The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats
Abstract
:1. Introduction
2. Results
2.1. Morphology and Physicochemical Parameters of Nano-Cu
2.2. Concentration of Copper in the Spleen
2.3. Nano-Cu Altered the Number of Blood Cells in Rats
2.4. Nano-Cu Exposure Altered Lymphocyte Subpopulation in the Spleen
2.5. Nano-Cu Exposure Affected the Antibody Production
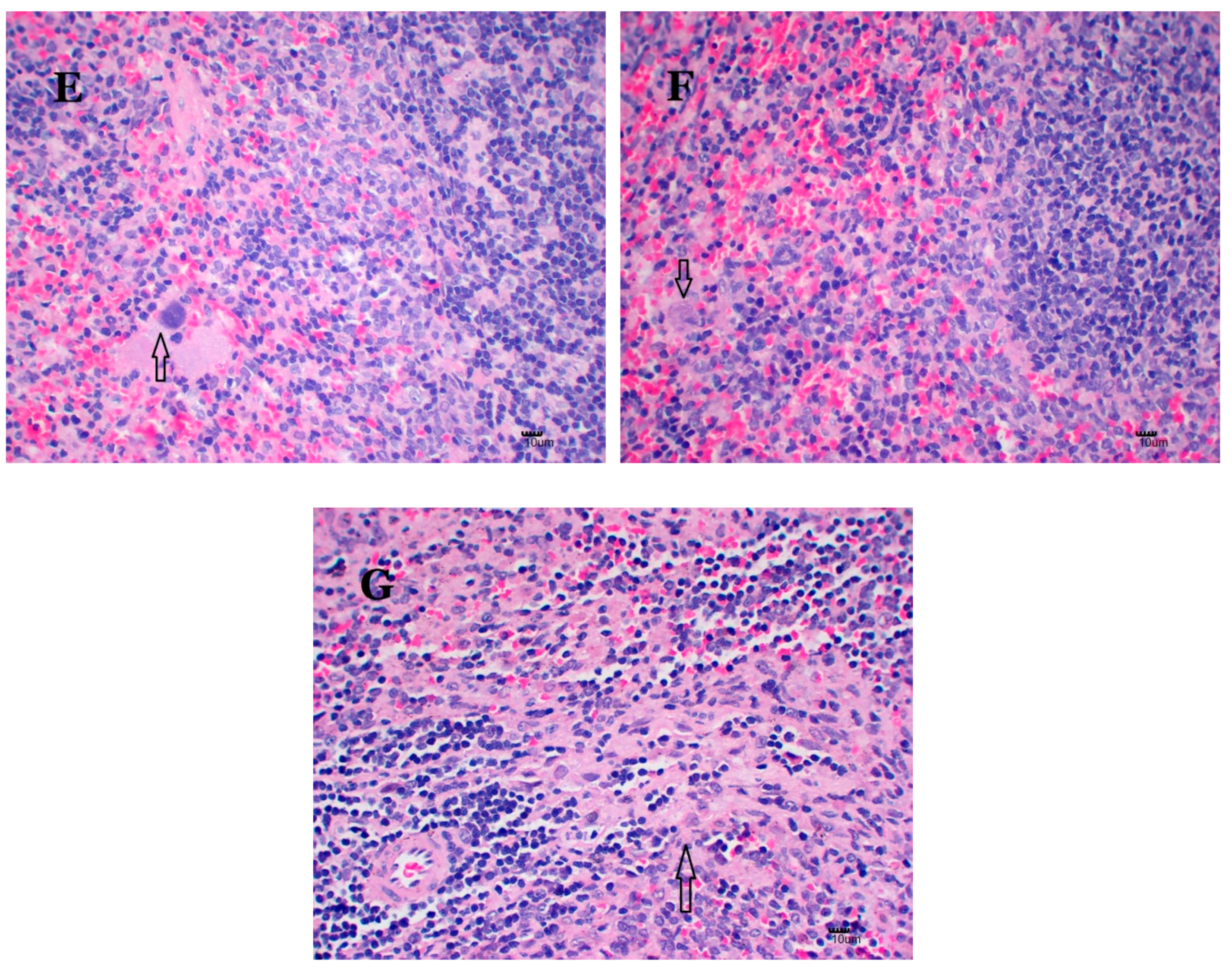
2.6. Nano-Cu Exposure Induced Obvious Histopathology Changes
2.7. Analysis of the Oxidative Stress in the Spleen
2.8. Nano-Cu Exposure Induced Inflammatory Responses in the Spleen
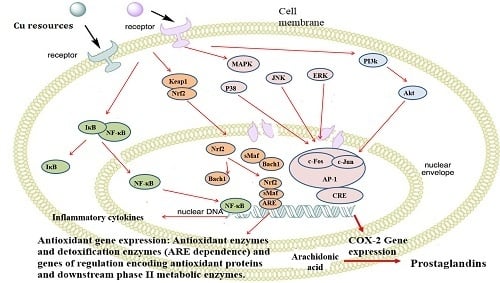
2.9. Nano-Cu Exposure Activated MAPK, NrF2, and PI3K in the Spleen
3. Discussion
4. Material and Methods
4.1. Characterization of Nano-Cu
4.2. Animals and Experimental Procedures
4.3. Copper Concentration in the Spleen
4.4. Hematologic Analysis
4.5. Lymphocyte Subpopulation Analysis
4.6. Immunoglobulin Analysis
4.7. Histological Examination
4.8. Oxidative Stress Analysis
4.9. mRNA and Protein Expression of Cytokines
4.10. mRNA and Protein Expression of Nrf2, PI3K/Akt, and MAPKs Signalling Pathways
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RBC | Red blood cell count |
| WBC | White blood cell count |
| PLT | Platelets |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| CAT | Cata-lase |
| GSH-Px | Glutathione peroxidase |
| NF-κB | Nuclear factor-kappa B |
| TNF-α | Tumor necrosis factor alpha |
| INF-γ | Interferon-gamma |
| MCP-1 | Monocyte chemotactic protein-1 |
| MIP-1α | Macrophage inflammatory protein-1 alpha |
| IL-1β/-2/-4/-6 | Interleukin -1beta/-2/-4/-6 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | nuclear factor erythroid-2p45-related factor2 |
| Bach1 | BTB and CNC homology 1 |
| ARE | Antioxidant response element |
| HO-1 | Heme oxygenase1 |
| PI3-K | Phosphatidylinositol 3-kinase |
| ERK1/2 | Extracellular regulated protein kinases1/2 |
| JNK | c-Jun N-terminal kinase |
| p38 | p38 mitogen-activated protein kinase |
| CREB | cAMP-response element binding protein |
| AP-1 | Activating protein-1 |
| COX-2 | Cyclooxygenase 2 |
References
- Tarantino, G.; Porcu, C.; Arciello, M.; Andreozzi, P.; Balsano, C. Serum copper bioavailability predicts carotid intima-media tickness in obese patients with low prevalence of co-morbidities. J. Gastroenterol. Hepatol. 2018, 33, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Braude, R. Some observations on the need for copper in the diet of fattening pigs. J. Agric. Sci. 1945, 35, 163–167. [Google Scholar] [CrossRef]
- Burnell, T.W.; Cromwell, G.L.; Stahly, T.S. Effects of dried whey and copper sulfate on the growth responses to organic acid in diets for weanling pigs. J. Anim. Sci. 1998, 66, 1100. [Google Scholar] [CrossRef]
- Anyagu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. LANGMUIR 2008, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Eguia, A.; Fu, C.M.; Lu, F.Y.; Lien, T.F. Effects of nanocopper on copper availability and nutrients digestibility, growth performance and serum traits of piglets. Livest. Sci. 2009, 126, 122–129. [Google Scholar] [CrossRef]
- Joshua, P.P.; Valli, C.; Balakrishnan, V. Effect of in ovo supplementation of nano forms of zinc, copper, and selenium on post-hatch performance of broiler chicken. Vet. World 2016, 9, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Pineda, L.; Sawosz, E.; Vadalasetty, K.P.; Chwalibog, A. Effect of copper nanoparticles on metabolic rate and development of chicken embryos. Anim. Feed Sci. Technol. 2013, 186, 125–129. [Google Scholar] [CrossRef]
- Rao, P.V.; Prasad, T.; Sheikh, R.A.; Balam, S.K.; Narasimhulu, G.; Suresh, R.C.; Ab, I.R.; Hua, S.G. Biogenic silver nanoparticles usingRhinacanthus nasutusleaf extract: Synthesis, spectral analysis, and antimicrobial studies. Int. J. Nanomed. 2013, 8, 3355–3364. [Google Scholar]
- Mroczek-Sosnowska, N.; Lukasiewicz, M.; Wnuk, A.; Sawosz, E.; Niemiec, J. Effect of copper nanoparticles and copper sulfate administered in ovo on copper content in breast muscle, liver and spleen of broiler chickens. Folia Morphol. 2014, 52, 135–142. [Google Scholar]
- Dreher, K.L. Health and Environmental Impact of Nanotechnology: Toxicological Assessment of Manufactured Nanoparticles. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 77, 3–5. [Google Scholar] [CrossRef]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 2011, 42, 821–826. [Google Scholar] [CrossRef]
- Kaewamatawong, T.; Kawamura, N.; Okajima, M.; Sawada, M.; Morita, T.; Shimada, A. Acute pulmonary toxicity caused by exposure to colloidal silica: Particle size dependent pathological changes in mice. Toxicol. Pathol. 2005, 33, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long circulating and target specific nanoparticles: Theory to practice. Pharm. Rev 2001, 53, 283–318. [Google Scholar] [PubMed]
- Jong, W.H.D.; Loveren, H.V. Screening of xenobiotics for direct immuneotoxicity in an animal study. Methods 2007, 41, 3–8. [Google Scholar] [CrossRef]
- Reddy, R.V.; Taylor, M.J.; Sharma, R.P. Evaluation of citrinin toxicity on the immune functions of mice. J. Food Prot. 1988, 51, 32–36. [Google Scholar] [CrossRef]
- Sizova, E.; Glushchenko, N.; Miroshnikov, S.; Skalny, A. Influence of cu10x copper nanoparticles intramuscular injection on mineral composition of rat spleen. J. Trace Elem. Med. Biol. 2011, 25, S84–S89. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Zheng, L.; Sun, Q.; Li, N.; Cui, Y.; Hu, R.; Gao, G.; Cheng, Z.; Cheng, J.; Gui, S.; et al. The chronic spleen injury of mice following long-term exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 894–902. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Tang, H.; Xu, M.; Zhou, X.; Zhang, Y.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Li, Y. Acute toxicity and biodistribution of different sized copper nano-particles in rats after oral administration. Mater. Sci. Eng. C 2018, 93, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Shin, N.R.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar] [Green Version]
- Zhang, H.; Wu, X.; Mehmood, K.; Chang, Z.; Li, K.; Jiang, X.; Nabi, F.; Ijaz, M.; Rehman, M.U.; Javed, M.T.; et al. Intestinal epithelial cell injury induced by copper containing nanoparticles in piglets. Environ. Toxicol. Pharm. 2017, 56, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cholewinska, E.; Ognik, K.; Fotschki, B.; Zdunczyk, Z.; Juskiewicz, J. Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS ONE 2018, 13, e0197083. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Hajivar, S.; Zakeri-Milani, P.; Mohammadi, H.; Niazi, M.; Soleymani-Goloujeh, M.; Baradaran, B.; Valizadeh, H. Overview on experimental models of interactions between nanoparticles and the immune system. Biomed. Pharmacother. 2016, 83, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, C.; Clemente, E.; Amato, V.; Pedata, P.; Sabbioni, E.; Bernardini, G.; Paganelli, R. Engineered metal based nanoparticles and innate immunity. Clin. Mol. Allergy 2015, 13, 13. [Google Scholar] [CrossRef]
- Mantovani, A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010, 10, 369–373. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Pullner, A.; King, S.B.S.; Drexler, I.; Meier, S.; Brocker, T.; Voehringer, D. Constitutive ablation of dendritic cells breaks self-tolerance of cd4 t cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009, 206, 549–559. [Google Scholar] [CrossRef]
- Barbano, R.; Muscarella, L.A.; Pasculli, B.; Valori, V.M.; Fontana, A.; Coco, M.; Torre, A.I.; Balsamo, T.; Poeta, M.L.; Marangi, G.F.; et al. Aberrant Keap1 methylation in breast cancer and association with clinicopathological features. Epigenetics 2013, 8, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Shah, A.; Gangwani, M.R.; Silverstein, P.S.; Fu, M.; Kumar, A. Hiv-1 nef induces ccl5 production in astrocytes through p38-mapk and pi3k/akt pathway and utilizes nf-kb, cebp and ap-1 transcription factors. Sci. Rep. 2014, 4, 4450. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kuo, C.T.; Cheng, C.Y.; Wu, C.Y.; Lee, C.W.; Hsieh, H.L.; Lee, I.T.; Yang, C.M. Il-1β promotes A549 cell migration via MAPKs/AP-1- and nf-κB-dependent matrix metalloproteinase-9 expression. Cell. Signal. 2009, 21, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Pirillo, A.; Pellegatta, F.; Inoue, H.; Catapano, A.L. Native LDL and oxidized LDL modulate cyclooxygenase-2 expression in HUVECsthrough a p38-MAPK, NF-kappaB, CRE dependent pathway and affect PGE2 synthesis. Int. J. Mol. Med. 2004, 14, 353–359. [Google Scholar] [PubMed]
- Subbaramaiah, K.; Cole-PADannenberg, A.J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by creb-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2013, 62, 2522–2530. [Google Scholar]
- Tuan, T.N.M.D.; Vincent, A.Z.P.D.; Shigeo-Uematsu, M.D.; Jerry, L.M.P.D.; Gerald-Weinstein, M.D. New model of a scaling dermatosis: Induction of hyperproliferation in hairless mice with eicosa-5,8,11-trienoic acid. J. Investig. Dermatol. 1981, 76, 384–387. [Google Scholar]
- Inoue, H.; Nanayama, T.; Hara, S.; Yokoyama, C.; Tanabe, T. The cyclic AMP response element plays an essential role in the expression of the human prostaglandin-endoperoxide synthase 2 gene in differentiated U937 monocytic cells. FEBS Lett. 1994, 350, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Driessen, G.J.; Ijspeert, H.; Wentink, M.; Yntema, H.G.; van Hagen, P.M.; Van, S.A.; Bucciol, G.; Cogulu, O.; Trip, M.; Nillesen, W.; et al. Increased pi3k/akt activity and deregulated humoral immune response in human pten deficiency. J. Allergy Clin. Immunol. 2016, 138, 1744–1747. [Google Scholar] [CrossRef]
- NIH (National Institutes of Health). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996.
- Liu, W.H.; Saint, D.A. Validation of a quantitative method for real time PCR kinetics. Biochem. Biophys. Res. Commun. 2002, 294, 347–353. [Google Scholar] [CrossRef]
- Tan, C.Y.; Immermann, F.W.; Shite, S.; Pride, M.W.; Danka, P.; Belanger, K.A.; Wendy, W.; Daniel, A.S.; Mohinder, S.; Kathrin, U.J.; et al. Evaluation of a Validated Luminex-Based Multiplex Immunoassay for Measuring Immunoglobulin G Antibodies in Serum to Pneumococcal Capsular Polysaccharides. mSphere 2018, 3, e00127-18. [Google Scholar] [CrossRef]










| Particles | Average Size | Size Distribution | Purity (%) |
|---|---|---|---|
| Nano-Cu | 80 nm | 75 ± 35 nm | 99.9 |
| Micro-Cu | 1 μm | 0.85 ± 0.40 μm | 99.9 |
| Index | Control | Micro-Cu | CuCl2·2H2O | Nano-Cu 80–100 nm | ||
|---|---|---|---|---|---|---|
| 1%HPMC | 200 (mg/kg) | 200 (mg/kg) | 50 (mg/kg) | 100 (mg/kg) | 200 (mg/kg) | |
| RBC (1012/L) | 7.8 ± 0.5 | 7.2 ± 0.4 | 7.2 ± 0.3 | 7.1 ± 0.3 | 6.9 ± 0.4 * | 6.7 ± 0.5 * |
| WBC (109/L) | 9.2 ± 2.5 | 13.5 ± 2.6 ** | 17.1 ± 3.5 ** | 10.1 ± 2.5 | 10.9 ± 2.1 * | 12.9 ± 2.3 ** |
| lymphocytes (%) | 8.8 ± 2.7 | 11.6 ± 3.4 ** | 12.7 ± 3.1 ** | 8.4 ± 1.6 | 9.5 ± 1.4 | 10.9 ± 2.3 ** |
| PLT (109/L) | 1337.6 ± 100.5 | 1395 ± 104.6 | 1292.1 ± 135.4 | 1368.6 ± 100.6 | 1393.7 ± 65.1 | 1424.2 ± 129.9 * |
| HGB (g/dL) | 159.3 ± 5.3 | 155 ± 8.1 | 155.1 ± 6.7 | 154.9 ± 9.9 | 156.7 ± 4.5 | 155.6 ± 12.3 |
| HCT (%) | 46 ± 2.8 | 42.8 ± 2.4 | 42.7 ± 2 | 48.6 ± 2.6 | 40.7 ± 1.3 * | 40.9 ± 2.6 * |
| Index | Control | Micro-Cu | CuCl2·2H2O | Nano-Cu 80–100 nm | ||
|---|---|---|---|---|---|---|
| 1%HPMC | 200 (mg/kg) | 200 (mg/kg) | 50 (mg/kg) | 100 (mg/kg) | 200 (mg/kg) | |
| MDA (nmol/mgprot) | 1.38 ± 0.07 | 1.55 ± 0.2 * | 1.4 ± 0.04 | 1.31 ± 0.09 | 1.61 ± 0.11 ** | 1.75 ± 0.02 ** |
| SOD (U/mgprot) | 58.77 ± 6.35 | 67.46 ± 5.53 ** | 65.12 ± 3.51 * | 57.38 ± 4.23 | 65.17 ± 5.39* | 69.58 ± 6.59 ** |
| NO (μmol/gprot) | 0.135 ± 0.004 | 0.147 ± 0.014 * | 0.155 ± 0.006 * | 0.129 ± 0.008 | 0.149 ± 0.003** | 0.154 ± 0.005 ** |
| iNOS (U/mgprot) | 0.55 ± 0.14 | 0.6 ± 0.03 | 0.67 ± 0.15 * | 0.59 ± 0.02 | 0.69 ± 0.09 * | 0.72 ± 0.11 ** |
| CAT (U/mgprot) | 10.8 ± 2.4 | 14.6 ± 2 ** | 14.2 ± 0.6 ** | 10.1 ± 2.3 | 15.9 ± 1.4 ** | 15.7 ± 0.8 ** |
| GSH-Px (U/mgprot) | 506.36 ± 41.07 | 613.74 ± 75.21 ** | 589.42 ± 16.08 * | 545.04 ± 78.91 | 672.40 ± 42.94 ** | 751.45 ± 96.60 ** |
| Gene | Control | Micro-Cu | CuCl2·2H2O | Nano-Cu 80–100 nm | ||
|---|---|---|---|---|---|---|
| 1%HPMC | 200 (mg/kg) | 200 (mg/kg) | 50 (mg/kg) | 100 (mg/kg) | 200 (mg/kg) | |
| IFN-γ | 1.00 ± 0.00 | 1.08 ± 0.07 | 1.19 ± 0.06 * | 1.03 ± 0.01 | 1.17 ± 0.03 * | 1.68 ± 0.08 ** |
| TNF-α | 1.00 ± 0.00 | 1.34 ± 0.10 ** | 1.45 ± 0.05 ** | 1.04 ± 0.01 | 1.43 ± 0.06 ** | 1.63 ± 0.08 ** |
| MIP-1α | 1.00 ± 0.00 | 1.37 ± 0.03 * | 1.48 ± 0.04 ** | 1.01 ± 0.02 | 1.22 ± 0.02 * | 1.74 ± 0.08 ** |
| MCP-1 | 1.00 ± 0.00 | 1.05 ± 0.03 | 1.16 ± 0.03 * | 1.05 ± 0.08 | 1.15 ± 0.08 * | 1.44 ± 0.08 ** |
| MIF | 1.00 ± 0.00 | 1.09 ± 0.60 | 1.18 ± 0.20 * | 1.06 ± 0.03 | 1.34 ± 0.50 ** | 1.57 ± 0.90 ** |
| IL-1β | 1.00 ± 0.00 | 1.31 ± 0.03 * | 1.73 ± 0.02 ** | 1.00 ± 0.02 | 1.13 ± 0.04 * | 1.54 ± 0.05 ** |
| IL-2 | 1.00 ± 0.00 | 1.17 ± 0.01 * | 1.23 ± 0.04 * | 1.00 ± 0.01 | 1.18 ± 0.01 * | 1.43 ± 0.03 ** |
| IL-4 | 1.00 ± 0.00 | 1.16 ± 0.03 * | 1.19 ± 0.05 * | 1.08 ± 0.02 | 1.16 ± 0.02 * | 1.51 ± 0.02 ** |
| IL-6 | 1.00 ± 0.00 | 1.13 ± 0.05 * | 1.17 ± 0.05 * | 1.01 ± 0.02 | 1.22 ± 0.01 * | 1.48 ± 0.03 ** |
| Protein (pg/mL) | Control | Micro-Cu | CuCl2·2H2O | Nano-Cu 80–100 nm | ||
|---|---|---|---|---|---|---|
| 1%HPMC | 200 (mg/kg) | 200 (mg/kg) | 50 (mg/kg) | 100 (mg/kg) | 200 (mg/kg) | |
| IFN-γ | 156.3 ± 5.7 | 165.0 ± 4.6 * | 174.7 ± 8.5 * | 159.3 ± 5.5 | 169.3 ± 11.5 * | 252.0 ± 11.0 ** |
| TNF-α | 91.6 ± 2.2 | 153.1 ± 4.6 ** | 142.8 ± 5.0 ** | 91.7 ± 8.2 | 163.6 ± 4.7 ** | 205.3 ± 3.3 ** |
| MIP-1α | 19.4 ± 2.9 | 27.4 ± 4.7 * | 28.9 ± 3.3 ** | 19.8 ± 1.9 | 23.6 ± 4.7 * | 33.5 ± 5.6 ** |
| MCP-1 | 103.8 ± 1.8 | 107.6 ± 1.7 | 111.9 ± 2.1 | 104.1 ± 2.1 | 109.2 ± 1.7 | 119.0 ± 2.0 * |
| MIF | 36.5 ± 3.2 | 38.6 ± 2.5 | 42.5 ± 6.1 ** | 37.1 ± 1.8 | 39.5 ± 2.2 * | 58.88 ± 1.4 ** |
| IL-1β | 149.5 ± 3.6 | 194.3 ± 2.5 ** | 254.9 ± 13.8 ** | 148.3 ± 3 | 171.8 ± 7.5 * | 198.5 ± 19.1 ** |
| IL-2 | 14.8 ± 2.4 | 16.5 ± 3.1 * | 18.2 ± 2.1 * | 14.5 ± 1.5 | 16.6 ± 1.7 * | 18.4 ± 3.7 ** |
| IL-4 | 26.0 ± 2.7 | 22.1 ± 3.7 ** | 27.5 ± 3.8 | 25.0 ± 2.7 | 27.7 ± 4.1 | 30.2 ± 3.7 ** |
| IL-6 | 96.5 ± 8.2 | 90.6 ± 5.3 * | 94.0 ± 6.7 | 96.0 ± 8.5 | 103.1 ± 7.9 * | 113.1 ± 10.7 ** |
| Primer name | Primer Sequence (5′ to 3′) | Product Size (bp) | |
|---|---|---|---|
| GAPDH | F: CCTTCCGTGTTCCTACCCC | R: GCCCAGGATGCCCTTTAGTG | 131 |
| IFN-γ | F: TTCGAGGTGAACAACCCACA | R: CACTCTCTACCCCAGAATCAGC | 131 |
| TNF-α | F: AAGGGAATTGTGGCTCTGGG | R: ACTTCAGCGTCTCGTGTGTT | 83 |
| MIP-1α | F: GCCTGAGATTAGAGGCAGCA | R: AGGTGGCAGGAATGTTCTGG | 89 |
| MCP-1 | F: GGGCCTGTTGTTCACAGTTG | R: TGAGTAGCAGCAGGTGAGTG | 88 |
| MIF | F: GGCCTCACTTACCTGCACC | R: AACCATTTATTTCTCCCGACC | 108 |
| IL-1β | F: TTGAGTCTGCACAGTTCCCC | R: ATGTCCCGACCATTGCTGTT | 91 |
| IL-2 | F: AACAAGTCTGGGGTTCTCGG | R: TGTTGTGAGCGTGGACTCAT | 102 |
| IL-4 | F: AACAAGTCTGGGGTTCTCGG | R: TGTTGTGAGCGTGGACTCAT | 102 |
| IL-6 | F: CTGGTCTTCTGGAGTTCCGTT | R: AGAGCATTGGAAGTTGGGGT | 175 |
| Keap1 | F: CCAGGTACATAGGTCTGGCTG | R: CCAGTACGCCTCTAGCTGAA | 72 |
| Bach1 | F: AAGCTGAGTTTGGAGGCAGA | R: GTGCAAACCCACAATGGACC | 88 |
| Nrf2 | F: GGCTGTGTGTTCTGAGTATCG | R: TCCATGTCCGTTGTAAGCCA | 86 |
| HO-1 | F: AGCACAGGGTGACAGAAGAG | R: AACTCTGTCTGTGAGGGACT | 118 |
| PI3K | F: GTGCCTTAGCTCTCTCTGCT | R: ACTGGGTTTCCTCATGGCTG | 163 |
| ERK1/2 | F: TCCTTGGGAGGGAAGATACC | R: ATGACAATCCCGTAGCTCCA | 101 |
| JNK | F: TGATGACGCCTTACGTGGTA | R: GGCAAACCATTTCTCCCATA | 120 |
| p38 | F: AGACGAATGGAAGAGCCTGA | R: GGGATGGACAGAACAGAAGC | 109 |
| CREB | F: GAGAAGCCGAGTGTTGGTGA | R: ACTCTGCTGGTTGTCTGCTC | 176 |
| AP-1 | F: AAGTAGCCCCCAACCTCTCT | R: CACCCCAGCATACAGACACT | 85 |
| COX-2 | F: TTTCAATGTGCAAGACCCGC | R: TACAGCTCAGTTGAACGCCT | 120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhao, L.; Luo, J.; Tang, H.; Xu, M.; Wang, Y.; Yang, X.; Chen, H.; Li, Y.; Ye, G.; et al. The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats. Int. J. Mol. Sci. 2019, 20, 1469. https://doi.org/10.3390/ijms20061469
Zhou X, Zhao L, Luo J, Tang H, Xu M, Wang Y, Yang X, Chen H, Li Y, Ye G, et al. The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats. International Journal of Molecular Sciences. 2019; 20(6):1469. https://doi.org/10.3390/ijms20061469
Chicago/Turabian StyleZhou, Xuerong, Ling Zhao, Jie Luo, Huaqiao Tang, Min Xu, Yanyan Wang, Xiaoyu Yang, Helin Chen, Yinglun Li, Gang Ye, and et al. 2019. "The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats" International Journal of Molecular Sciences 20, no. 6: 1469. https://doi.org/10.3390/ijms20061469
APA StyleZhou, X., Zhao, L., Luo, J., Tang, H., Xu, M., Wang, Y., Yang, X., Chen, H., Li, Y., Ye, G., Shi, F., Lv, C., & Jing, B. (2019). The Toxic Effects and Mechanisms of Nano-Cu on the Spleen of Rats. International Journal of Molecular Sciences, 20(6), 1469. https://doi.org/10.3390/ijms20061469