory function in a rat model of estrogen deficiency. Chin. Med. J. 2019, 132, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K.; Chan, K. Evaluation of the pharmaceutical properties and value of Astragali radix. Medicines 2018, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, T.; Li, Q.; Chen, X.; Bi, K. Simultaneous determination of five isoflavonoids in commercial radix Astragali by high performance liquid chromatography. Chin. J. Chromatogr. 2006, 24, 486–488. [Google Scholar] [CrossRef]
- Viktorov, G.I. The cardiovascular system in infectious diseases. Ter. Arkh. 1988, 60, 144–148. [Google Scholar] [PubMed]
- Cui, Y.; Wang, Q.; Sun, R.; Guo, L.; Wang, M.; Jia, J.; Xu, C.; Wu, R. Astragalus membranaceus (Fisch.) Bunge repairs intestinal mucosal injury induced by LPS in mice. BMC Complement. Altern. Med. 2018, 18, 230. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, W.; Wei, Q.; Chen, P.; Li, Y. Cytoprotective effects of the medicinal herb Astragalus membranaceus on lipopolysaccharideexposed cells. Mol. Med. Rep. 2018, 18, 4321–4327. [Google Scholar]
- Shi, J.; Zheng, L.; Lin, Z.; Hou, C.; Liu, W.; Yan, T.; Zhu, L.; Wang, Y.; Lu, L.; Liu, Z. Study of pharmacokinetic profiles and characteristics of active components and their metabolites in rat plasma following oral administration of the water extract of Astragali radix using UPLC-MS/MS. J. Ethnopharmacol. 2015, 169, 183–194. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Zeng, X.; Wu, H.; Su, W.; He, J. Identification and pharmacokinetics of multiple potential bioactive constituents after oral administration of radix Astragali on cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Mol. Sci. 2015, 16, 5047–5071. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhao, J.B.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Simultaneous determination of calycosin-7-O-beta-d-glucoside, ononin, calycosin, formononetin, astragaloside IV and astragaloside II in rat plasma after oral administration of radix Astragali extraction for their pharmacokinetic studies by ultra-pressure liquid chromatography with tandem mass spectrometry. Cell Biochem. Biophys. 2014, 70, 677–686. [Google Scholar] [PubMed]
- Xu, F.; Zhang, Y.; Xiao, S.; Lu, X.; Yang, D.; Yang, X.; Li, C.; Shang, M.; Tu, P.; Cai, S. Absorption and metabolism of Astragali radix decoction: In silico, in vitro and a case study in vivo. Drug Metab. Dispos. 2006, 34, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 2007, 3, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sparidans, R.W.; Wang, Y.; Lebre, M.C.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. P-glycoprotein (MDR1/ABCB1) restricts brain accumulation and cytochrome P450-3A (CYP3A) limits oral availability of the novel ALK/ROS1 inhibitor lorlatinib. Int. J. Cancer 2018, 143, 2029–2038. [Google Scholar] [CrossRef]
- Benet, L.Z.; Izumi, T.; Zhang, Y.; Silverman, J.A.; Wacher, V.J. Intestinal MDR transport proteins and P-450 enzymes as barriers to oral drug delivery. J. Control. Release 1999, 62, 25–31. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus Membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef]
- Song, J.Z.; Mo, S.F.; Yip, Y.K.; Qiao, C.F.; Han, Q.B.; Xu, H.X. Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in radix Astragali by high performance liquid chromatography. J. Sep. Sci. 2007, 30, 819–824. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Zhang, K.; Pugliese, M.; Pugliese, A.; Passantino, A. Biological active ingredients of traditional Chinese herb Astragalus membranaceus on treatment of diabetes: A systematic review. Mini Rev. Med. Chem. 2015, 15, 315–329. [Google Scholar]
- Wang, D.; Zhuang, Y.; Tian, Y.; Thomas, G.N.; Ying, M.; Tomlinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid. Med. Cell. Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef] [PubMed]
- Baratta, M.T.; Ruberto, G. Cycloartane triterpene glycosides from Astragalus siculus. Planta Med. 1997, 63, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Yesilada, E.; Bedir, E.; Calis, I.; Takaishi, Y.; Ohmoto, Y. Effects of triterpene saponins from Astragalus species on in vitro cytokine release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Calis, I.; Yuruker, A.; Tasdemir, D.; Wright, A.D.; Sticher, O.; Luo, Y.D.; Pezzuto, J.M. Cycloartane triterpene glycosides from the roots of Astragalus melanophrurius. Planta Med. 1997, 63, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhao, L.G.; Liang, J.; Guo, L.; Yang, Y.L.; Hu, F.; Zhu, R.J.; Feng, S.L. Component analysis and structure identification of active substances for anti-gastric ulcer effects in radix Astragali by liquid chromatography and tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 960, 43–51. [Google Scholar] [CrossRef]
- Qi, L.W.; Yu, Q.T.; Yi, L.; Ren, M.T.; Wen, X.D.; Wang, Y.X.; Li, P. Simultaneous determination of 15 marker constituents in various radix Astragali preparations by solid-phase extraction and high-performance liquid chromatography. J. Sep. Sci. 2008, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Teyeb, H.; Masullo, M.; Mari, A.; Douki, W.; Piacente, S. LC-ESI-MS quali-quantitative determination of phenolic constituents in different parts of wild and cultivated Astragalus gombiformis. J. Pharm. Biomed. Anal. 2013, 72, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Guerrini, M.; El-Sebakhy, N.A.; Asaad, A.M.; Toaima, S.M.; Abou-Sheer, M.E.; Luo, Y.D.; Pezzuto, J.M. Cycloartane saponins from Astragalus peregrinus as modulators of lymphocyte proliferation. Fitoterapia 2001, 72, 894–905. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Li, J.; Tang, S.; Wang, M.; Huang, W.; Yao, W.; Gao, X. A polysaccharide extracted from Astragalus membranaceus residue improves cognitive dysfunction by altering gut microbiota in diabetic mice. Carbohydr. Polym. 2019, 205, 500–512. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef]
- Yin, J.; Chan, B.; Yu, H.; Lau, I.; Han, X. Separation, structure characterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef]
- Shao, B.M.; Xu, W.; Dai, H.; Tu, P.; Li, Z.; Gao, X.M. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem. Biophys. Res. Commun. 2004, 320, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ji, H.; Dong, X.; Feng, Y.; Liu, A. Apoptosis of human gastric carcinoma MGC-803 cells induced by a novel Astragalus membranaceus polysaccharide via intrinsic mitochondrial pathways. Int. J. Biol. Macromol. 2019, 126, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Structural characterization of an intestinal immune system-modulating arabino-3,6-galactan-like polysaccharide from the above-ground part of Astragalus membranaceus (Bunge). Carbohydr. Polym. 2016, 136, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Bedir, E.; Calis, I.; Aquino, R.; Piacente, S.; Pizza, C. Cycloartane triterpene glycosides from the roots of Astragalus brachypterus and Astragalus microcephalus. J. Nat. Prod. 1998, 61, 1469–1472. [Google Scholar] [CrossRef]
- Horo, I.; Bedir, E.; Masullo, M.; Piacente, S.; Ozgokce, F.; Alankus-Caliskan, O. Saponins from Astragalus hareftae (NAB.) SIRJ. Phytochemistry 2012, 84, 147–153. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Li, Q.; Li, P.; Qin, X.M.; Chen, S. A practical quality control method for saponins without UV absorption by UPLC-QDA. Front. Pharmacol. 2018, 9, 1377. [Google Scholar] [CrossRef]
- Gong, L.; Chang, H.; Zhang, J.; Guo, G.; Shi, J.; Xu, H. Astragaloside IV protects rat cardiomyocytes from hypoxia-induced injury by down-regulation of miR-23a and miR-92a. Cell. Physiol. Biochem. 2018, 49, 2240–2253. [Google Scholar] [CrossRef]
- Wen, W.; Chen, J.; Ding, L.; Luo, X.; Zheng, X.; Dai, Q.; Gu, Q.; Liu, C.; Liang, M.; Guo, X.; et al. Astragaloside exerts anti-photoaging effects in UVB-induced premature senescence of rat dermal fibroblasts through enhanced autophagy. Arch. Biochem. Biophys. 2018, 657, 31–40. [Google Scholar] [CrossRef]
- Costa, I.M.; Lima, F.O.V.; Fernandes, L.C.B.; Norrara, B.; Neta, F.I.; Alves, R.D.; Cavalcanti, J.; Lucena, E.E.S.; Cavalcante, J.S.; Rego, A.C.M.; et al. Astragaloside IV supplementation promotes a neuroprotective effect in experimental models of neurological disorders: A systematic review. Curr. Neuropharmacol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Matkowski, A.; Wozniak, D.; Lamer-Zarawska, E.; Oszmianski, J.; Leszczynska, A. Flavonoids and phenol carboxylic acids in the oriental medicinal plant Astragalus membranaceus acclimated in Poland. Z. Naturforsch. C 2003, 58, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; He, X.G.; Lindenmaier, M.; Nolan, G.; Yang, J.; Cleary, M.; Qiu, S.X.; Cordell, G.A. Liquid chromatography-electrospray ionization mass spectrometry study of the flavonoids of the roots of Astragalus mongholicus and A. membranaceus. J. Chromatogr. A 2000, 876, 87–95. [Google Scholar] [CrossRef]
- Choi, S.I.; Heo, T.R.; Min, B.H.; Cui, J.H.; Choi, B.H.; Park, S.R. Alleviation of osteoarthritis by calycosin-7-O-beta-d-glucopyranoside (CG) isolated from Astragali radix (AR) in rabbit osteoarthritis (OA) model. Osteoarthritis Cartil. 2007, 15, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, X.; Ma, Y.F.; Huang, Y.F. Protective effect of calycosin-7-O-beta-D-glucopyranoside against oxidative stress of BRL-3A cells induced by thioacetamide. Pharmacogn. Mag. 2015, 11, 524–532. [Google Scholar]
- Gao, J.; Liu, Z.J.; Chen, T.; Zhao, D. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of radix Astragali. Pharm. Biol. 2014, 52, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, F.; Niu, Y.; Wu, X.; Pan, Y. A comparative study on the effect of promoting the osteogenic function of osteoblasts using isoflavones from radix Astragalus. Phytother. Res. 2018, 32, 115–124. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.; Yang, Z.; Liu, A. Relationship between structural properties and antitumor activity of Astragalus polysaccharides extracted with different temperatures. Int. J. Biol. Macromol. 2019, 124, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Liu, H.B.; Wang, J. Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed. Pharmacother. 2018, 97, 1468–1478. [Google Scholar] [CrossRef]
- Leng, B.; Tang, F.; Lu, M.; Zhang, Z.; Wang, H.; Zhang, Y. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-kappaB signaling pathway. Life Sci. 2018, 209, 111–121. [Google Scholar] [CrossRef]
- Jin, C.; Dai, R.H. Effect of Astragalus membranaceus on erythrocyte sodium content and sodium transport in the coronary heart disease. Zhong Xi Yi Jie He Za Zhi 1991, 11, 651–653. [Google Scholar]
- Qin, H.; Liu, P.; Lin, S. Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE(-/-) mice induced by hyperlipaemia. Evid. Based Complement. Altern. Med. 2015, 2015, 385154. [Google Scholar] [CrossRef]
- Jia, G.; Leng, B.; Wang, H.; Dai, H. Inhibition of cardiotrophin1 overexpression is involved in the antifibrotic effect of astrogaloside IV. Mol. Med. Rep. 2017, 16, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.L.; Liang, X.C. Astragalus membranaceus injection combined with conventional treatment for viral myocarditis: A systematic review of randomized controlled trials. Chin. J. Integr. Med. 2014, 20, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Lairez, O. Hypertrophic cardiomyopathies. Rev. Med. Interne 2019. [Google Scholar] [CrossRef] [PubMed]
- Gerondakis, S.; Grumont, R.; Gugasyan, R.; Wong, L.; Isomura, I.; Ho, W.; Banerjee, A. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene 2006, 25, 6781–6799. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Baltimore, D. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 2006, 210, 171–186. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
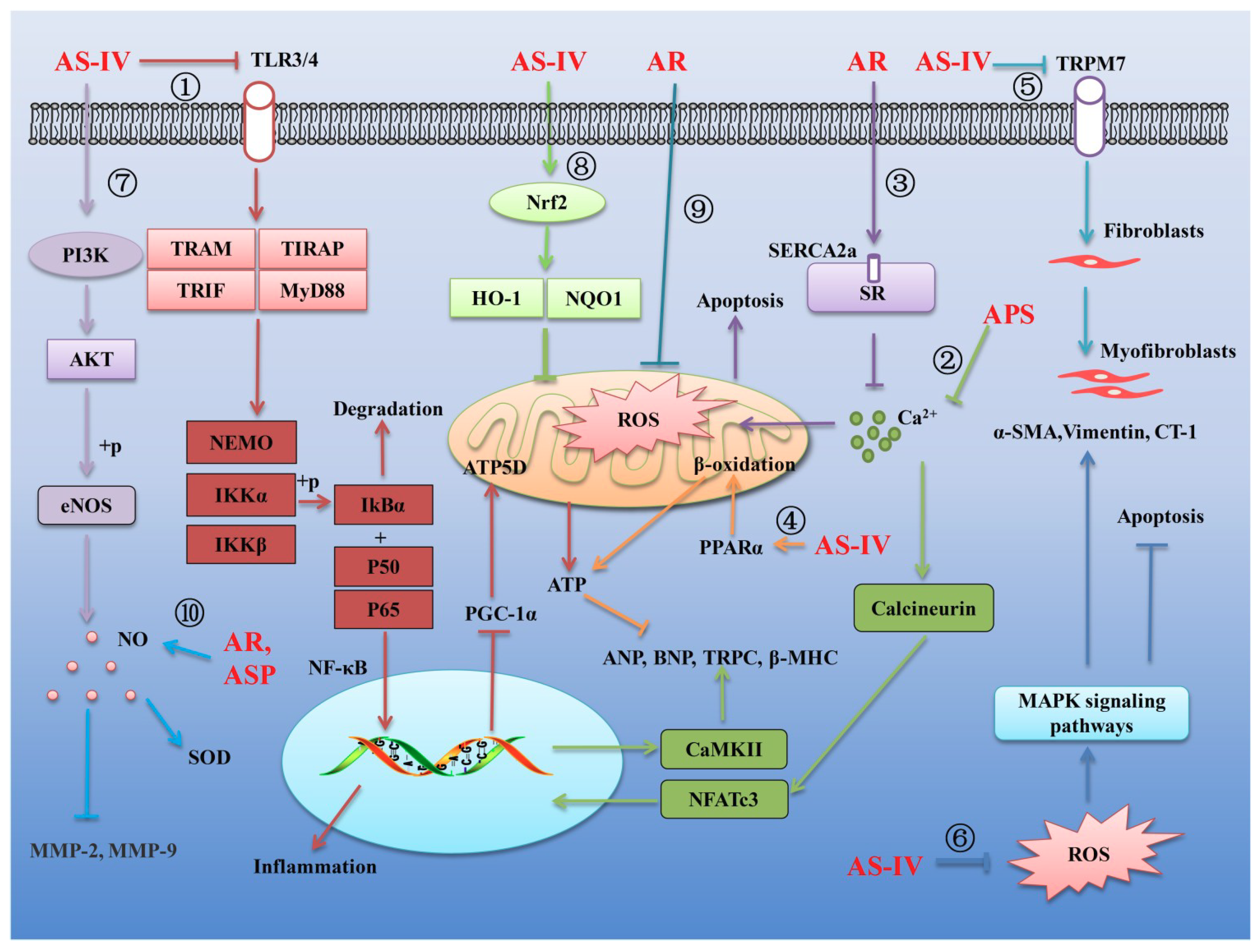
- Yang, J.; Wang, H.X.; Zhang, Y.J.; Yang, Y.H.; Lu, M.L.; Zhang, J.; Li, S.T.; Zhang, S.P.; Li, G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-small ka, CyrillicB signaling pathway in isoproterenol-induced myocardial hypertrophy. J. Ethnopharmacol. 2013, 150, 1062–1070. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, F.; Yang, Y.; Lu, M.; Luan, A.; Zhang, J.; Yang, J.; Wang, H. Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-kappaB/PGC-1alpha signaling mediated energy biosynthesis. PLoS ONE 2015, 10, e0118759. [Google Scholar]
- Dai, H.; Jia, G.; Liu, X.; Liu, Z.; Wang, H. Astragalus polysaccharide inhibits isoprenaline-induced cardiac hypertrophy via suppressing Ca2+-mediated calcineurin/NFATc3 and CaMKII signaling cascades. Environ. Toxicol. Pharmacol. 2014, 38, 263–271. [Google Scholar] [CrossRef]
- Su, D.; Li, H.Y.; Yan, H.R.; Liu, P.F.; Zhang, L.; Cheng, J.H. Astragalus improved cardiac function of adriamycin-injured rat hearts by upregulation of SERCA2a expression. Am. J. Chin. Med. 2009, 37, 519–529. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, P.; Xu, M.; Zhang, C.; Guo, W.; Chen, H.; Tian, J.; Wei, H.; Lu, R.; Cao, T. Astragaloside IV alleviates heart failure via activating PPARalpha to switch glycolysis to fatty acid beta-oxidation. Sci. Rep. 2017, 7, 2691. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Q.Y.; Zhou, Y.; Lu, X.C.; Liu, Y.H.; Wu, Y.; Guo, Q.; Ma, Y.T.; Tang, Y.Q. Astragaloside against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine 2017, 30, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Jia, G.; Lu, M.; Liang, C.; Wang, Y.; Wang, H. Astragaloside IV inhibits isoprenalineinduced cardiac fibrosis by targeting the reactive oxygen species/mitogenactivated protein kinase signaling axis. Mol. Med. Rep. 2017, 15, 1765–1770. [Google Scholar] [CrossRef]
- Lin, X.P.; Cui, H.J.; Yang, A.L.; Luo, J.K.; Tang, T. Astragaloside IV improves vasodilatation function by regulating the PI3K/Akt/eNOS signaling pathway in rat aorta endothelial cells. J. Vasc. Res. 2018, 55, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Iantorno, M.; Hays, A.G.; Schar, M.; Krishnaswamy, R.; Soleimanifard, S.; Steinberg, A.; Stuber, M.; Gerstenblith, G.; Weiss, R.G. Simultaneous noninvasive assessment of systemic and coronary endothelial function. Circ. Cardiovasc. Imaging 2016, 9, e003954. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; deMuinck, E.D.; Zhuang, Z.; Drinane, M.; Kauser, K.; Rubanyi, G.M.; Qian, H.S.; Murata, T.; Escalante, B.; Sessa, W.C. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment and blood flow reserve. Proc. Natl. Acad. Sci. USA 2005, 102, 10999–11004. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, F.; Jin, J.; Wu, H.; Zhang, B.; Wang, Z.; Shi, H.; Wu, X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018, 340, 58–66. [Google Scholar] [CrossRef]
- Huang, Y.; Kwan, K.K.L.; Leung, K.W.; Wang, H.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K. The extracts and major compounds derived from Astragali radix alter mitochondrial bioenergetics in cultured cardiomyocytes: Comparison of various polar solvents and compounds. Int. J. Mol. Sci. 2018, 19, 1574. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.H.; Zhang, B.Q.; Lian, M.J.; Xie, X.J.; Chen, P. Vascular protective effects of Astragalus membranaceus and its main constituents in rats with chronic hyperhomocysteinemia. Exp. Ther. Med. 2017, 14, 2401–2407. [Google Scholar] [CrossRef]
- Cho, J.; D’Antuono, M.; Glicksman, M.; Wang, J.; Jonklaas, J. A review of clinical trials: Mesenchymal stem cell transplant therapy in type 1 and type 2 diabetes mellitus. Am. J. Stem Cells 2018, 7, 82–93. [Google Scholar] [PubMed]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef]
- van den Broek, T.; Borghans, J.A.M.; van Wijk, F. The full spectrum of human naive T cells. Nat. Rev. Immunol. 2018, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Qiu, S.D.; Chen, H.X.; Tian, H.; Liu, G.Q. Effect of Astragalus polysaccharide on pancreatic cell mass in type 1 diabetic mice. Zhongguo Zhong Yao Za Zhi 2007, 32, 2169–2173. [Google Scholar] [PubMed]
- Sang, Z.; Zhou, L.; Fan, X.; McCrimmon, R.J. Radix Astragali (huangqi) as a treatment for defective hypoglycemia counterregulation in diabetes. Am. J. Chin. Med. 2010, 38, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Qiu, S.D.; Chen, H.X.; Tian, H.; Wang, H.X. The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol. Pharm. Bull. 2007, 30, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Yu, M. Astragalus polysaccharides: An effective treatment for diabetes prevention in NOD mice. Exp. Clin. Endocrinol. Diabetes 2008, 116, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Y.; Yang, G.; Li, F. Increased galectin-1 expression in muscle of Astragalus polysaccharide-treated Type 1 diabetic mice. J. Nat. Med. 2011, 65, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, X.; Zhang, T.; Li, Q.; Zhang, J.; Zhao, J. Astragalus polysaccharide improves insulin sensitivity via AMPK activation in 3T3-L1 Adipocytes. Molecules 2018, 23, 2711. [Google Scholar] [CrossRef]
- Zou, F.; Mao, X.Q.; Wang, N.; Liu, J.; Ou-Yang, J.P. Astragalus polysaccharides alleviates glucose toxicity and restores glucose homeostasis in diabetic states via activation of AMPK. Acta Pharmacol. Sin. 2009, 30, 1607–1615. [Google Scholar] [CrossRef]
- Ke, B.; Ke, X.; Wan, X.; Yang, Y.; Huang, Y.; Qin, J.; Hu, C.; Shi, L. Astragalus polysaccharides attenuates TNF-alpha-induced insulin resistance via suppression of miR-721 and activation of PPAR-gamma and PI3K/AKT in 3T3-L1 adipocytes. Am. J. Transl. Res. 2017, 9, 2195–2206. [Google Scholar] [PubMed]
- Jope, R.S.; Johnson, G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004, 29, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Z.; Gao, J.; Shi, W.; Li, L.; Jiang, S.; Hu, H.; Liu, Z.; Xu, D.; Wu, L. The key roles of GSK-3beta in regulating mitochondrial activity. Cell. Physiol. Biochem. 2017, 44, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ou-Yang, J.P.; Wu, K.; Wang, Y.; Zhou, Y.F.; Wen, C.Y. Hypoglycemic effect of Astragalus polysaccharide and its effect on PTP1B. Acta Pharmacol. Sin. 2005, 26, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Q.; Wu, Y.; Wu, K.; Liu, M.; Zhang, J.F.; Zou, F.; Ou-Yang, J.P. Astragalus polysaccharide reduces hepatic endoplasmic reticulum stress and restores glucose homeostasis in a diabetic KKAy mouse model. Acta Pharmacol. Sin. 2007, 28, 1947–1956. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, D.; Mao, X.; Zou, F.; Jin, H.; Ou-Yang, J. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol. Cell. Endocrinol. 2009, 307, 89–98. [Google Scholar] [CrossRef]
- Wei, Z.; Weng, S.; Wang, L.; Mao, Z. Mechanism of Astragalus polysaccharides in attenuating insulin resistance in Rats with type 2 diabetes mellitus via the regulation of liver microRNA203a3p. Mol. Med. Rep. 2018, 17, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ju, J.; Yang, Y.; Wang, H.; Chen, W.; Zhao, X.; Ye, H.; Zhang, Y. Astragalus polysaccharides protect cardiac stem and progenitor cells by the inhibition of oxidative stress-mediated apoptosis in diabetic hearts. Drug Des. Dev. Ther. 2018, 12, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, S.; Dai, M.; Jia, X.; Wang, Q.; Zhang, Z.; Mao, Y. The effect of Astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complement. Altern. Med. 2017, 17, 310. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Zhu, L.; Hu, M.; Lei, L.; Long, Q. Neuregulin-1/ErbB signaling is impaired in the rat model of diabetic cardiomyopathy. Cardiovasc. Pathol. 2012, 21, 414–420. [Google Scholar] [CrossRef]
- Li, B.; Zheng, Z.; Wei, Y.; Wang, M.; Peng, J.; Kang, T.; Huang, X.; Xiao, J.; Li, Y.; Li, Z. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc. Diabetol. 2011, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Ennequin, G.; Capel, F.; Caillaud, K.; Chavanelle, V.; Etienne, M.; Teixeira, A.; Li, X.; Boisseau, N.; Sirvent, P. Neuregulin 1 improves complex 2-mediated mitochondrial respiration in skeletal muscle of healthy and diabetic mice. Sci. Rep. 2017, 7, 1742. [Google Scholar] [CrossRef] [PubMed]
- Odiete, O.; Hill, M.F.; Sawyer, D.B. Neuregulin in cardiovascular development and disease. Circ. Res. 2012, 111, 1376–1385. [Google Scholar] [CrossRef]
- Rupert, C.E.; Coulombe, K.L. The roles of neuregulin-1 in cardiac development, homeostasis and disease. Biomark. Insights 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Lu, K.; Wang, L.; Lv, M.; Fu, W. Astraglaus polysaccharide protects diabetic cardiomyopathy by activating NRG1/ErbB pathway. BioSci. Trends 2018, 12, 149–156. [Google Scholar] [CrossRef]
- Lopaschuk, G.D. Metabolic abnormalities in the diabetic heart. Heart Fail. Rev. 2002, 7, 149–159. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; McNulty, P.; Young, M.E. Adaptation and maladaptation of the heart in diabetes: Part I: General concepts. Circulation 2002, 105, 1727–1733. [Google Scholar] [CrossRef]
- Buchanan, J.; Mazumder, P.K.; Hu, P.; Chakrabarti, G.; Roberts, M.W.; Yun, U.J.; Cooksey, R.C.; Litwin, S.E.; Abel, E.D. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005, 146, 5341–5349. [Google Scholar] [CrossRef]
- Thrainsdottir, I.S.; von Bibra, H.; Malmberg, K.; Ryden, L. Effects of trimetazidine on left ventricular function in patients with type 2 diabetes and heart failure. J. Cardiovasc. Pharmacol. 2004, 44, 101–108. [Google Scholar] [CrossRef]
- Chen, W.; Xia, Y.; Zhao, X.; Wang, H.; Chen, W.; Yu, M.; Li, Y.; Ye, H.; Zhang, Y. The critical role of Astragalus polysaccharides for the improvement of PPARalpha [correction of PPRAalpha]-mediated lipotoxicity in diabetic cardiomyopathy. PLoS ONE 2012, 7, e45541. [Google Scholar]
- Auwardt, R.B.; Mudge, S.J.; Chen, C.; Power, D.A. Inhibition with antisense oligonucleotide suggests that IkappaB-alpha does not form a negative autoregulatory loop for NF-kappaB in mesangial cells. Exp. Nephrol. 2000, 8, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Wu, C.Y.; Cheng, J.T. Merit of Astragalus polysaccharide in the improvement of early diabetic nephropathy with an effect on mRNA expressions of NF-kappaB and IkappaB in renal cortex of streptozotoxin-induced diabetic rats. J. Ethnopharmacol. 2007, 114, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.; Huang, J.; Guo, Y.; Chen, J.; Chen, Y.; Xiao, W.; Liu, X.; Wang, N. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-kappaB-mediated inflammatory genes expression. Cytokine 2013, 61, 970–977. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.; Ren, Q.; Liu, Y.; Yang, X. Astragaloside effect on TGF-beta1, SMAD2/3 and alpha-SMA expression in the kidney tissues of diabetic KKAy mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6828–6834. [Google Scholar] [PubMed]
- Ding, Y.; Yuan, S.; Liu, X.; Mao, P.; Zhao, C.; Huang, Q.; Zhang, R.; Fang, Y.; Song, Q.; Yuan, D.; et al. Protective effects of astragaloside IV on db/db mice with diabetic retinopathy. PLoS ONE 2014, 9, e112207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Tian, N.; Zou, D.; Shi, Y.; Zhang, N. Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice. Drug Des. Dev. Ther. 2018, 12, 2431–2442. [Google Scholar] [CrossRef]
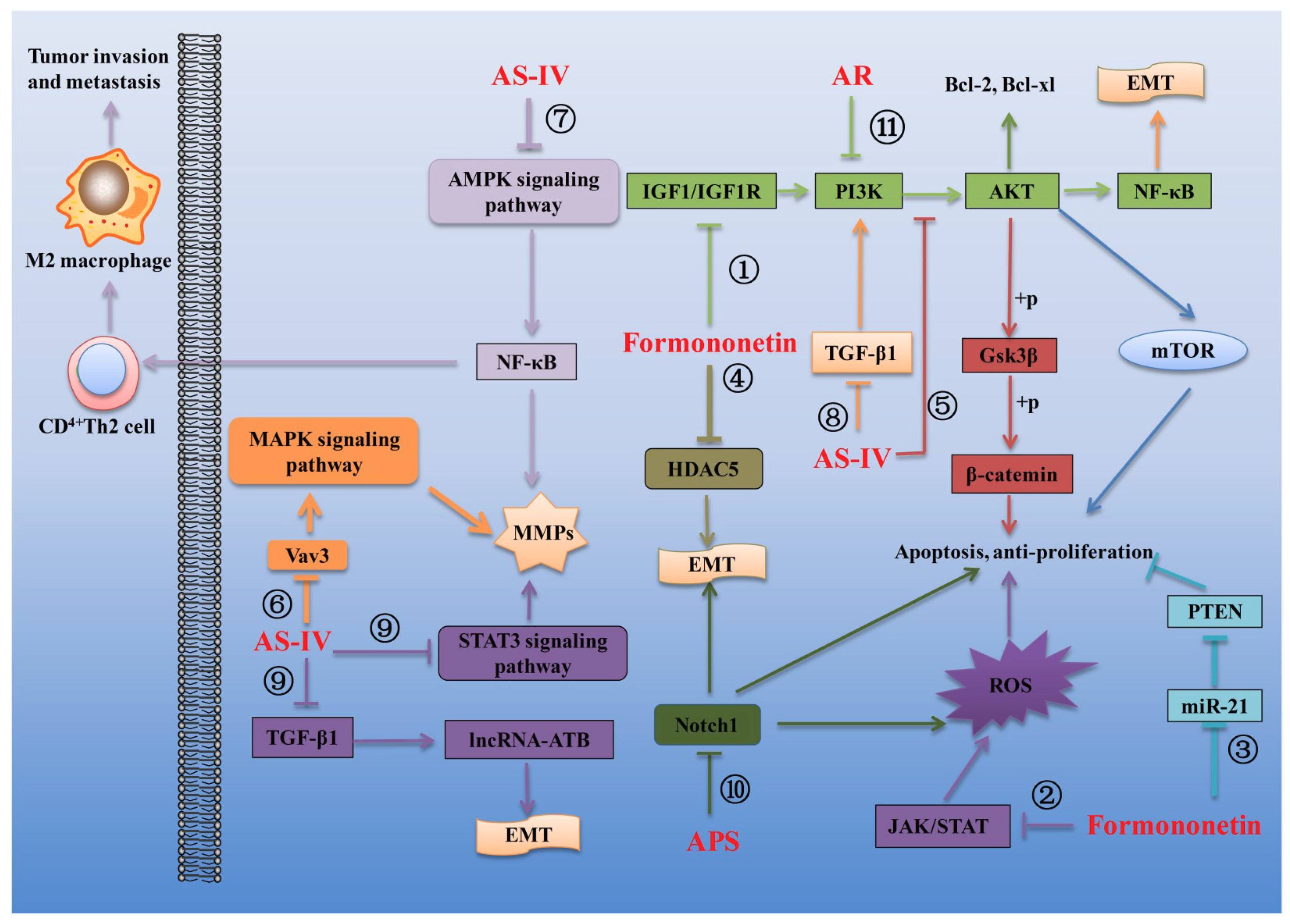
- Zhang, J.; Liu, L.; Wang, J.; Ren, B.; Zhang, L.; Li, W. Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J. Ethnopharmacol. 2018, 221, 91–99. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef]
- Jin, Y.M.; Xu, T.M.; Zhao, Y.H.; Wang, Y.C.; Cui, M.H. In vitro and in vivo anti-cancer activity of formononetin on human cervical cancer cell line HeLa. Tumour Biol. 2014, 35, 2279–2284. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, J.; Xin, M.; Huang, W.; Chen, X. Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Horm. Metab. Res. 2011, 43, 681–686. [Google Scholar] [CrossRef]
- Li, T.; Zhao, X.; Mo, Z.; Huang, W.; Yan, H.; Ling, Z.; Ye, Y. Formononetin promotes cell cycle arrest via downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer cells. Cell. Physiol. Biochem. 2014, 34, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.; Ai, X.; Cheng, B.; Lu, S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8453–8461. [Google Scholar] [PubMed]
- Hajji, K.; Mteyrek, A.; Sun, J.; Cassar, M.; Mezghani, S.; Leprince, J.; Vaudry, D.; Masmoudi-Kouki, O.; Birman, S. Neuroprotective effects of PACAP against paraquat-induced oxidative stress in the Drosophila central nervous system. Hum. Mol. Genet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Xie, W.; Sun, E.; Sun, Y.; Tian, D.; Liu, C.; Han, R.; Li, N.; Liu, M.; Han, R.; et al. microRNA-21 regulates cell proliferation and migration and cross talk with PTEN and p53 in bladder cancer. DNA Cell Biol. 2015, 34, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Li, Z.; Yan, H.; Qin, J.; Li, T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017, 8, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.R.; Xie, Q.; Li, F.; Zhang, Y.; Ma, J.W.; Xie, S.M.; Li, H.Y.; Zhong, X.Y. Epithelial-to-mesenchymal transition is involved in BCNU resistance in human glioma cells. Neuropathology 2014, 34, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, J.M.; Chen, J.K.; Yan, X.L.; Chen, H.M.; Nong, W.X.; Huang, H.Q. Histone deacetylase 5 promotes the proliferation of glioma cells by upregulation of Notch 1. Mol. Med. Rep. 2014, 10, 2045–2050. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Zheng, J.M.; Yan, X.L.; Chen, H.M.; Chen, J.K.; Huang, H.Q. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int. J. Clin. Exp. Pathol. 2015, 8, 6434–6441. [Google Scholar] [PubMed]
- Jia, L.; Lv, D.; Zhang, S.; Wang, Z.; Zhou, B. Astragaloside IV inhibits the progression of non-small cell lung cancer through Akt/GSK-3beta/beta-catenin pathway. Oncol. Res. 2017, 8, 868. [Google Scholar]
- Trenkle, T.; Hakim, S.G.; Jacobsen, H.C.; Sieg, P. Differential gene expression of the proto-oncogene VAV3 and the transcript variant VAV3.1 in oral squamous cell carcinoma. Anticancer Res. 2015, 35, 2593–2600. [Google Scholar] [PubMed]
- Jiang, K.; Lu, Q.; Li, Q.; Ji, Y.; Chen, W.; Xue, X. Astragaloside IV inhibits breast cancer cell invasion by suppressing Vav3 mediated Rac1/MAPK signaling. Int. Immunopharmacol. 2017, 42, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, F.; Liu, N.; Shen, W.; Huang, T. Astragaloside IV inhibits progression of glioma via blocking MAPK/ERK signaling pathway. Biochem. Biophys. Res. Commun. 2017, 491, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cui, W.Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.L.; Gong, W.Y.; Dong, J.C.; Liu, B.J. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J. Exp. Clin. Cancer Res. 2018, 37, 207. [Google Scholar] [CrossRef] [PubMed]
- Liang, X. EMT: New signals from the invasive front. Oral Oncol. 2011, 47, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wen, K. Astragaloside IV inhibits TGF-beta1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-kappaB pathway in gastric cancer cells. Phytother. Res. 2018, 32, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gu, J.; Zhang, M.; Yuan, J.; Zhao, B.; Jiang, J.; Jia, X. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-alpha-ERK1/2-NF-kappaB pathway. Int. Immunopharmacol. 2014, 23, 304–313. [Google Scholar] [CrossRef]
- Hu, T.; Fei, Z.; Wei, N. Chemosensitive effects of Astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharmacol. Rep. 2017, 69, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Yang, F.; Wang, F.; Ma, J.Z.; Guo, Y.J.; Tao, Q.F.; Liu, F.; Pan, W.; Wang, T.T.; Zhou, C.C.; et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014, 25, 666–681. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Y.; Chen, H. Astragaloside IV inhibits cell migration and viability of hepatocellular carcinoma cells via suppressing long noncoding RNA ATB. Biomed. Pharmacother. 2018, 99, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Ke, Y.; Zeng, Y.F.; Zhang, Y.W.; Yu, H.J. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Wael, H.; Yoshida, R.; Kudoh, S.; Hasegawa, K.; Niimori-Kita, K.; Ito, T. Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer 2014, 85, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, L.; Wang, L.; Xin, X. Down-regulation of Notch1 by gamma-secretase inhibition contributes to cell growth inhibition and apoptosis in ovarian cancer cells A2780. Biochem. Biophys. Res. Commun. 2010, 393, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Liao, W.R.; Sun, R.X. Astragalus polysaccharide induces the apoptosis of human hepatocellular carcinoma cells by decreasing the expression of Notch1. Int. J. Mol. Med. 2016, 38, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Fang, Y.; Chi, J.; Li, J.; Zhang, D.; Zou, Y.; Wang, Z. Astragalus polysaccharides decrease proliferation, migration and invasion but increase apoptosis of human osteosarcoma cells by up-regulation of microRNA-133a. Braz. J. Med. Biol. Res. 2018, 51, e7665. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef]
- Tseng, A.; Yang, C.H.; Chen, C.H.; Chen, C.H.; Hsu, S.L.; Lee, M.H.; Lee, H.C.; Su, L.J. An in vivo molecular response analysis of colorectal cancer treated with Astragalus membranaceus extract. Oncol. Rep. 2016, 35, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Soudani, N.; Caniza, M.A.; Assaf-Casals, A.; Shaker, R.; Lteif, M.; Su, Y.; Tang, L.; Akel, I.; Muwakkit, S.; Chmaisse, A.; et al. Prevalence and characteristics of acute respiratory virus infections in pediatric cancer patients. J. Med. Virol. 2019. [Google Scholar] [CrossRef]
- Chen, I.Y.; Moriyama, M.; Chang, M.F.; Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Reddy, A.P.; Gupta, M.R. Management of asthma: The current US and European guidelines. Adv. Exp. Med. Biol. 2014, 795, 81–103. [Google Scholar]
- Wang, J.; Jin, R.G.; Xiao, L.; Wang, Q.J.; Yan, T.H. Anti-asthma effects of synthetic salidroside through regulation of Th1/Th2 balance. Chin. J. Nat. Med. 2014, 12, 500–504. [Google Scholar] [CrossRef]
- Rathnayake, S.N.H.; Van den Berge, M.; Faiz, A. Genetic profiling for disease stratification in chronic obstructive pulmonary disease and asthma. Curr. Opin. Pulm. Med. 2019. [Google Scholar] [CrossRef]
- Sweilam, M.; Helmy, A.; El-Sharnoby, J.A.; El-Bendary, A.; Erfan, A.A.; Asy, H.M. Role of Foxp3 and regulatory CD4+CD25+ T-lymphocytes in bronchial asthma. Egypt. J. Immunol. 2008, 15, 113–123. [Google Scholar]
- Huang, X.; Tang, L.; Wang, F.; Song, G. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in ovalbumin-induced asthma. Immunobiology 2014, 219, 565–571. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Rev. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef]
- Mushaben, E.M.; Kramer, E.L.; Brandt, E.B.; Khurana Hershey, G.K.; Le Cras, T.D. Rapamycin attenuates airway hyperreactivity, goblet cells and IgE in experimental allergic asthma. J. Immunol. 2011, 187, 5756–5763. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Li, B.; Cai, C.; Ye, J.; Xia, J.; Ma, S. Astragaloside IV ameliorates airway inflammation in an established murine model of asthma by inhibiting the mTORC1 signaling pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 4037086. [Google Scholar] [CrossRef] [PubMed]
- Dufey, E.; Sepulveda, D.; Rojas-Rivera, D.; Hetz, C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am. J. Physiol. Cell Physiol. 2014, 307, C582–C594. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xing, Q.Q.; Xu, J.Y.; Ding, D.; Zhao, X. Astragalus polysaccharide modulates ER stress response in an OVA-LPS induced murine model of severe asthma. Int. J. Biol. Macromol. 2016, 93, 995–1006. [Google Scholar] [CrossRef]
- Liu, M.; Qin, J.; Hao, Y.; Liu, M.; Luo, J.; Luo, T.; Wei, L. Astragalus polysaccharide suppresses skeletal muscle myostatin expression in diabetes: Involvement of ROS-ERK and NF-kappaB pathways. Oxid. Med. Cell. Longev. 2013, 2013, 782497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tan, S.; Zhang, H.; Liu, P.; Tan, Y.Z.; Li, J.C.; Jia, D.; Shen, X.F. Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother. Res. 2018, 32, 1521–1529. [Google Scholar] [CrossRef]
- Willis, B.C.; Liebler, J.M.; Luby-Phelps, K.; Nicholson, A.G.; Crandall, E.D.; du Bois, R.M.; Borok, Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: Potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005, 166, 1321–1332. [Google Scholar] [CrossRef]
- Al-Tamari, H.M.; Dabral, S.; Schmall, A.; Sarvari, P.; Ruppert, C.; Paik, J.; DePinho, R.A.; Grimminger, F.; Eickelberg, O.; Guenther, A.; et al. FoxO3 an important player in fibrogenesis and therapeutic target for idiopathic pulmonary fibrosis. EMBO Mol Med. 2018, 10, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Cai, X.; Qian, Q.; Zhang, W.; Wang, D. Astragaloside IV modulates TGF-beta1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J. Cell. Mol. Med. 2018, 22, 4354–4365. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, S.; Zhang, Z.; Wang, B.; Wan, L. Astragalus injection attenuates bleomycin-induced pulmonary fibrosis via down-regulating Jagged1/Notch1 in lungs. J. Pharm. Pharmacol. 2016, 68, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; He, J.; Chen, H.; Chen, J.; Qian, X.; Huang, W. Erythropoietin attenuates hyperoxia-induced lung injury by upregulating epidermal growth factor-like domain 7 in newborn rats. Biomed. Rep. 2017, 6, 32–38. [Google Scholar] [CrossRef]
- Wang, X.H.; Huang, W.M. Astragalus polysaccharides exert protective effects in newborn rats with bronchopulmonary dysplasia by upregulating the expression of EGFL7 in lung tissue. Int. J. Mol. Med. 2014, 34, 1529–1536. [Google Scholar] [CrossRef]
- Chen, T.; Wang, R.; Jiang, W.; Wang, H.; Xu, A.; Lu, G.; Ren, Y.; Xu, Y.; Song, Y.; Yong, S.; et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation 2016, 39, 483–492. [Google Scholar] [CrossRef]
- Su, G.; Chen, X.; Liu, Z.; Yang, L.; Zhang, L.; Lundborg, C.S.; Wen, Z.; Guo, X.; Qin, X.; Liang, J.; et al. Oral Astragalus (Huang qi) for preventing frequent episodes of acute respiratory tract infection in children. Cochrane Database Syst. Rev. 2016, 12, Cd011958. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Zanardelli, M.; Bartolucci, G.; Karioti, A.; Bilia, A.R.; Vannacci, A.; Mugelli, A.; Ghelardini, C. In vitro evidence for the use of Astragali radix extracts as adjuvant against oxaliplatin-induced neurotoxicity. Planta Med. 2015, 81, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Shang, A.Q.; Wang, W.W.; Yang, J.P. Astragaloside suppresses tumor necrosis factor receptor-associated factor 5 signaling pathway and alleviates neurodegenerative changes in retinal pigment epithelial cells induced by isoflurane. J. Cell. Biochem. 2019, 120, 1028–1037. [Google Scholar] [CrossRef]
- Nederlof, R.; Eerbeek, O.; Hollmann, M.W.; Southworth, R.; Zuurbier, C.J. Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br. J. Pharmacol. 2014, 171, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Luo, H.; Zhou, X.; Cheng, C.Y.; Lin, L.; Liu, B.L.; Liu, K.; Li, P.; Yang, H. Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochimica et biophysica acta. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Zhao, Y.; Zhang, J.; Liu, B.; Jiao, S.; Zhang, X. Astragaloside IV reduces neuronal apoptosis and parthanatos in ischemic injury by preserving mitochondrial hexokinase-II. Free Radic. Biol. Med. 2019, 131, 251–263. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Fang, C.; Li, Q.; Cui, J.; Sun, J.; Li, L. miR-124 Regulates the expression of BACE1 in the hippocampus under chronic cerebral hypoperfusion. Mol. Neurobiol. 2017, 54, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xia, J.; Jiang, B.; Xu, Y.; Li, Y. TUG1 alleviates hypoxia injury by targeting miR-124 in H9c2 cells. Biomed. Pharmacother. 2018, 103, 1669–1677. [Google Scholar] [CrossRef]
- Sun, M.; Li, M.; Huang, Q.; Han, F.; Gu, J.H.; Xie, J.; Han, R.; Qin, Z.H.; Zhou, Z. Ischemia/reperfusion-induced upregulation of TIGAR in brain is mediated by SP1 and modulated by ROS and hormones involved in glucose metabolism. Neurochem. Int. 2015, 80, 99–109. [Google Scholar] [CrossRef]
- Yeh, S.H.; Yang, W.B.; Gean, P.W.; Hsu, C.Y.; Tseng, J.T.; Su, T.P.; Chang, W.C.; Hung, J.J. Translational and transcriptional control of Sp1 against ischaemia through a hydrogen peroxide-activated internal ribosomal entry site pathway. Nucleic Acids Res. 2011, 39, 5412–5423. [Google Scholar] [CrossRef]
- Yu, W.; Lv, Z.; Zhang, L.; Gao, Z.; Chen, X.; Yang, X.; Zhong, M. Astragaloside IV reduces the hypoxia-induced injury in PC-12 cells by inhibiting expression of miR-124. Biomed. Pharmacother. 2018, 106, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhao, B. Astragalus polysaccharide protects hypoxia-induced injury by up-regulation of miR-138 in rat neural stem cells. Biomed. Pharmacother. 2018, 102, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Wang, S.; Qiu, J.; Yu, C. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting alpha-synuclein expression via the p38 MAPK pathway. Int. J. Mol. Med. 2017, 40, 1772–1780. [Google Scholar] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Li, S.P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008, 22, 954–965. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, L.; Ong, Q.; Lin, Z.; Varman, P.M.; Sung, K.; Cui, B. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PLoS ONE 2014, 9, e92917. [Google Scholar] [CrossRef]
- Cheung, E.C.; Slack, R.S. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE 2004, 2004, PE45. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Perrone-Capano, C.; di Porzio, U. Chronic activation of ERK and neurodegenerative diseases. BioEssays 2003, 25, 1085–1095. [Google Scholar] [CrossRef]
- Yue, R.; Li, X.; Chen, B.; Zhao, J.; He, W.; Yuan, H.; Yuan, X.; Gao, N.; Wu, G.; Jin, H.; et al. Astragaloside IV attenuates glutamate-induced neurotoxicity in PC12 cells through Raf-MEK-ERK pathway. PLoS ONE 2015, 10, e0126603. [Google Scholar] [CrossRef]
- Castellano, G.; Torrens, F. Quantitative structure-antioxidant activity models of isoflavonoids: A theoretical study. Int. J. Mol. Sci. 2015, 16, 12891–12906. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Duan, Y.; Bao, Y.; Wei, C.; An, L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J. Ethnopharmacol. 2005, 98, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Yao, C.H.; Wang, S.H.; Lai, Y.L.; Tsai, C.C.; Chen, Y.S. Effect of Astragalus membranaceus in rats on peripheral nerve regeneration: In vitro and in vivo studies. J. Trauma 2010, 68, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, L.C.; Yao, C.H.; Hsu, Y.M.; Lin, J.H.; Yang, T.Y.; Chen, Y.H.; Chen, Y.S. Increased calcitonin gene-related peptide and macrophages are involved in Astragalus membranaceus-mediated peripheral nerve regeneration in rats. Am. J. Chin. Med. 2018, 46, 69–86. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Da Costa, I.M.; Cavalcanti, J.; de Queiroz, D.B.; de Azevedo, E.P.; do Rego, A.C.M.; Araujo Filho, I.; Parente, P.; Botelho, M.A.; Guzen, F.P. Supplementation with herbal extracts to promote behavioral and neuroprotective effects in experimental models of Parkinson’s disease: A systematic review. Phytother. Res. 2017, 31, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, J.Y.; Yang, C.B.; Malampati, S.; Huang, Y.Y.; Li, M.X.; Li, M.; Song, J.X. Neuroprotective natural products for the treatment of Parkinson’s disease by targeting the autophagy-lysosome pathway: A systematic review. Phytother. Res. 2017, 31, 1119–1127. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Rev. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, C.L.; Missima, F.; Pagliarone, A.C.; Bachiega, T.F.; Bufalo, M.C.; Araujo, J.P., Jr.; Sforcin, J.M. Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother. Res. 2010, 24, 1141–1146. [Google Scholar]
- Drouin-Ouellet, J.; St-Amour, I.; Saint-Pierre, M.; Lamontagne-Proulx, J.; Kriz, J.; Barker, R.A.; Cicchetti, F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Yang, J.; Jia, M.; Zhang, X.; Wang, P. Calycosin attenuates MPTP-induced Parkinson’s disease by suppressing the activation of TLR/NF-kappaB and MAPK pathways. Phytother. Res. 2019, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Guo, C.; Tang, W.; Liu, W.; Liu, Y. Astragalus polysaccharide protects neurons and stabilizes mitochondrial in a mouse model of Parkinson disease. Med. Sci. Monit. 2018, 24, 5192–5199. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, R.; Ding, F.; Wang, H.; Han, W.; Ma, F.; Hu, M.; Ma, C.W.; Huang, Z. Astragalus polysaccharide suppresses 6-hydroxydopamine-induced neurotoxicity in caenorhabditis elegans. Oxid. Med. Cell. Longev. 2016, 2016, 4856761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, X.; Gong, X.; Yang, Y.; Chen, C.; Shan, G.; Yao, Q. Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-small ka, CyrillicB in vivo and in vitro. Int. Immunopharmacol. 2017, 42, 18–24. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Han, W.; Cheng, J.; Qin, Y. Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol. Med. Rep. 2017, 15, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-kappaB activation and Nrf2 response. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.T.; Kuo, C.Y.; Kuan, Y.D.; Lin, H.C.; Wu, L.H.; Lee, C.H. The extracts of Astragalus membranaceus inhibit melanogenesis through the ERK signaling pathway. Int. J. Med. Sci. 2017, 14, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Guo, Z.Z.; Zhu, Y.F.; Huang, Z.J.; Gong, X.; Li, Y.H.; Son, W.J.; Li, X.Y.; Lou, Y.M.; Zhu, L.J.; et al. A systematic review of pharmacokinetic studies on herbal drug Fuzi: Implications for Fuzi as personalized medicine. Phytomedicine 2018, 44, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.A. Overview of enzymes of drug metabolism. J. Pharmacokinet. Biopharm. 1996, 24, 449–459. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II drug metabolizing enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Republic 2010, 154, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [PubMed]
- Ghersi-Egea, J.F.; Saudrais, E.; Strazielle, N. Barriers to drug distribution into the perinatal and postnatal brain. Pharm. Res. 2018, 35, 84. [Google Scholar] [CrossRef] [PubMed]
- Hu, M. Commentary: Bioavailability of flavonoids and polyphenols: Call to arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Jia, X.; Bajimaya, S.; Lin, H.; Tam, V.H.; Hu, M. Disposition of flavonoids via recycling: Comparison of intestinal versus hepatic disposition. Drug Metab. Dispos. 2005, 33, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Yadav, D.K.; Rawat, P.; Maurya, R.; Jain, G.K. Quantitative determination of formononetin and its metabolite in rat plasma after intravenous bolus administration by HPLC coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 391–397. [Google Scholar] [CrossRef]
- Singh, S.P.; Tewari, D.; Pradhan, T.; Jain, G.K. PAMPA permeability, plasma protein binding, blood partition, pharmacokinetics and metabolism of formononetin, a methoxylated isoflavone. Food Chem. Toxicol. 2011, 49, 1056–1062. [Google Scholar] [CrossRef]
- Luo, L.Y.; Fan, M.X.; Zhao, H.Y.; Li, M.X.; Wu, X.; Gao, W.Y. Pharmacokinetics and bioavailability of the isoflavones formononetin and ononin and their in vitro absorption in ussing chamber and Caco-2 cell models. J. Agric. Food Chem. 2018, 66, 2917–2924. [Google Scholar] [CrossRef]
- Jeong, E.J.; Jia, X.; Hu, M. Disposition of formononetin via enteric recycling: Metabolism and excretion in mouse intestinal perfusion and Caco-2 cell models. Mol. Pharm. 2005, 2, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Chen, J.; Jia, X.; Tam, V.H.; Hu, M. Disposition of flavonoids via enteric recycling: Structural effects and lack of correlations between in vitro and in situ metabolic properties. Drug Metab. Dispos. 2006, 34, 1837–1848. [Google Scholar] [CrossRef]
- Chen, J.; Lin, H.; Hu, M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother. Pharmacol. 2005, 55, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, J.; Lin, H.; Hu, M. Disposition of flavonoids via enteric recycling: Enzyme-transporter coupling affects metabolism of biochanin A and formononetin and excretion of their phase II conjugates. J. Pharmacol. Exp. Ther. 2004, 310, 1103–1113. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, H.; Yu, J.; Zhu, L.; Yan, T.; Wu, P.; Lu, L.; Wang, Y.; Hu, M.; Liu, Z. SGLT-1 Transport and deglycosylation inside intestinal cells are key steps in the absorption and disposition of calycosin-7-O-β-d-Glucoside in rats. Drug Metab. Dispos. 2016, 44, 283–296. [Google Scholar] [CrossRef]
- Ye, G.; Ma, R.R.; Li, Z.X.; Wang, H.; Zhu, H.Y.; Sun, Z.L.; Huang, C.G. Determination of calycosin-7-O-beta-D-glucopyranoside in rat plasma and urine by HPLC. Biomed. Chromatogr. 2007, 21, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.S.; Chen, T.B.; Sun, W.X.; Chen, L.; Luo, P.; Zhang, Z.F.; Ding, L.S. Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble astragaloside IV derivative (astragalosidic acid, LS-102). Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 251–259. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, C.; Liu, R.H.; Li, H.L.; Zhang, J.T.; Mao, C.; Moran, S.; Chen, C.L. Preclinical pharmacokinetics and tissue distribution of a natural cardioprotective agent astragaloside IV in rats and dogs. Life Sci. 2006, 79, 808–815. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Pan, G.; Fawcett, J.P.; Sun, J. Transport and bioavailability studies of astragaloside IV, an active ingredient in radix Astragali. Basic. Clin. Pharmacol. Toxicol. 2004, 95, 295–298. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Fawcett, J.P. Determination of astragaloside IV in rat plasma by liquid chromatography electrospray ionization mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 801, 285–288. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, X.; Yuan, B.; Yu, W.; Suo, H.; Li, Z.; Xu, H. Disposition of astragaloside IV via enterohepatic circulation is affected by the activity of the intestinal microbiome. J. Agric. Food Chem. 2015, 63, 6084–6093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.L.; Chen, G.G.; Du, Y. Pharmacokinetics of astragaloside iv in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2007, 32, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, S.; Qian, D.W.; Shang, E.X.; Guan, H.L.; Ren, H.; Zhu, Z.H.; Duan, J.A. The interaction between ononin and human intestinal bacteria. Yao Xue Xue Bao 2014, 49, 1162–1168. [Google Scholar] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Liu, R.; Li, H.; Zhang, J.; Mao, C.; Chen, C. Quantitative determination of astragaloside IV, a natural product with cardioprotective activity, in plasma, urine and other biological samples by HPLC coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 822, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.R.; Wang, G.J.; Wu, X.L.; Li, H.; Xie, H.T.; Lv, H.; Sun, J.G. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur. J. Drug Metab. Pharmacokinet. 2006, 31, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.D.; Wei, M.G. Profiling the metabolism of astragaloside IV by ultra performance liquid chromatography coupled with quadrupole/time-of-flight mass spectrometry. Molecules 2014, 19, 18881–18896. [Google Scholar] [CrossRef]
- Zhou, R.N.; Song, Y.L.; Ruan, J.Q.; Wang, Y.T.; Yan, R. Pharmacokinetic evidence on the contribution of intestinal bacterial conversion to beneficial effects of astragaloside IV, a marker compound of astragali radix, in traditional oral use of the herb. Drug Metab. Pharmacokinet. 2012, 27, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Y.Q.; Fu, Y.L. Analysis of clinical dosage of Astragalus membranaceus in past dynasties. J. Tradit. Chin. Med. 2015, 56, 519–520. [Google Scholar]
- Chen, R.B.; Zhang, M.M.; Zhang, Z.Q.; Li, H.; Gai, G.Z. Data mining analysis of clinical dosage of Astragalus membranaceus and related applications. Glob. Tradit. Chin. Med. 2011, 4, 438–441. [Google Scholar]
- Du, X.H.; Du, X.J.; Zhang, K.X.; Wang, J.T.; Guo, G.Y. Clinical observation of radix Astragali injection combined with chemotherapy in the treatment of malignant tumors. Shanghai Med. Pharm. J. 2000, 21, 16–18. [Google Scholar]
- Tang, L.; Wu, M.H. Clinical Observation of Astragalus injection combined with chemotherapy in the treatment of malignant tumors. J. Chin. Phys. 2006, 8, 425–426. [Google Scholar]
- Hu, Z.Y.; Yu, Q.; Zhao, Y.S. Dose-dependent association between UGT1A1*28 polymorphism and irinotecan-induced diarrhoea: A meta-analysis. Eur. J. Cancer. 2010, 46, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Garcia Gil, S.; Ramos Diaz, R.; Nazco Casariego, G.J.; Llanos Munoz, M.; Vina Romero, M.M.; Martin Calero, B.; Perez Perez, J.A.; Gutierrez Nicolas, F. Effect of UGT, SLCO, ABCB and ABCC polymorphisms on irinotecan toxicity. Med. Clin. 2018, 151, 425–430. [Google Scholar] [CrossRef]
- Petrenaite, V.; Ohman, I.; Ekstrom, L.; Saebye, D.; Hansen, T.F.; Tomson, T.; Sabers, A. UGT polymorphisms and lamotrigine clearance during pregnancy. Epilepsy Res. 2018, 140, 199–208. [Google Scholar] [CrossRef]
- Hu, D.G.; Mackenzie, P.I.; McKinnon, R.A.; Meech, R. Genetic polymorphisms of human UDP-glucuronosyltransferase (UGT) genes and cancer risk. Drug Metab. Rev. 2016, 48, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.J.; Yoon, S.H.; Yu, K.S.; Cho, J.Y.; Park, S.I.; Lee, E.; Lee, J.O.; Koh, Y.; Song, K.H.; Choe, P.G.; et al. The genetic polymorphism UGT1A4*3 is associated with low posaconazole plasma concentrations in hematological malignancy patients receiving the oral suspension. Antimicrob. Agents Chemother. 2018, 62, e02230-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, X.; Deng, Y.S.; Qiu, X.W. ABCB1 1199G > A Polymorphism impacts transport ability of P-gp-mediated antipsychotics. DNA Cell Biol. 2018, 37, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Tandia, M.; Mhiri, A.; Paule, B.; Saffroy, R.; Cailliez, V.; Noe, G.; Farinotti, R.; Bonhomme-Faivre, L. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): Monocentric study. Cancer Chemother. Pharmacol. 2017, 79, 759–766. [Google Scholar] [CrossRef]
- Cuppen, B.V.; Pardali, K.; Kraan, M.C.; Marijnissen, A.C.; Yrlid, L.; Olsson, M.; Bijlsma, J.W.; Lafeber, F.P.; Fritsch-Stork, R.D. Polymorphisms in the multidrug-resistance 1 gene related to glucocorticoid response in rheumatoid arthritis treatment. Rheumatol. Int. 2017, 37, 531–536. [Google Scholar] [CrossRef]
- Vos, K.; Sciuto, C.L.; Piedade, R.; Ashton, M.; Bjorkman, A.; Ngasala, B.; Martensson, A.; Gil, J.P. MRP2/ABCC2 C1515Y polymorphism modulates exposure to lumefantrine during artemether-lumefantrine antimalarial therapy. Pharmacogenomics 2017, 18, 981–985. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, H.; Peng, R.; Huang, C.; Bai, R. ABCC2 (1249G > A) polymorphism implicates altered transport activity for sorafenib. Xenobiotica 2017, 47, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Satoh, S.; Inoue, K.; Saito, M.; Habuchi, T.; Suzuki, T. Telmisartan pharmacokinetics in Japanese renal transplant recipients. Clin. Chim. Acta 2009, 399, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Hira, D.; Terada, T. BCRP/ABCG2 and high-alert medications: Biochemical, pharmacokinetic, pharmacogenetic and clinical implications. Biochem. Pharmacol. 2018, 147, 201–210. [Google Scholar] [CrossRef]
- Asher, G.N.; Corbett, A.H.; Hawke, R.L. Common herbal dietary supplement-drug interactions. Am. Fam. Phys. 2017, 96, 101–107. [Google Scholar]
- Ramos-Esquivel, A.; Viquez-Jaikel, A.; Fernandez, C. Potential drug-drug and herb-drug interactions in patients with cancer: A prospective study of medication surveillance. J. Oncol. Pract. 2017, 13, e613–e622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L. Potential herb-drug interaction in the prevention of cardiovascular diseases during integrated traditional and Western medicine treatment. Chin. J. Integr. Med. 2015, 21, 3–9. [Google Scholar] [CrossRef]





| NO. | Name | Categories | References |
|---|---|---|---|
| 1 | Astragaloside I–VIII | Astragalus triterpene saponins | [27,29] |
| 2 | Acetylastragaloside | [29] | |
| 3 | Isoastragaloside I–IV | [27,29,30] | |
| 4 | Acetylastragaloside I | [31] | |
| 5 | Astramembrannin II | [29] | |
| 6 | Cycloastragenol | [29] | |
| 7 | Cyclosieversigenis | [29] | |
| 8 | Soyasaponin I, II | [29,30] | |
| 9 | Soyasapogenol B | [29] | |
| 10 | Lupeol | [29] | |
| 11 | Malonylastragaloside I | [29] | |
| 12 | Agroastragaloside I–IV | [30] | |
| 13 | Monghocoside I, II | [30] | |
| 14 | Atramembrannin I,II | [30] | |
| 15 | Asernestioside A, B, C | [30] | |
| 16 | Astrasieversianin II,X | [30,32] | |
| 17 | Astrojanoside | [30] | |
| 18 | Astrojanoside A | [33] | |
| 19 | Azukisaponin II, V | [30] | |
| 20 | Brachyoside A, B, C | [30,33] | |
| 21 | β-daucosterol | [30] | |
| 22 | β-sitosterol | [30] | |
| 23 | Cloversaponin IV | [30] | |
| 24 | Cycloaraloside A | [30] | |
| 25 | Cyclocanthoside A, B, E, G | [30,34] | |
| 26 | Cyclocephaloside I, II | [30] | |
| 27 | Cyclodissectoside | [30] | |
| 28 | Cyclounifolioside B | [30] | |
| 29 | Dehydroazukisaponin V | [30] | |
| 30 | Calycosin-7-O-β-d-glucoside | [30] | |
| 31 | Calycosin-3-O-β-d-glucoside | [35] | |
| 32 | Formononetin-7-O-β-d-glucoside | [30] | |
| 33 | Hareftoside A, B, C, D, E | [30] | |
| 34 | Isoliquiritigenin | [30] | |
| 35 | Macrophyllosaponin B | [30] | |
| 36 | Melilotus-saponinO2 | [30] | |
| 37 | Mongholicoside A, B | [30] | |
| 38 | Oleifoloside B | [30] | |
| 39 | Quercetin-3-glucoside | [30] | |
| 40 | Rhamnocitrin-3-glucoside | [30] | |
| 41 | Trojanoside A, B, H | [30] | |
| 42 | Wistariasaponin B2, D | [30] | |
| 43 | 2′-hydroxy-3′,4′-dimethoxyisoflavone-7-O-β-d-glucopyranoside | [30] | |
| 44 | 2′-hydroxy-3,4′-dimethoxyisoflavan-7-O-β-d-glucoside | [35] | |
| 45 | 3′,4′-dimethoxyisoflavone-7-O-β-d-glucoside | [30] | |
| 46 | 3′-methoxy-5′-hydroxy-isoflavone-7-O-β-d-glucoside | [30] | |
| 47 | 3-O-β-d-xylopyranosyl-6,25-di-O-β-d-glucopyranosyl-3β,6α,16β,24(S),25-pentahydroxycycloartane | [30] | |
| 48 | 3-O-β-d-xylopyraosyl-24S-cycloart-3β,6α,16β,24,25-pentaol-25-O-β-d-glucopyranoside | [30] | |
| 49 | 3-O-[α-l-rhamnopyranosyl-(1→2)-β-d-xylopyranosyl-(1→2)-β-d-glucuronopyranosyl]-3β,21β,22α,24,29-pentahydroxyolean-12-ene | [30] | |
| 50 | 3-O-β-d-glucuronopyranosyl-soyasapogenin B | [30] | |
| 51 | 6,3′-dihydroxy-2′,4′-dimethoxyisoflavean-6-O-β-d-glucopyranoside | [30] | |
| 52 | 7,3′-dihydroxyl-6,4′-dimethoxyisoflavon-7-O-β-d-glucopyranoside | [30] | |
| 53 | 7,2′-dihydroxy-3′,4′-dimethoxyisoflavan-7-O-β-d-glucoside | [30] | |
| 54 | (6αR,1lαR)-3-hydroxy-9,10-dimethoxypterocarpan-3-O-β-d-glucoside | [29,30] | |
| 55 | (3R)-2′-hydroxy-3′,4′-dimethoxyisoflavan-7-O-β-d-glucoside | [30,36] | |
| 56 | (6αR,11αR)-9,10-dimethoxypterocarpan-3-O-β-d-glucoside | [30] | |
| 57 | (3R,4R)-3-(2-hydroxy-3,4-dimethoxyphenyl)chroman-4,7-diol-7-O-β-d-glucopyranoside | [30] | |
| 58 | 7-methylquercetin-3-O-α-l-rhamnopyranosyl-(1→2)-[6-O-(3-hydroxy-3-methylglutaryl)-β-d-galactopyranoside] | [37] | |
| 59 | kaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-[6-O-(3-hydroxy-3-methylglutaryl)-β-d-galactopyranoside] | [37] | |
| 60 | 7-methylkaempferol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside | [37] | |
| 61 | 7-methylkaempferol-3-O-α-l-rhamnopyranosyl-(1→2)-[6-O-(3-hydroxy-3-methylglutaryl)-β-d-galactopyranoside] | [37] | |
| 62 | 7-methylque-rcetin 3-O-β-d-galactopyranoside | [37] | |
| 63 | 20(R),24(S)-epoxy-9β,19-cyclolanostane-3β,6α,16β,25-tetrol 3-O-α-l-rhamnopyranosyl-(1→4)-β-d-glucopyranoside | [33,38] | |
| 64 | 20(R),24(S)-epoxy-9β,19-cyclolanostane-3β,6α,16β,25-tetrol 3-O-α-l-rhamnopyranosyl-(1→2)-β-d-glucopyranoside | [33,38] | |
| 65 | 20(R),24(S)-epoxy-9β,19-cyclolanostane-3β,6α,16β,25-tetrol 3-O-β-d-glucopyranoside | [38] | |
| 66 | 20(R), 25-epoxy-9β, 19-cyclolanostane-3β, 6α, 16β, 24(S)-tetrol (24-O-acetyl)-3-O-α-l-rhamnopyranosyl-(1→2)-(6′-O-acetyl)-β-d-glucopyranoside | [38] | |
| 67 | Calycosin-7-O-β-d-glucoside-6″-O-acetate | Astragalus flavonoids | [30] |
| 68 | Calycosin-7-O-β-d-glucoside-6″-O-malonate | [29] | |
| 69 | Calycosin | [29] | |
| 70 | Ononin | [29] | |
| 71 | Formononetin-7-O-β-d-glucoside-6″-O-malonate | [29] | |
| 72 | Formononetin | [29] | |
| 73 | Dimethoxy-dihydrogen-isoflavones | [30] | |
| 74 | Astrapterocarpan-glucoside-6″-O-malonate | [29] | |
| 75 | Astraisoflavan-7-O-β-d-glucoside-6″-O-malonate | [35] | |
| 76 | Sulfuretin | [27] | |
| 77 | Pendulone | [27] | |
| 78 | Isoliquiritigenin | [27] | |
| 79 | Rutin | [36] | |
| 80 | Cascara citrin | [30] | |
| 81 | (3R)-8,2′-dihydroxy-7,4′-dimethoxyisoflavan | [36] | |
| 82 | Dimethoxy isoflavones | [30] | |
| 83 | Isoliquiritigenin,dimethoxy ispflavan | [30] | |
| 84 | Isorhamnetin | [30] | |
| 85 | Kaempferol | [30] | |
| 86 | Kumatakehin | [30] | |
| 87 | l-3-hydroxv-9-methoxypterocarpan | [30] | |
| 88 | Pterocarpans | [30] | |
| 89 | Quercetin | [30] | |
| 90 | Rhamnocitrin | [30] | |
| 91 | Sphondin | [30] | |
| 92 | Kaempferol | [37] | |
| 93 | 2′-hydroxy-3′,4′-dimethoxyisoflavone-7-O-β-d-glucopyranoside | [30] | |
| 94 | 2′-hydroxy-3′,4′,7-trimethoxyisoflavone | [30] | |
| 95 | 2′,3′,7-trihydroxy-4′-methoxyisoflavone | [30] | |
| 96 | 2′,4′-dihydroxv-5,6-dlmethoxvlsoflavane | [30] | |
| 97 | 4,2′,4′-trihydroxy chalcone | [30] | |
| 98 | 5,7,4′-trihydroxyisoflavone | [30] | |
| 99 | 8,2′-dihydroxy-4′,7-dimethoxyisoflavone | [30] | |
| 100 | (3R)-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan | [30] | |
| 101 | (3R)-2′,3′-dihydroxy-4′,7-dimethoxyisoflavone | [30] | |
| 102 | 3,9,10-trimethoxypterocarpan,(6αR,1lαR)-10-hydroxy-3,9-dimethoxypterocarpan | [30] | |
| 103 | 9,10-dimethoxypterocarpan-7-O-β-d-glucopyranoside | [30] | |
| 104 | 3-hydroxy-9,10-dimethoxypterocarpan | [35] | |
| 105 | APS A,B, C, D | Astragalus polysaccharides | [29] |
| 106 | AERP1 (Molecular weight: 2.01 × 106 Da) | [39] | |
| 107 | AERP2 (Molecular weight: 2.11 × 103 Da) | [39] | |
| 108 | APS (Glc, Ara, Gal and Rha) | [40] | |
| 109 | APS (Glc) | [40] | |
| 110 | APS (Glc, Molecular weight: 2.1 × 104 Da) | [40] | |
| 111 | APS-I (Ara: Xyl: Glc in the ratio of 0.54: 1: 18.14, Molecular weight: 4.8×106 Da) | [40] | |
| 112 | APS-II (Ara: Xyl: Glc in the ratio of 0.23: 1: 29.39, Molecular weight: 8.7×103 Da) | [40] | |
| 113 | APS (Glc, Molecular weight: 3.6×104 Da) | [40] | |
| 114 | APS-I (Glc: Gal: Ara in the ratio of 1.75: 1.63: 1, Molecular weight: 3.6×104 Da) | [40] | |
| 115 | APS-II (Glc, Molecular weight: 1.2 × 104 Da) | [40] | |
| 116 | APS-III (Glc, Molecular weight: 3.5 × 104 Da) | [40] | |
| 117 | APS (Man, Gal, Fru, Fuc and Xyl) | [40] | |
| 118 | Astragalan (Glc, Molecular weight: 1.5 × 104 Da) | [40] | |
| 119 | APS (GIc: Gal: Ara in the ratio of 1.75: 1.63: 1, Molecular weight: 3.6×104 Da) | [40] | |
| 120 | APS (Glc, Molecular weight: 3.6 × 104 Da) | [40] | |
| 121 | APS (Rha: Glc: Gal: Ara in the ratio of 1.19: 72.01: 5.85: 20.95, Molecular weight: 1.1×104 Da) | [40] | |
| 122 | AMon-S (Ara: Gal: GalA: GlcA in the ratio of 18: 18: 1: 1, Molecular weight: 7.6 × 104 Da) | [40] | |
| 123 | F-8 (Rha: Rib: Fuc: Ara: Xyl: Man: Gal: GIc in the ratio of 2: 2: 1: 2: 6: 2: 3: 100, Molecular weight: 2.2 × 104 Da) | [40] | |
| 124 | F-9 (Fuc: Xyl: GIc in the ratio of 1: 2: 100, Molecular weight: 2.2 × 104 Da) | [40] | |
| 125 | APS (Rha: Xyl: GIc: Gal: Man: Fru in the ratio of 4.9: 4.7: 8.3: 122.2: 2.2: 3.1) | [40] | |
| 126 | APSID3(Ara: Rha: Gal: Glc in the ratio of 2: 2: 5: 6, Molecular weight: 5.8 × 105 Da) | [40] | |
| 127 | APS-I (Ara: GIc in the ratio of 1: 3.45, Molecular weight: 1.7 × 106 Da) | [40] | |
| 128 | APS-II (Rha: Ara: GIc in the ratio of 1: 6.25: 17.86, Molecular weight: 1.2 × 106 Da) | [40] | |
| 129 | APS (Ara: Man: GIc: Gal in the ratio of 0.10: 1.26: 1: 0.01) | [40] | |
| 130 | APS (Molecular weight: 6.9 × 104 Da) | [40] | |
| 131 | APS (Rha: Xyl: GIc: Gal in the ratio of 1: 4: 5: 1.5, Molecular weight: 3.0 × 105 Da) | [40] | |
| 132 | RAP (Rha: Ara: Glc: Gal: GalA in a molar ratio of 0.03: 1.00:0.27: 0.36: 0.30, Molecular weight: 1.334 × 106 Da) | [41] | |
| 133 | APS (Rha: Xyl: Gle: Gal: Man: Fru in a molar ratio of 4.9: 4.7: 8.3: 122.2: 2.2: 3.1, Molecular weight: 3500~ 1.58 × 106 Da) | [42] | |
| 134 | APS4 (Rha: Ara: Xyl: Man: Gal in a molar ratio of 12.1: 0.3: 0.6: 1.0: 1.0: 1.7, Molecular weight: 1.61 × 106 Da) | [43] | |
| 135 | Arabino-3,6-galactan | [44] | |
| 136 | alcohol-soluble polysaccharide (ASP) (Ara: Gal: Glu: Man in a molar ratio of 1.00: 0.98: 3.01: 1.52, Molecular weight: 2100 Da) | [5] |
| Administration | Species | Dose | Detected Compounds | Pharmacokinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (min) | AUC(0-t) (min·ng/mL) | AUC(0-∞) (min·ng/mL) | MRT (h) | T1/2 (min) | CL/F (L·h−1·kg−1) | ||||
| HQ aqueous extract, p.o., single treatment [20] | Rat | 4 g/kg | CG | 2.67 ± 1.17 | 28.33 ± 4.08 | 355.48 ± 96.91 | 399.66 ± 138.44 | ND | 177.24 ± 73.98 | ND |
| Ononin | 3.97 ± 0.83 | 22.50 ± 6.12 | 425.26 ± 59.89 | 451.147 ± 65.53 | ND | 163.22 ± 34.44 | ND | |||
| Formononetin | 4.24 ± 1.62 | 18.33 ± 6.06 | 341.31 ± 108.69 | 385.78 ± 114.41 | ND | 213.09 ± 55.57 | ND | |||
| AS-IV | 8.40 ± 5.64 | 45.00 ± 25.10 | 2777.4 ± 1220.25 | 3321.99 ± 1032.04 | ND | 291.83 ± 125.58 | ND | |||
| C-3′-G | 1841.99 ± 391.56 | 33.33 ± 13.66 | 370,570.07 ± 118,683.13 | 423,856.39 ± 128,163.25 | ND | 232.59 ± 112.86 | ND | |||
| F-7-G | 141.23 ± 54.67 | 33.33 ± 9.83 | 27,808.62 ± 5918.16 | 30,076.63 ± 6376.79 | ND | 182.50 ± 56.30 | ND | |||
| CG-3′-G | 88.94 ± 40.61 | 45.00 ± 9.49 | 10,550.06 ± 5895.49 | 10,580.22 ± 5897.22 | ND | 70.56 ± 9.35 | ND | |||
| D-7-G | 19.65 ± 10.15 | 35.83 ± 14.29 | 2145.60 ± 574.75 | 2232.42 ± 614.78 | ND | 140.30 ± 25.94 | ND | |||
| HQ aqueous extract, p.o., single treatment [20] | Rat | 16 g/kg | CG | 17.27 ± 10.19 | 25.00 ± 5.48 | 1256.42 ± 555.23 | 1405.16 ± 505.53 | ND | 313.14 ± 188.96 | ND |
| Ononin | 9.52 ± 4.20 | 24.17 ± 6.65 | 865.61 ± 349.91 | 858.61 ± 352.27 | ND | 68.71 ± 15.26 | ND | |||
| Formononetin | 26.01 ± 9.75 | 10.83 ± 2.04 | 2534.14 ± 942.59 | 3053.52 ± 1243.25 | ND | 280.86 ± 115.56 | ND | |||
| AS-IV | 26.63 ± 13.54 | 21.67 ± 1.67 | 3237.24 ± 993.96 | 4116.24 ± 1078.26 | ND | 319.91 ± 58.10 | ND | |||
| C-3′-G | 3301.60 ± 1113.35 | 25.00 ± 10.95 | 746,605.22 ± 105,842.50 | 860,702.31 ± 269,532.48 | ND | 185.70 ± 176.90 | ND | |||
| F-7-G | 282.21 ± 131.2 | 48.33 ± 24.63 | 64,267.55 ± 20,197.31 | 66,083.40 ± 20,987.58 | ND | 125.20 ± 26.86 | ND | |||
| CG-3′-G | 97.08 ± 38.95 | 42.50 ± 6.12 | 16,864.73 ± 6773.30 | 16,945.86 ± 6736.94 | ND | 95.84 ± 57.09 | ND | |||
| D-7-G | 50.75 ± 32.36 | 50.00 ± 20.49 | 9847.21 ± 7727.72 | 10,978.84 ± 7912.12 | ND | 255.71 ± 124.07 | ND | |||
| HQ aqueous extract, p.o., single treatment [21] | Mice | 6 g/kg | CG | 33.41 | 90.00 | 6489.60 ± 2228.40 | 7630.20 ± 2418.60 | ND | 156.60 ± 40.20 | ND |
| Ononin | 51.38 | 90.00 | 13,670.40 ± 3581.40 | 14,354.40 ± 2842.80 | ND | 117.60 ± 20.40 | ND | |||
| Calycosin | 32.98 | 90.00 | 8152.80 ± 2484.6 | 9292.20 ± 2261.40 | ND | 132.60 ± 55.20 | ND | |||
| Formononetin | 47.93 | 120.00 | 14,002.80 ± 2869.80 | 15,385.20 ± 2740.80 | ND | 239.40 ± 61.80 | ND | |||
| AS-IV | 128.95 | 90.00 | 41,722.20 ± 10,714.20 | 43,349.40 ± 11,791.20 | ND | 208.80 ± 69.00 | ND | |||
| HQ 95 % ethanol extract, p.o., single treatment [22] | Rat | 11.36 g/kg | CG | 0.205 | 90.00 | ND | 56.22 | 4.273 | 162.18 | 97,008.817 |
| Ononin | 0.074 | 90.00 | ND | 760.38 | 4.104 | 55.86 | 572,786.673 | |||
| Calycosin | 0.700 | 60.00 | ND | 122.64 | 3.422 | 232.80 | 44,469.181 | |||
| Formononetin | 0.097 | 150.00 | ND | 14.94 | 4.369 | 72.42 | 365,548.079 | |||
| AS-IV | 0.5879 | 90.00 | ND | 107.256 | 5.210 | 76.518 | 50,848.964 | |||
| Astragaloside II | 0.016 | 60.00 | ND | 4.20 | 4.223 | 41.76 | 1,301,557.754 | |||
| Administration | Species | Dose | Detected compounds | Pharmacokinetic parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC(0-t) (h·ng/mL) | AUC(0-∞) (h·ng/mL) | MRT (min) | T1/2 (h) | CL (L·h-1·kg-1) | Vd (L/kg) | Vss (L/kg) | ||||
| Formononetin, p.o. [217] | Rat | 50 mg/kg | Formononetin | 16.67 ± 6.60 | 1.00 ± 0.00 | 251.44 ± 63.67 | 278.27 ± 66.68 | ND | ND | ND | ND | NA |
| Daidzin | 8.99 ± 3.29 | ND | 145.58 ± 34.70 | ND | ND | ND | ND | ND | NA | |||
| Daidzin + Daidzin conjugates | 301.33 ± 149.92 | ND | 5032.25 ± 530.22 | ND | ND | ND | ND | ND | NA | |||
| Formononetin conjugates | 311.97 ± 97.36 | ND | 5231.46 ± 1219.91 | ND | ND | ND | ND | ND | NA | |||
| Daidzin conjugates | 292.70 ± 146.20 | ND | 4886.67 ± 1498.49 | ND | ND | ND | ND | ND | NA | |||
| Formononetin + Formononetin conjugates | 327.67 ± 100.72 | 3.42 ± 2.74 | 5482.90 ± 1276.11 | 6509.66 ± 720.47 | ND | ND | ND | ND | NA | |||
| Formononetin, i.v. [217] | Rat | 10 mg/kg | Formononetin | 4548.50 ± 321.73 | NA | 1949.10 ± 295.09 | 1975.88 ± 317.15 | ND | 1.95 ± 0.48 | 5.13 ± 0.82 | 14.16 ± 1.26 | NA |
| Daidzin | 434 ± 19.80 | NA | 279.72 ± 52.03 | ND | ND | ND | ND | ND | NA | |||
| Daidzin + Daidzin conjugates | 494 ± 5.66 | NA | 1663.89 ± 5.72 | ND | ND | ND | ND | ND | NA | |||
| Formononetin conjugates | 962.00 ± 311.13 | NA | 3003.64 ± 321.79 | ND | ND | ND | ND | ND | NA | |||
| Daidzin conjugates | 308.20 ± 95.32 | NA | 1356.60 ± 42.89 | ND | ND | ND | ND | ND | NA | |||
| Formononetin + Formononetin conjugates | 5180 ± 367.70 | NA | 4976.60 ± 648.18 | 5098.57 ± 765.53 | ND | 5.09 ± 1.61 | ND | 14.23 ± 2.41 | NA | |||
| Formononetin, i.v. [216] | Rat | 10 mg/kg | Formononetin | 3207 | NA | 1683.86 | 1773.40 | ND | 10.34 | 5.63 | 84.14 | NA |
| Formononetin + Formononetin conjugates | 3676 | NA | 4317.62 | 4595.71 | ND | 7.18 | 2.17 | 22.54 | NA | |||
| Daidzin | 305.73 | NA | 243.68 | 263.86 | ND | 2.53 | ND | ND | NA | |||
| Daidzin + Daidzin conjugates | 347.73 | NA | 1625.02 | 1773.89 | ND | 6.85 | ND | ND | NA | |||
| Formononetin, p.o. [218] | Rat | 20 mg/kg | Formononetin | 81.04 ± 9.63 | 0.5 ± 0.0 | 191.38 ± 12.39 | 203.26 ± 12.93 | ND | 2.10 ± 0.28 | ND | NA | NA |
| Formononetin, i.v. [218] | Rat | 4 mg/kg | Formononetin | 349.48 ± 34.63 | NA | 174.99 ± 23.90 | 184.97 ± 24.71 | ND | 2.23 ± 0.6 | 4.3 ± 0.8 | 13.9 ± 1.0 | ND |
| CG, i.v. [223] | Rat | 0.5 mg/kg | CG | 446.1 ± 84.6 | NA | 89.33 ± 17.00 | 89.83 ± 17.00 | ND | 0.38 ± 0.14 | ND | ND | ND |
| CG glucuronide | 5.41 ± 1.22 | 0.57 ± 0.30 | 10.00 ± 5.17 | 12.83 ± 5.33 | ND | 0.95 ± 0.33 | ND | ND | ND | |||
| C-3′-G | 10.11 ± 3.17 | 0.32 ± 0.09 | 34.00 ± 23.83 | 54.83 ± 50.55 | ND | 4.11 ± 3.41 | ND | ND | ND | |||
| CG, p.o. [223] | Rat | 10 mg/kg | CG | 7.46 ± 3.76 | 0.28 ± 0.11 | 5.33 ± 1.83 | 5.50 ± 2.67 | ND | 1.52 ± 0.46 | ND | ND | NA |
| CG glucuronide | 51.29 ± 15.7 | 1.00 ± 0.39 | 106.33 ± 55.17 | 106.67 ± 55.50 | ND | 1.03 ± 0.24 | ND | ND | NA | |||
| C-3′-G | 1189.66 ± 346.95 | 0.96 ± 0.43 | 1574.50 ± 346.00 | 1852.67 ± 569.67 | ND | 2.86 ± 1.21 | ND | ND | NA | |||
| CG, i.p. [223] | Rat | 10 mg/kg | CG | 965.24 ± 133.53 | 0.25 ± 0.06 | 1199.67 ± 373.83 | 1210.67 ± 368.00 | ND | 1.29 ± 0.30 | ND | ND | NA |
| CG glucuronide | 322.24 ± 125.53 | 1.10 ± 0.42 | 1681.33 ± 642.67 | 2422.50 ± 1118.33 | ND | 6.65 ± 4.85 | ND | ND | NA | |||
| C-3′-G | 221.62 ± 86.60 | 1.10 ± 0.42 | 1151.83 ± 443.33 | 1651.83 ± 765.00 | ND | 6.57 ± 4.82 | ND | ND | NA | |||
| CG, p.o. [224] | Rat | 120 mg/kg | CG | 1870.00 ± 360.00 | 0.67 ± 0.08 | 1595.33 ± 472.33 | 1679.67 ± 509.00 | ND | 0.19 ± 0.02 (Ka); 0.26 ± 0.02 (Ke) | 71.40 ± 16.20 | ND | NA |
| Ononin, p.o. [218] | Rat | 20 mg/kg | Ononin | (1) 2.11 ± 11.23 (2) 4.63 ± 3.62 | (1) 0.5 ± 0.0 (2) 4.0 ± 0.0 | 68.95 ± 16.83 | 74.59 ± 18.55 | ND | 1.82 ± 0.56 | ND | ND | NA |
| Formononetin | (1) 44 ± 2.71 (2) 0.52 ± 3.35 | (1) 1.0 ± 0.0 (2) 5.7 ± 0.0 | 85.09 ± 13.17 | ND | ND | NA | ND | ND | NA | |||
| Ononin, i.v. [218] | Rat | 4 mg/kg | Ononin | 491.61 ± 44.42 | NA | 189.17 ± 23.29 | 199.62 ± 25.87 | ND | 1.92 ± 0.8 | 6.6 ± 0.9 | 18.4 ± 1.5 | ND |
| AS-IV, p.o. [225] | Rat | 20 mg/kg | AS-IV | 92.4 ± 14.2 | 1.0 ± 0.5 | 419.50 ± 126.65 | 420.41 ± 129.01 | ND | t1/2α = 0.89 ± 0.37; t1/2β = 22.02 ± 8.26 | 0.045 ± 0.013 | 0.19 ± 0.15 | NA |
| AS-IV, i.v. [226] | Rat (male) | 0.75 mg/kg | AS-IV | 3790 | NA | ND | 4816.67 | 122 | 1.64 | 0.3 | 0.39 | 0.63 |
| AS-IV, i.v. [226] | Rat (female) | 0.75 mg/kg | AS-IV | 5180 | NA | ND | 2550 | 47.4 | 0.57 | 0.3 | 0.14 | 0.23 |
| AS-IV, i.v. [226] | Rat (male) | 1.5 mg/kg | AS-IV | 6980 | NA | ND | 6700 | 95.2 | 1.12 | 0.42 | 0.43 | 0.71 |
| AS-IV, i.v. [226] | Rat (female) | 1.5 mg/kg | AS-IV | 4800 | NA | ND | 4966.67 | 90.1 | 1.12 | 0.18 | 0.16 | 0.23 |
| AS-IV, i.v. [226] | Rat (male) | 3 mg/kg | AS-IV | 7790 | NA | ND | 8666.67 | 98.7 | 1.20 | 0.36 | 0.38 | 0.57 |
| AS-IV, i.v. [226] | Rat (female) | 3 mg/kg | AS-IV | 7240 | NA | ND | 9183.33 | 149 | 2.48 | 0.18 | 0.21 | 0.41 |
| AS-IV, i.v. [226] | Dog (male) | 0.25 mg/kg | AS-IV | 1110 ± 280 | NA | ND | 1218.33 ± 516.67 | 71.5 ± 7.0 | 0.87 ± 0.14 | 0.24 ± 0.06 | 0.23 ± 0.05 | 0.32 ± 0.13 |
| AS-IV, i.v. [226] | Dog (female) | 0.25 mg/kg | AS-IV | 1130 ± 40 | NA | ND | 1096.67 ± 300.00 | 82.8 ± 29 | 1.05 ± 0.37 | 0.24 ± 0.06 | 0.22 ± 0.01 | 0.36 ± 0.04 |
| AS-IV, i.v. [226] | Dog (male) | 0.5 mg/kg | AS-IV | 4390 ± 2600 | NA | ND | 2600.00 ± 121.67 | 75.5 ± 6.9 | 1.00 ± 0.14 | 0.24 ± 0.06 | 0.14 ± 0.07 | 0.32 ± 0.09 |
| AS-IV, i.v. [226] | Dog (female) | 0.5 mg/kg | AS-IV | 3480 ± 1600 | NA | ND | 2883.33 ± 766.67 | 78.0 ± 8.5 | 1.12 ± 0.13 | 0.24 ± 0.06 | 0.16 ± 0.07 | 0.31 ± 0.06 |
| AS-IV, i.v. [226] | Dog (male) | 1 mg/kg | AS-IV | 7920 ± 3700 | NA | ND | 6033.33 ± 1316.67 | 83.0 ± 23 | 1.15 ± 0.35 | 0.24 ± 0.06 | 0.14 ± 0.07 | 0.28 ± 0.08 |
| AS-IV, i.v. [226] | Dog (female) | 1 mg/kg | AS-IV | 8860 ± 2500 | NA | ND | 5916.67 ± 1366.67 | 64.8 ± 16 | 0.84 ± 0.22 | 0.18 ± 0.06 | 0.12 ± 0.04 | 0.23 ± 0.04 |
| AS-IV, i.v. [227] | Rat | 2.5 mg/kg | AS-IV | ND | NA | ND | 5990 | ND | 3.37 | 0.42 | 0.77 | ND |
| AS-IV, p.o. [228] | Rat | 20 mg/kg | AS-IV | 374 | 0.43 | ND | 1062 | ND | t1/2α = 0.30; t1/2β = 4.65; | 0.43 | 0.56 | NA |
| AS-IV, p.o. [229] | Rat | 10 mg/kg | AS-IV | 27.08 ± 16.17 | 2.00 ± 63 | 85.56 ± 43.17 | ND | ND | 1.71 ± 1.16 | ND | ND | NA |
| Cycloastragenol | 3.48 ± 2.34 | 9.67 ± 0.82 | 13.15 ± 8,78 | ND | ND | ND | ND | ND | NA | |||
| Iso-cycloastragenol | 7.02 ± 3.68 | 8.00 ± 0.00 | 38.23 ± 17.22 | ND | ND | 4.77 ± 3.26 | ND | ND | NA | |||
| AS-IV, i.v. [229] | Rat | 1.5 mg/kg | AS-IV | 7865.40 ± 570.67 | NA | 7917.21 ± 1038.52 | ND | ND | 1.29 ± 0.41 | ND | ND | ND |
| Cycloastragenol | 1.21 ± 0.22 | 8.33 ± 0.82 | 2.73 ± 0.79 | ND | ND | ND | ND | ND | ND | |||
| Iso-cycloastragenol | 2.98 ± 0.86 | 8.00 ± 0.00 | 15.80 ± 2.60 | ND | ND | 3.05 ± 1.06 | ND | ND | ND | |||
| AS-IV, i.v. [230] | Dog | 0.5 mg/kg | AS-IV | ND | NA | 1998.22 | 2104 | 222.61 | 2.953 | 0.24 | ND | 0.74 |
| AS-IV, i.v. [230] | Dog | 1 mg/kg | AS-IV | ND | NA | 4380 | 4604.67 | 232.48 | 3.28 | 0.24 | ND | 0.61 |
| AS-IV, i.v. [230] | Dog | 2 mg/kg | AS-IV | ND | NA | 11706.67 | 12075.17 | 241.80 | 4.03 | 0.18 | ND | 0.61 |
| AS-IV, p.o. [230] | Dog | 10 mg/kg | AS-IV | ND | NA | 3368.33 | 3400.83 | 261.14 | 3.83 | 0.6 | ND | 2.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Lou, Y.; Kong, M.; Luo, Q.; Liu, Z.; Wu, J. A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine. Int. J. Mol. Sci. 2019, 20, 1463. https://doi.org/10.3390/ijms20061463
Guo Z, Lou Y, Kong M, Luo Q, Liu Z, Wu J. A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine. International Journal of Molecular Sciences. 2019; 20(6):1463. https://doi.org/10.3390/ijms20061463
Chicago/Turabian StyleGuo, Zhenzhen, Yanmei Lou, Muyan Kong, Qing Luo, Zhongqiu Liu, and Jinjun Wu. 2019. "A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine" International Journal of Molecular Sciences 20, no. 6: 1463. https://doi.org/10.3390/ijms20061463
APA StyleGuo, Z., Lou, Y., Kong, M., Luo, Q., Liu, Z., & Wu, J. (2019). A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine. International Journal of Molecular Sciences, 20(6), 1463. https://doi.org/10.3390/ijms20061463




