Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro
Abstract
1. Introduction
2. Results
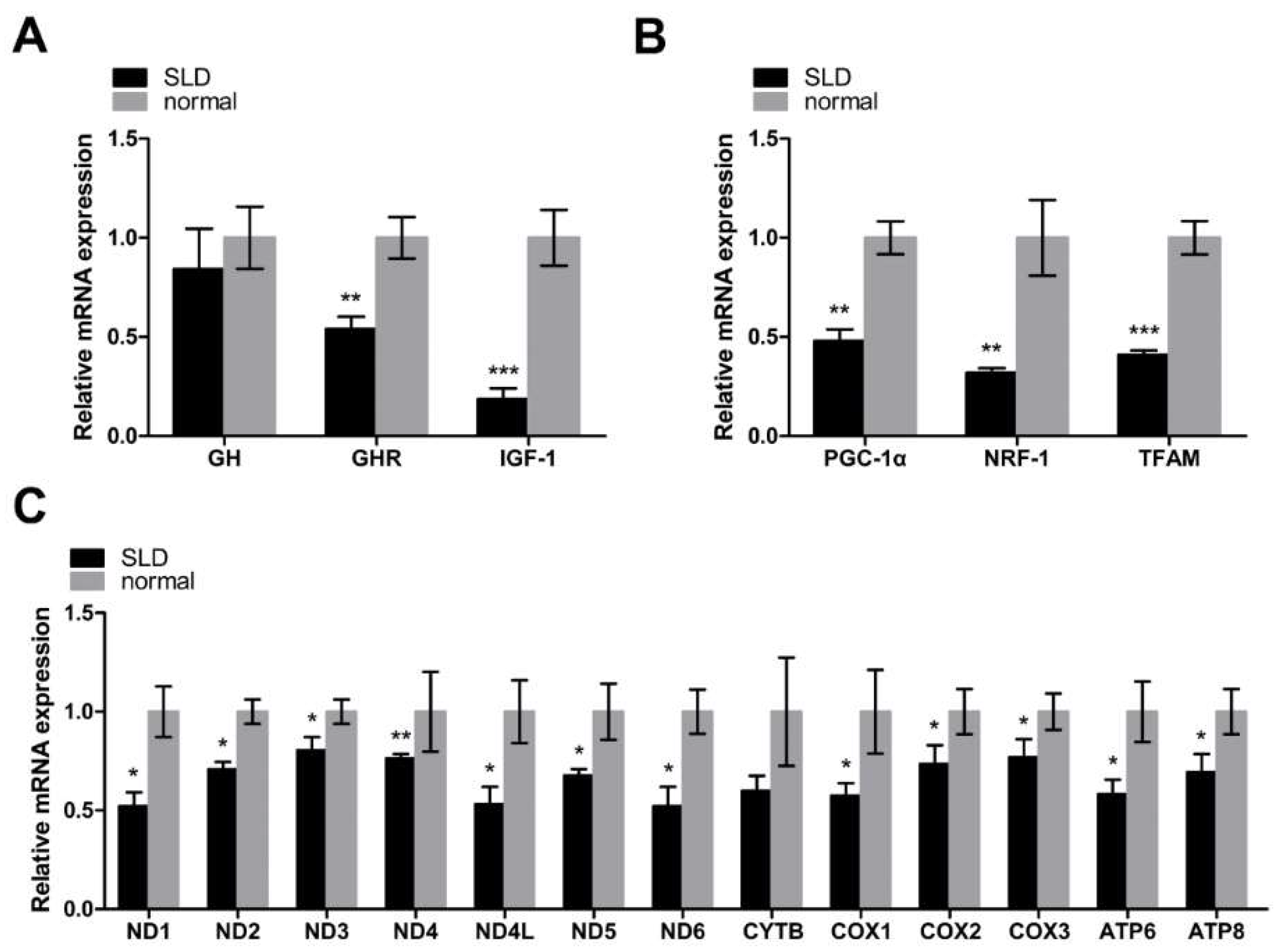
2.1. Low Expression of Key Regulators of Mitochondrial Biogenesis and mtDNA-Encoded OXPHOS Genes in SLD Chicken Skeletal Muscle
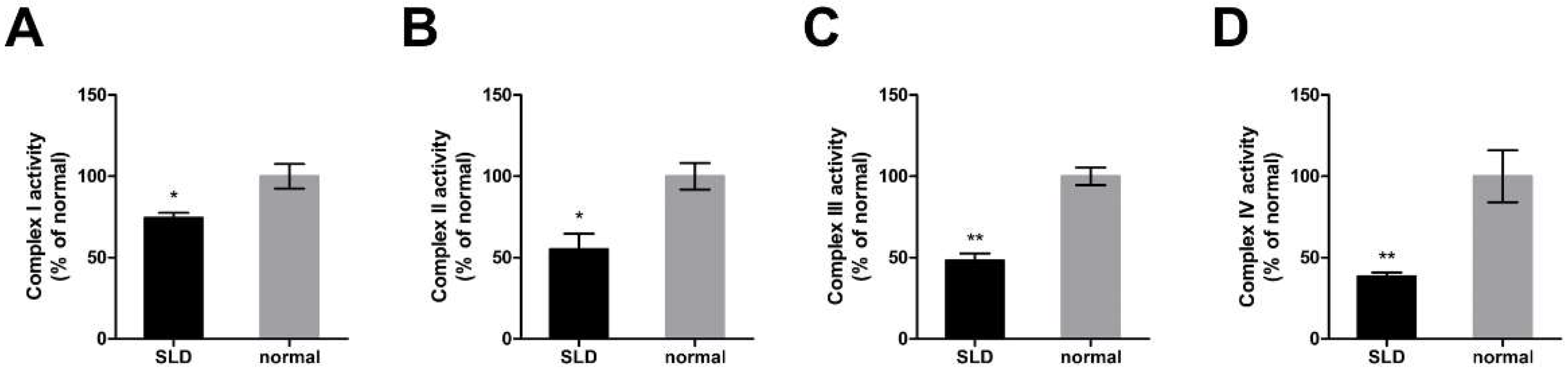
2.2. Reduced Enzymatic Activity of OXPHOS Complexes in SLD Chicken Skeletal Muscle
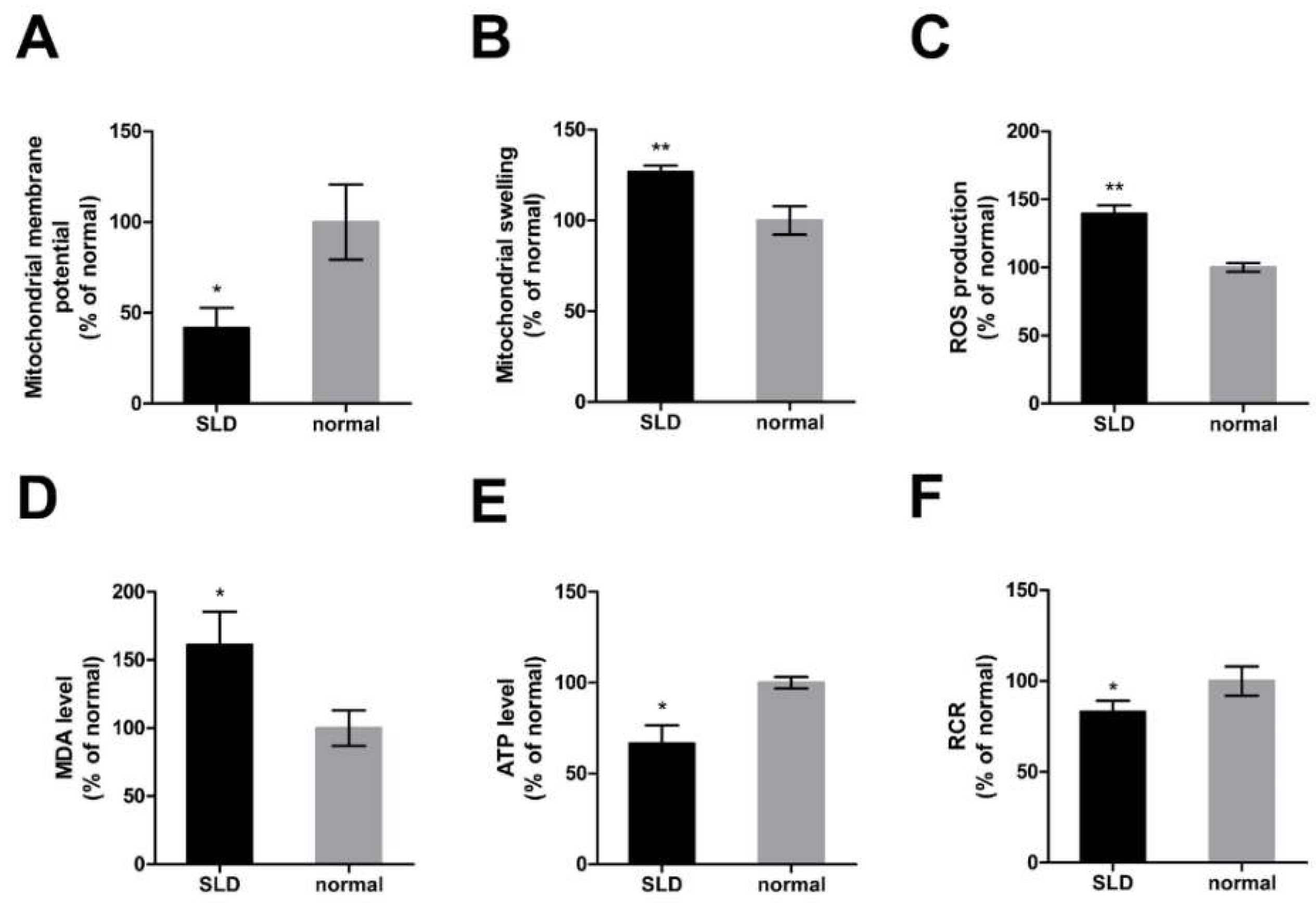
2.3. Impaired Mitochondrial Function in SLD Chicken Skeletal Muscle
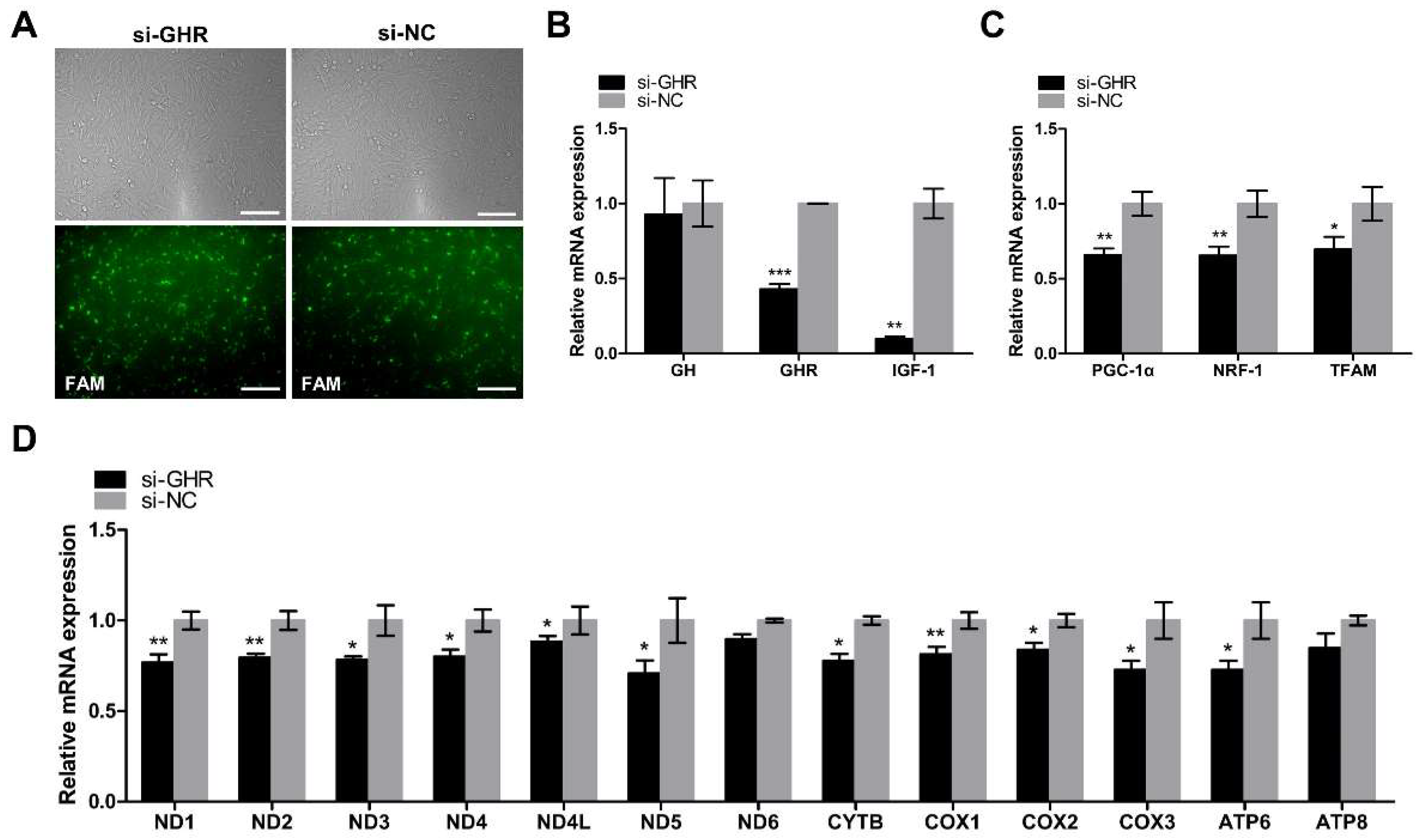
2.4. Knockdown of GHR Downregulated the Expression of Key Regulators of Mitochondrial Biogenesis and mtDNA-Encoded OXPHOS Genes in DF-1 Cells
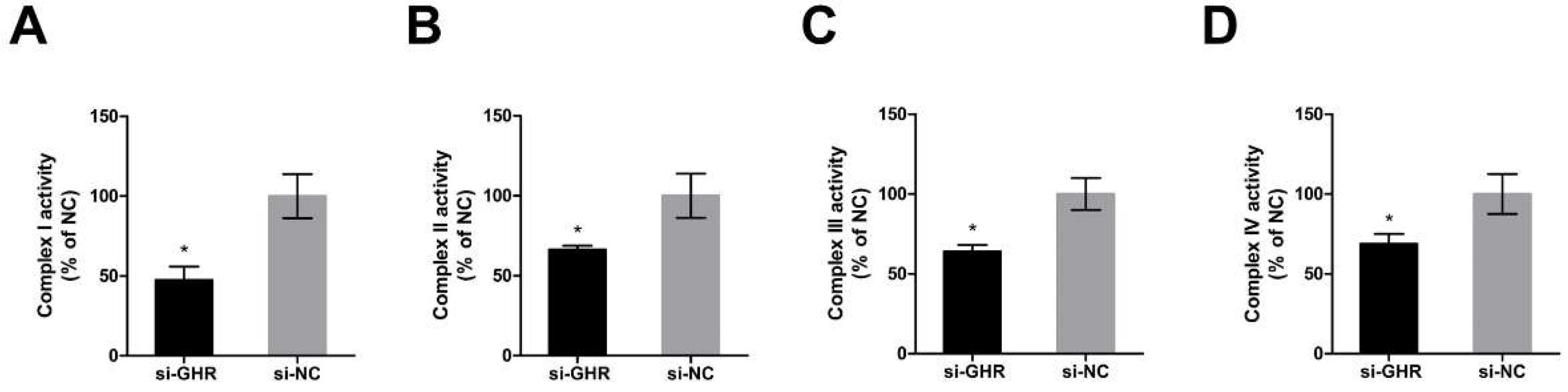
2.5. Knockdown of GHR Reduced the Enzymatic Activity of OXPHOS Complexes in DF-1 Cells
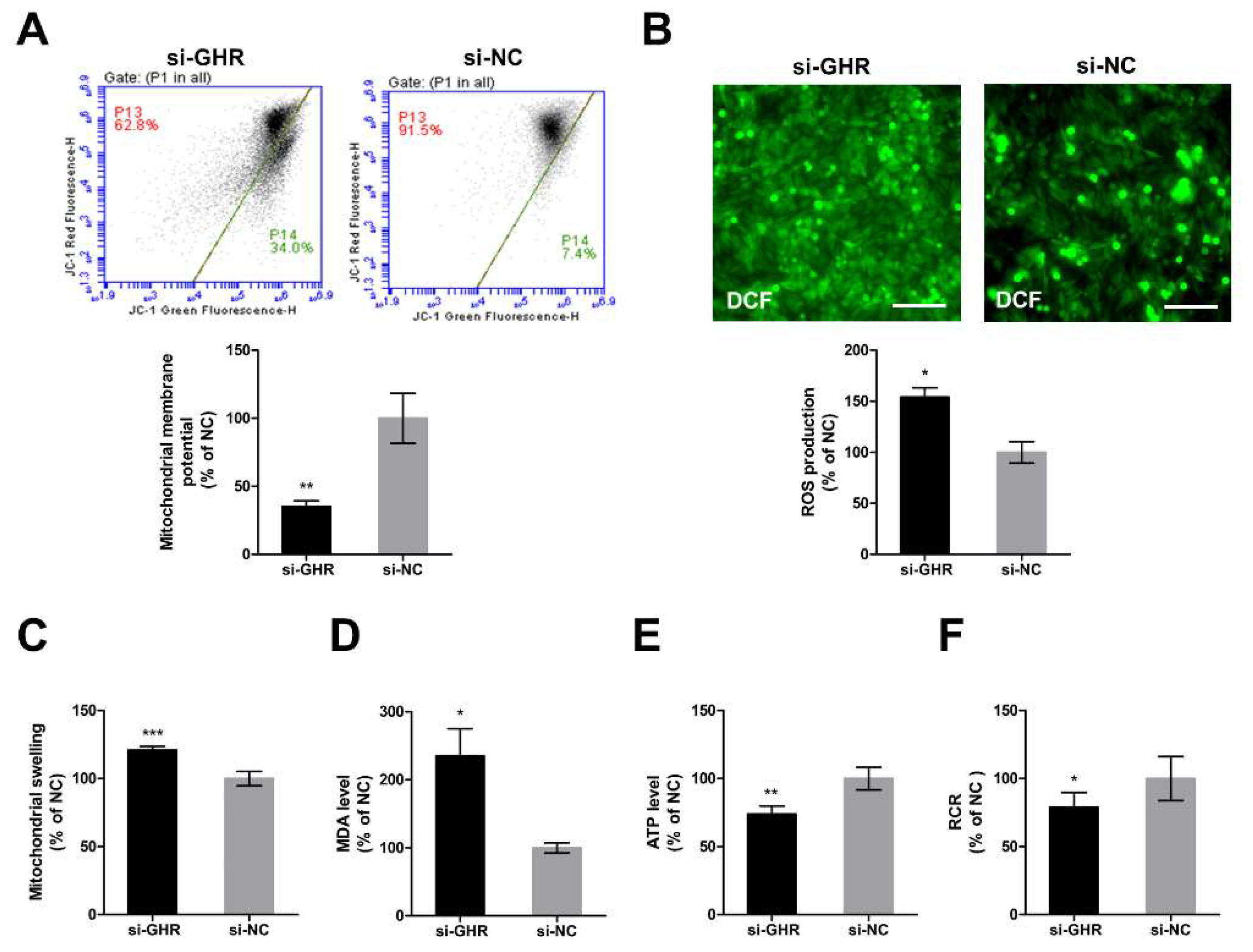
2.6. Knockdown of GHR Impaired Mitochondrial Function in DF-1 Cells
2.7. Knockdown of GHR Altered Mitochondrial Structure and Reduced Mitochondrial Number in DF-1 Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Cell Culture and RNA Interference
4.4. Quantitative Real-Time PCR
4.5. Transmission Electron Microscopy
4.6. MitoTracker Green Staining and Hoechst 33342 Staining
4.7. Mitochondria Isolation and Mitochondrial Protein Concentration Measurement
4.8. Measurement of Enzymatic Activity of Mitochondrial OXPHOS Complexes
4.9. Measurement of Adenosine Triphosphate Level
4.10. Measurement of Malondialdehyde Level
4.11. Measurement of Mitochondrial Membrane Potential
4.12. Measurement of Mitochondrial Swelling
4.13. Measurement of Reactive Oxygen Species Production
4.14. Measurement of Mitochondrial Respiratory Control Ratio
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahman, J.; Rahman, S. Mitochondrial medicine in the omics era. Lancet 2018, 391, 2560–2574. [Google Scholar] [CrossRef]
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2.0: An updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016, 44, D1251–D1257. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, K.Z.; Chu, C.T. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013, 9, 1663–1676. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Perret-Vivancos, C.; Abbate, A.; Ardail, D.; Raccurt, M.; Usson, Y.; Lobie, P.E.; Morel, G. Growth hormone activity in mitochondria depends on GH receptor Box 1 and involves caveolar pathway targeting. Exp. Cell Res. 2006, 312, 215–232. [Google Scholar]
- Brown-Borg, H.M.; Johnson, W.T.; Rakoczy, S.G. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. Age 2012, 34, 43–57. [Google Scholar] [CrossRef]
- Gesing, A.; Masternak, M.M.; Wang, F.; Joseph, A.M.; Leeuwenburgh, C.; Westbrook, R.; Lewinski, A.; Karbownik-Lewinska, M.; Bartke, A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1062–1076. [Google Scholar] [CrossRef]
- Zawada, I.; Masternak, M.M.; List, E.O.; Stout, M.B.; Berryman, D.E.; Lewinski, A.; Kopchick, J.J.; Bartke, A.; Karbownik-Lewinska, M.; Gesing, A. Gene expression of key regulators of mitochondrial biogenesis is sex dependent in mice with growth hormone receptor deletion in liver. Aging 2015, 7, 195–204. [Google Scholar] [CrossRef]
- Radovic, S.M.; Starovlah, I.M.; Cap, I.; Miljkovic, D.; Nef, S.; Kostic, T.S.; Andric, S.A. Insulin/IGF1 signalling regulates the mitochondrial biogenesis markers in steroidogenic cells of prepubertal testis, but not ovary. Biol. Reprod. 2019, 100, 253–267. [Google Scholar] [CrossRef]
- Lyons, A.; Coleman, M.; Riis, S.; Favre, C.; O’Flanagan, C.H.; Zhdanov, A.V.; Papkovsky, D.B.; Hursting, S.D.; O’Connor, R. Insulin-like growth factor 1 signaling is essential for mitochondrial biogenesis and mitophagy in cancer cells. J. Biol. Chem. 2017, 292, 16983–16998. [Google Scholar] [CrossRef]
- Tahara, K.; Tsukada, A.; Hanai, T.; Okumura, K.; Yamada, K.; Murai, A.; Yamamoto, R.; Maeno, M.; Saito, N.; Shimada, K. Identification of two types of growth hormone receptor mutations in two strains of sex-linked dwarf chickens. J. Poult. Sci. 2009, 46, 249–256. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Cogburn, L.A.; Burnside, J. Comparison of gene expression in normal and growth hormone receptor-deficient dwarf chickens reveals a novel growth hormone regulated gene. Biochem. Biophys. Res. Commun. 1995, 206, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.H.; Xie, L.; Nie, Q.H.; Zeng, H.; Peng, Z.J.; Zhang, D.X.; Zhang, X.Q. The effects of different Sex-Linked dwarf variations on chinese native chickens. J. Integr. Agr. 2012, 11, 1500–1508. [Google Scholar] [CrossRef]
- Knizetova, H. Effects of the sex-linked dwarf gene (dw) on skeletal muscle cellularity in broiler chickens. Br. Poult. Sci. 1993, 34, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Lin, S.; Li, G.; Nie, Q.; Zhang, X. Integrative analyses of miRNA-mRNA interactions reveal let-7b, miR-128 and MAPK pathway involvement in muscle mass loss in Sex-Linked dwarf chickens. Int. J. Mol. Sci. 2016, 17, 276. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, H.; Liu, J.; Cheng, L. Pyrroloquinoline quinone inhibits oxygen/glucose deprivation-induced apoptosis by activating the PI3K/AKT pathway in cardiomyocytes. Mol. Cell Biochem. 2014, 386, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Junnila, R.K.; List, E.O.; Berryman, D.E.; Murrey, J.W.; Kopchick, J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013, 9, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Mu, H.; Luo, W.; Li, Y.; Jia, X.; Wang, S.; Jia, X.; Nie, Q.; Li, Y.; et al. Let-7b regulates the expression of the growth hormone receptor gene in deletion-type dwarf chickens. BMC Genomics 2012, 13, 306. [Google Scholar] [CrossRef]
- Puche, J.E.; Garcia-Fernandez, M.; Muntane, J.; Rioja, J.; Gonzalez-Baron, S.; Castilla, C.I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology 2008, 149, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Little, J.P.; Stokl, A.J.; Hettinga, B.P.; Akhtar, M.; Tarnopolsky, M.A. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J. Biol. Chem. 2011, 286, 10605–10617. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.R.; Khan, S.M.; Portell, F.R.; Smigrodzki, R.M.; Bennett, J.J. Recombinant human mitochondrial transcription factor a stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion 2011, 11, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Collu-Marchese, M.; Shuen, M.; Pauly, M.; Saleem, A.; Hood, D.A. The regulation of mitochondrial transcription factor a (Tfam) expression during skeletal muscle cell differentiation. Biosci. Rep. 2015, 35, e00221. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman, A.R.; Mohamed, I.N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Javadov, S.; Baetz, D.; Rajapurohitam, V.; Zeidan, A.; Kirshenbaum, L.A.; Karmazyn, M. Antihypertrophic effect of Na+/H+ exchanger isoform 1 inhibition is mediated by reduced mitogen-activated protein kinase activation secondary to improved mitochondrial integrity and decreased generation of mitochondrial-derived reactive oxygen species. J. Pharmacol. Exp. Ther. 2006, 317, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Karmazyn, M.; Escobales, N. Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J. Pharmacol. Exp. Ther. 2009, 330, 670–678. [Google Scholar] [CrossRef]
- Bai, F.; Guo, F.; Jiang, T.; Wei, H.; Zhou, H.; Yin, H.; Zhong, H.; Xiong, L.; Wang, Q. Arachidonyl-2-chloroethylamide alleviates cerebral ischemia injury through glycogen synthase kinase-3beta-mediated mitochondrial biogenesis and functional improvement. Mol. Neurobiol. 2017, 54, 1240–1253. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, L.; Yu, X.; Cai, L.; Lu, Y.; Zhang, J.; Li, T.; Li, J.; Xia, J.; Xu, F.; et al. Mitochondrial transplantation attenuates airway hyperresponsiveness by inhibition of cholinergic hyperactivity. Theranostics 2016, 6, 1244–1260. [Google Scholar] [CrossRef]
- Trinchese, G.; Paparo, L.; Aitoro, R.; Fierro, C.; Varchetta, M.; Nocerino, R.; Mollica, M.P.; Berni, C.R. Hepatic mitochondrial dysfunction and immune response in a murine model of peanut allergy. Nutrients 2018, 10, 744. [Google Scholar] [CrossRef]
- Liu, Z.; Solesio, M.E.; Schaffler, M.B.; Frikha-Benayed, D.; Rosen, C.J.; Werner, H.; Kopchick, J.J.; Pavlov, E.V.; Abramov, A.Y.; Yakar, S. Mitochondrial function is compromised in cortical bone osteocytes of Long-Lived growth hormone receptor null mice. J. Bone Miner. Res. 2019, 34, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Rambold, A.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Fan, G.; Qi, S.; Li, B. Reperfusion promotes mitochondrial biogenesis following focal cerebral ischemia in rats. PLoS ONE 2014, 9, e92443. [Google Scholar] [CrossRef]
- Zhu, Y.; Di, S.; Hu, W.; Feng, Y.; Zhou, Q.; Gong, B.; Tang, X.; Liu, J.; Zhang, W.; Xi, M.; et al. A new flavonoid glycoside (APG) isolated from Clematis tangutica attenuates myocardial ischemia/reperfusion injury via activating PKCepsilon signaling. Biochim. Biophys. Acta 2017, 1863, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Gong, K.; Niu, Y.; Liu, L.; Yan, Y.; Lu, G.; Zhang, L.; Hu, M.; Zhao, N.; Zhang, X.; et al. Inhibition of gamma-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: Implications for the treatment of Alzheimer’s disease. Free Radic. Biol. Med. 2009, 46, 1362–1375. [Google Scholar] [CrossRef]
- Thorburn, D.R.; Chow, C.W.; Kirby, D.M. Respiratory chain enzyme analysis in muscle and liver. Mitochondrion 2004, 4, 363–375. [Google Scholar] [CrossRef]
- Shen, Z.; Zheng, Y.; Wu, J.; Chen, Y.; Wu, X.; Zhou, Y.; Yuan, Y.; Lu, S.; Jiang, L.; Qin, Z.; et al. PARK2-dependent mitophagy induced by acidic postconditioning protects against focal cerebral ischemia and extends the reperfusion window. Autophagy 2017, 13, 473–485. [Google Scholar] [CrossRef]
- Zamzami, N.; Maisse, C.; Metivier, D.; Kroemer, G. Measurement of membrane permeability and permeability transition of mitochondria. Methods Cell Biol. 2001, 65, 147–158. [Google Scholar]
- Kim, T.S.; Yun, B.Y.; Kim, I.Y. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem. Pharmacol. 2003, 66, 2301–2311. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Hu, S.; Yang, M.; Liao, Z.; Zhang, D.; Luo, Q.; Zhang, X.; Li, H. Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro. Int. J. Mol. Sci. 2019, 20, 1608. https://doi.org/10.3390/ijms20071608
Hu B, Hu S, Yang M, Liao Z, Zhang D, Luo Q, Zhang X, Li H. Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro. International Journal of Molecular Sciences. 2019; 20(7):1608. https://doi.org/10.3390/ijms20071608
Chicago/Turabian StyleHu, Bowen, Shuang Hu, Minmin Yang, Zhiying Liao, Dexiang Zhang, Qingbin Luo, Xiquan Zhang, and Hongmei Li. 2019. "Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro" International Journal of Molecular Sciences 20, no. 7: 1608. https://doi.org/10.3390/ijms20071608
APA StyleHu, B., Hu, S., Yang, M., Liao, Z., Zhang, D., Luo, Q., Zhang, X., & Li, H. (2019). Growth Hormone Receptor Gene is Essential for Chicken Mitochondrial Function In Vivo and In Vitro. International Journal of Molecular Sciences, 20(7), 1608. https://doi.org/10.3390/ijms20071608




