Biosynthetic Gene Pyramiding Leads to Ascorbate Accumulation with Enhanced Oxidative Stress Tolerance in Tomato
Abstract
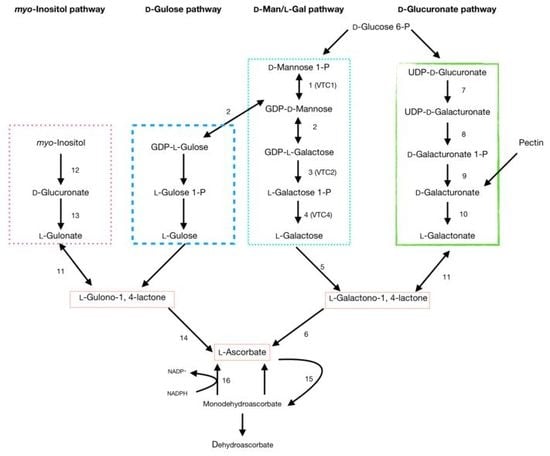
:1. Introduction
2. Results
2.1. Gene Pyramiding Altered Expression of AsA-Related Genes and AsA Concentration in Both Leaves and Fruits
2.2. Different Biosynthesis Pathways Contributed to the Total AsA Content in Leaves and Fruit of Pyramiding Lines
2.3. Light-Dependent Fluctuation of AsA Content in Wild Type and Pyramiding Lines
2.4. Pyramiding Biosynthetic Genes Increases AsA Transport Capacity
2.5. AsA Biosynthetic Pyramiding Improved Tolerance to Oxidative Stress in Tomato
2.6. The Fruit Shape, Fruit Size, and Soluble Solids Were Affected by Gene Pyramiding
3. Discussion
4. Methods
4.1. Plant Growth Conditions and Sampling
4.2. RNA Isolation and Gene Expression Analysis
4.3. Determination of AsA Content
4.4. Synthetic Precursor Feeding to Leaves and Fruits
4.5. Light Response Assay
4.6. Oxidative Stress Treatment
4.7. The AsA of Exudates of Leaf and Fruit Petioles Phloem
4.8. Histochemical Staining of AsA with Acidic AgNO3
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Gallie, D.R. L-ascorbic Acid: A multifunctional molecule supporting plant growth and development. Scientifica 2013, 2013, 795964. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Gest, N.; Garchery, C.; Gautier, H.; Jimenez, A.; Stevens, R. Light-dependent regulation of ascorbate in tomato by a monodehydroascorbate reductase localized in peroxisomes and the cytosol. Plant Biotechnol. J. 2013, 11, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, M.C.; Locato, V.; De Gara, L. Redox regulation in plant programmed cell death. Plant Cell Environ. 2012, 35, 234–244. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Kanellis, A.K. Altered apoplastic ascorbate redox state in tobacco plants via ascorbate oxidase overexpression results in delayed dark-induced senescence in detached leaves. Plant Physiol. Biochem. 2013, 73, 154–160. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Tetali, S.D.; Selinski, J.; Scheibe, R.; Padmasree, K. Importance of the alternative oxidase (AOX) pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of the cytochrome oxidase pathway in Arabidopsis thaliana. Ann. Bot. 2015, 116, 555–569. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Bonifacio, A.; Carvalho, F.E.; Andrade, C.M.; Passaia, G.; Schunemann, M.; Maraschin Fdos, S.; Martins, M.O.; Teixeira, F.K.; Rauber, R.; et al. The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci. 2014, 214, 74–87. [Google Scholar] [CrossRef]
- Pavet, V.; Olmos, E.; Kiddle, G.; Mowla, S.; Kumar, S.; Antoniw, J.; Alvarez, M.E.; Foyer, C.H. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol. 2005, 139, 1291–1303. [Google Scholar] [CrossRef]
- Stevens, R.; Buret, M.; Duffe, P.; Garchery, C.; Baldet, P.; Rothan, C.; Causse, M. Candidate genes and quantitative trait loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 2007, 143, 1943–1953. [Google Scholar] [CrossRef]
- Nishikirni, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Wolucka, B.A.; Van Montagu, M. GDP-mannose 3’,5’-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Gilot, C.; Persiau, G.; Ostergaard, J.; Han, Y.; Bauw, G.C.; Van Montagu, M.C. Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol. 1999, 121, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Valpuesta, V.; Botella, M.A. Biosynthesis of L-ascorbic acid in plants: New pathways for an old antioxidant. Trends Plant Sci. 2004, 9, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef]
- Laing, W.A.; Bulley, S.; Wright, M.; Cooney, J.; Jensen, D.; Barraclough, D.; MacRae, E. A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 16976–16981. [Google Scholar] [CrossRef] [Green Version]
- Gatzek, S.; Wheeler, G.L.; Smirnoff, N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002, 30, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Tabata, K.; Oba, K.; Suzuki, K.; Esaka, M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactone dehydrogenase. Plant J. 2001, 27, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Conklin, P.L.; Norris, S.R.; Wheeler, G.L.; Williams, E.H.; Smirnoff, N.; Last, R.L. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4198–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronje, C.; George, G.M.; Fernie, A.R.; Bekker, J.; Kossmann, J.; Bauer, R. Manipulation of L-ascorbic acid biosynthesis pathways in Solanum lycopersicum: Elevated GDP-mannose pyrophosphorylase activity enhances L-ascorbate levels in red fruit. Planta 2012, 235, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ouyang, B.; Yang, C.; Zhang, X.; Liu, H.; Zhang, Y.; Zhang, J.; Li, H.; Ye, Z. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLoS ONE 2013, 8, e61987. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Alhagdow, M.; Nunes-Nesi, A.; Quemener, B.; Guillon, F.; Bouchet, B.; Faurobert, M.; Gouble, B.; Page, D.; Garcia, V.; et al. GDP-D-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. Plant J. 2009, 60, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Liu, J.X.; Zhang, Y.Y.; Cai, X.F.; Gong, P.J.; Zhang, J.H.; Wang, T.T.; Li, H.X.; Ye, Z.B. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011, 30, 389–398. [Google Scholar] [CrossRef]
- Mounet-Gilbert, L.; Dumont, M.; Ferrand, C.; Bournonville, C.; Monier, A.; Jorly, J.; Lemaire-Chamley, M.; Mori, K.; Atienza, I.; Hernould, M.; et al. Two tomato GDP-D-mannose epimerase isoforms involved in ascorbate biosynthesis play specific roles in cell wall biosynthesis and development. J. Exp. Bot. 2016, 67, 4767–4777. [Google Scholar] [CrossRef] [Green Version]
- Torabinejad, J.; Donahue, J.L.; Gunesekera, B.N.; Allen-Daniels, M.J.; Gillaspy, G.E. VTC4 is a bifunctional enzyme that affects myoinositol and ascorbate biosynthesis in plants. Plant Physiol. 2009, 150, 951–961. [Google Scholar] [CrossRef]
- Ioannidi, E.; Kalamaki, M.S.; Engineer, C.; Pateraki, I.; Alexandrou, D.; Mellidou, I.; Giovannonni, J.; Kanellis, A.K. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J. Exp. Bot. 2009, 60, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, D.; Li, M.; Ma, F. Light and abiotic stresses regulate the expression of GDP-L-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 2013, 238, 535–547. [Google Scholar] [CrossRef]
- Haghjou, M.M.; Shariati, M.; Smirnoff, N. The effect of acute high light and low temperature stresses on the ascorbate-glutathione cycle and superoxide dismutase activity in two Dunaliella salina strains. Physiol. Plantarum 2009, 135, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Melino, V.J.; Hayes, M.A.; Soole, K.L.; Ford, C.M. The role of light in the regulation of ascorbate metabolism during berry development in the cultivated grapevine Vitis vinifera L. J. Sci. Food Agr. 2011, 91, 1712–1721. [Google Scholar] [CrossRef]
- Massot, C.; Stevens, R.; Genard, M.; Longuenesse, J.J.; Gautier, H. Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 2012, 235, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lorence, A.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/l-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009, 150, 942–950. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Ye, J.; Tao, P.; Li, H.; Zhang, J.; Zhang, Y.; Ye, Z. The tomato HD-Zip I transcription factor SlHZ24 modulates ascorbate accumulation through positive regulation of the D-mannose/L-galactose pathway. Plant J. 2016, 85, 16–29. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Tarlyn, N.M. L-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiol. 2002, 130, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Tavazza, R.; Diretto, G.; Beyer, P.; Taylor, M.A. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol. 2008, 26, 139–145. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by Engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef]
- Saltzman, A.; Birol, E.; Bouis, H.E.; Boy, E.; De Moura, F.F.; Islam, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future. Global Food Secur. 2013, 2, 9–17. [Google Scholar] [CrossRef]
- Maruthasalam, S.; Kalpana, K.; Kumar, K.K.; Loganathan, M.; Poovannan, K.; Raja, J.A.; Kokiladevi, E.; Samiyappan, R.; Sudhakar, D.; Balasubramanian, P. Pyramiding transgenic resistance in elite indica rice cultivars against the sheath blight and bacterial blight. Plant Cell Rep. 2007, 26, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Song, Y.; Yang, A.; Zhang, J. The transgene pyramiding tobacco with betaine synthesis and heterologous expression of AtNHX1 is more tolerant to salt stress than either of the tobacco lines with betaine synthesis or AtNHX1. Physiol. Plantarum 2009, 135, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.M.; Ashwin Narayan, J.; Syamaladevi, D.P.; Appunu, C.; Chakravarthi, M.; Ravichandran, V.; Tuteja, N.; Subramonian, N. Overexpression of EaDREB2 and pyramiding of EaDREB2 with the pea DNA helicase gene (PDH45) enhance drought and salinity tolerance in sugarcane (Saccharum spp. hybrid). Plant Cell Rep. 2015, 34, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Badejo, A.A.; Wada, K.; Gao, Y.; Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. J. Exp. Bot. 2012, 63, 229–239. [Google Scholar] [CrossRef]
- Agurto, M.; Schlechter, R.O.; Armijo, G.; Solano, E.; Serrano, C.; Contreras, R.A.; Zuniga, G.E. Arce-Johnson P: RUN1 and REN1 pyramiding in grapevine (Vitis vinifera cv. crimson seedless) displays an improved defense response leading to enhanced resistance to powdery mildew (Erysiphe necator). Front Plant Sci. 2017, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Li, X.; Tu, J. Pyramiding and evaluation of both a foreign Bacillus thuringiensis and a Lysine-rich protein gene in the elite indica rice 9311. Breeding Sci. 2016, 66, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Nakane, T.; Kume, S.; Shiomi, Y.; Maruta, T.; Ishikawa, T.; Shigeoka, S. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci. Biotechnol. Biochem. 2014, 78, 60–66. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Liang, D.; Cui, M.; Ma, F. Expression patterns and promoter characteristics of the gene encoding Actinidia deliciosa L-galactose-1-phosphate phosphatase involved in the response to light and abiotic stresses. Mol. Biol. Rep. 2013, 40, 1473–1485. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar]
- Gallie, D.R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2013, 64, 433–443. [Google Scholar] [CrossRef]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.-Q.; Liu, Y.-F.; Liu, P.; Lei, G.; He, S.-J.; Ma, B.; Zhang, W.-K.; Zhang, J.-S.; Chen, S.-Y. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, Q.; Deng, X.X. L-Ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J. Plant Physiol. 2014, 171, 1205–1216. [Google Scholar] [CrossRef] [PubMed]






| Lines | Feeding-leaf- total ascorbate content (μg g−1 FW) | ||||
| H2O | MI | GLc | GLA | AsA | |
| AC | 496.2 ± 6.3 | 422.5 ± 15.9 ** | 591.2 ± 7.6 ** | 215.6 ± 8.5 ** | 2139.9 ± 59.9 ** |
| GMP × GME | 554.6 ± 14.2 | 452.4 ± 14.2 ** | 742.4 ± 10.5 ** | 162.5 ± 9.9 ** | 2733.7 ± 291.6 ** |
| GGP × GPP | 579.8 ± 15.9 | 427.8 ± 3.2 ** | 623.8 ± 6.5 ** | 158.0 ± 9.8 ** | 2220.3 ± 71.9 ** |
| GMP × GME × GGP × GPP | 626.1 ± 10.2 | 508.1 ± 17.8 ** | 790.9 ± 6.4 ** | 283.1 ± 13.3 ** | 2584.4 ± 72.2 ** |
| Lines | Feeding-fruit- total ascorbate content (μg g−1 FW) | ||||
| H2O | MI | GLc | GLA | AsA | |
| AC | 254.3 ± 3.6 | 276.1 ± 1.4 * | 287.9 ± 3.0 ** | 259.9 ± 5.2 | 296.3 ± 1.2 ** |
| GMP × GME | 287.5 ± 4.0 | 384.6 ± 8.0 * | 396.2 ± 2.5 ** | 325.8 ± 4.9 ** | 368.4 ± 11.3 ** |
| GGP × GPP | 251.0 ± 2.8 | 265.9 ± 4.7 * | 350.1 ± 6.9 ** | 261.9 ± 6.1 | 351.5 ± 34.8 ** |
| GMP × GME × GGP × GPP | 301.9 ± 8.3 | 373.7 ± 5.9 ** | 390.4 ± 7.4 ** | 331.5 ± 5.4 ** | 354.0 ± 7.0 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ye, J.; Munir, S.; Yang, T.; Chen, W.; Liu, G.; Zheng, W.; Zhang, Y. Biosynthetic Gene Pyramiding Leads to Ascorbate Accumulation with Enhanced Oxidative Stress Tolerance in Tomato. Int. J. Mol. Sci. 2019, 20, 1558. https://doi.org/10.3390/ijms20071558
Li X, Ye J, Munir S, Yang T, Chen W, Liu G, Zheng W, Zhang Y. Biosynthetic Gene Pyramiding Leads to Ascorbate Accumulation with Enhanced Oxidative Stress Tolerance in Tomato. International Journal of Molecular Sciences. 2019; 20(7):1558. https://doi.org/10.3390/ijms20071558
Chicago/Turabian StyleLi, Xiaojing, Jie Ye, Shoaib Munir, Tao Yang, Weifang Chen, Genzhong Liu, Wei Zheng, and Yuyang Zhang. 2019. "Biosynthetic Gene Pyramiding Leads to Ascorbate Accumulation with Enhanced Oxidative Stress Tolerance in Tomato" International Journal of Molecular Sciences 20, no. 7: 1558. https://doi.org/10.3390/ijms20071558