Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function
Abstract
1. Introduction
2. Results
2.1. Nrf2 is Targeted by miR-153, miR-28 and miR-708
2.2. Oxidative Stress Condition Induced the Expression of Nrf2 and Suppressed the Expression of miR-153, miR-28 and miR-708 in Bovine Granulosa Cells
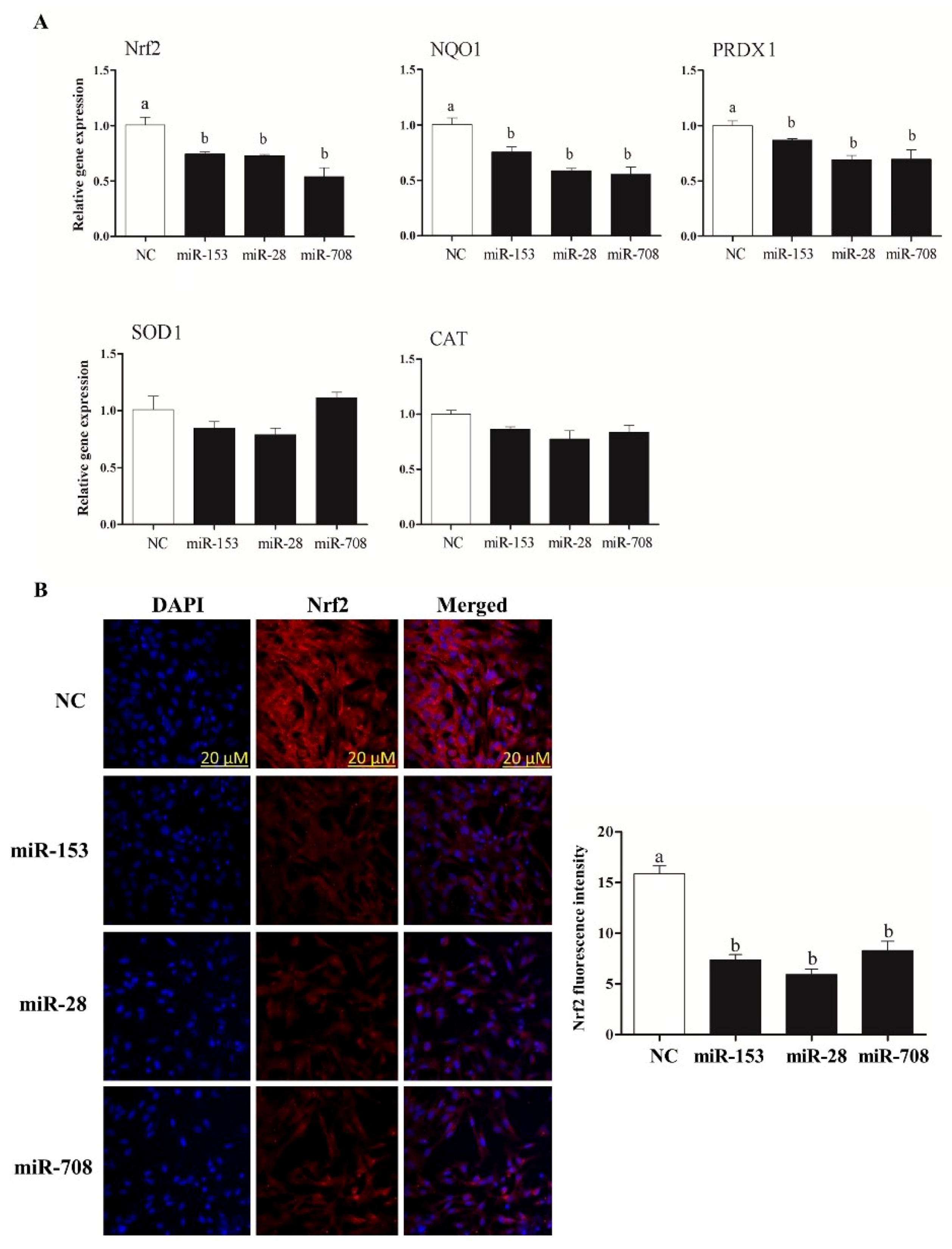
2.3. Overexpression of miR-153, miR-28 and miR-708 Suppressed the Expression of Nrf2 and Its Downstream Antioxidants in Bovine Granulosa Cells
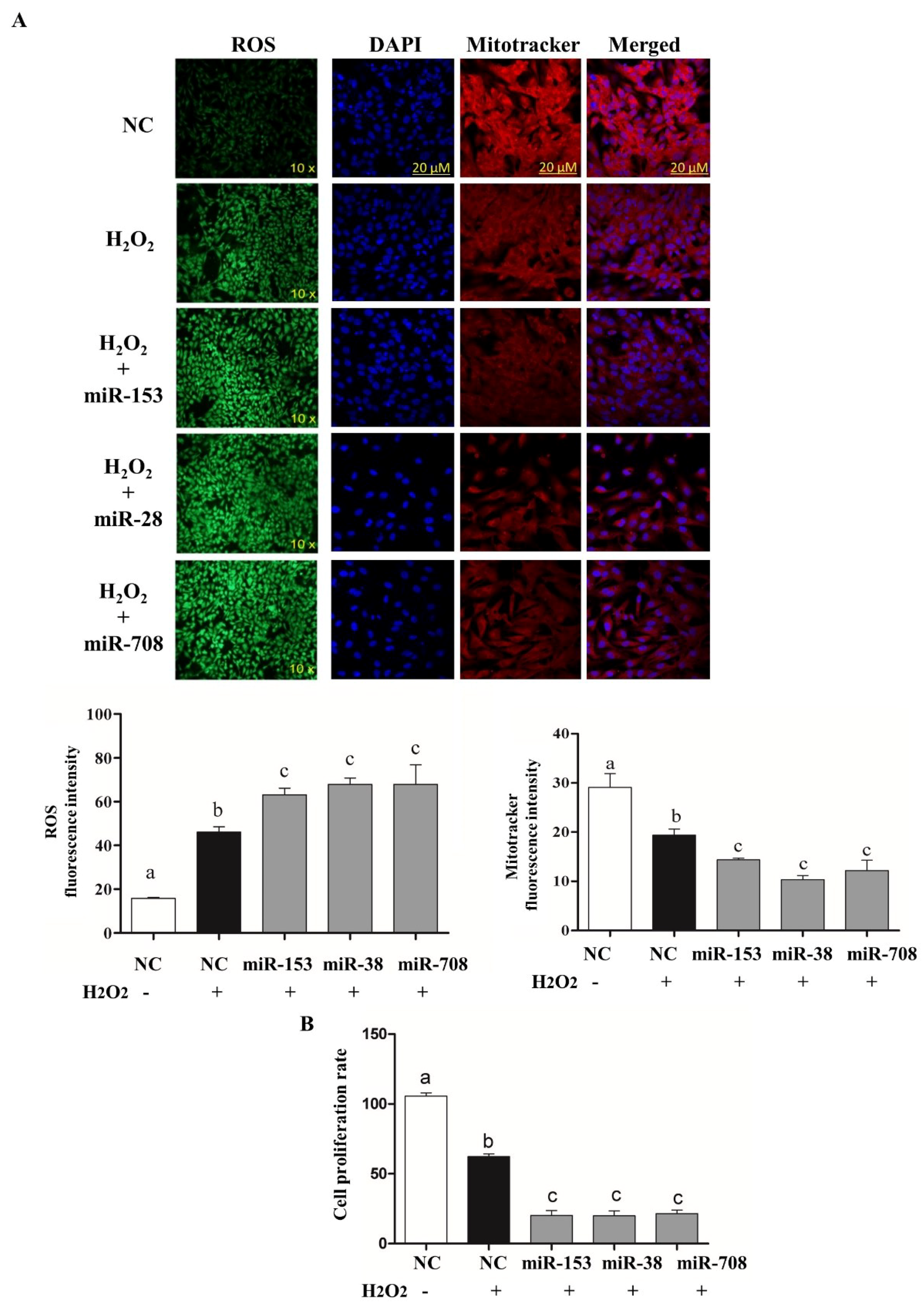
2.4. Overexpression of miR-153 or miR-28 and miR-708 Increased Intracellular ROS Level, Reduced Mitochondrial Activity and Cell Proliferation Rate in Bovine Granulosa Cells
2.5. Selective Knockdown of Bovine Nrf2 Impaired Bovine Granulosa Cell Functions
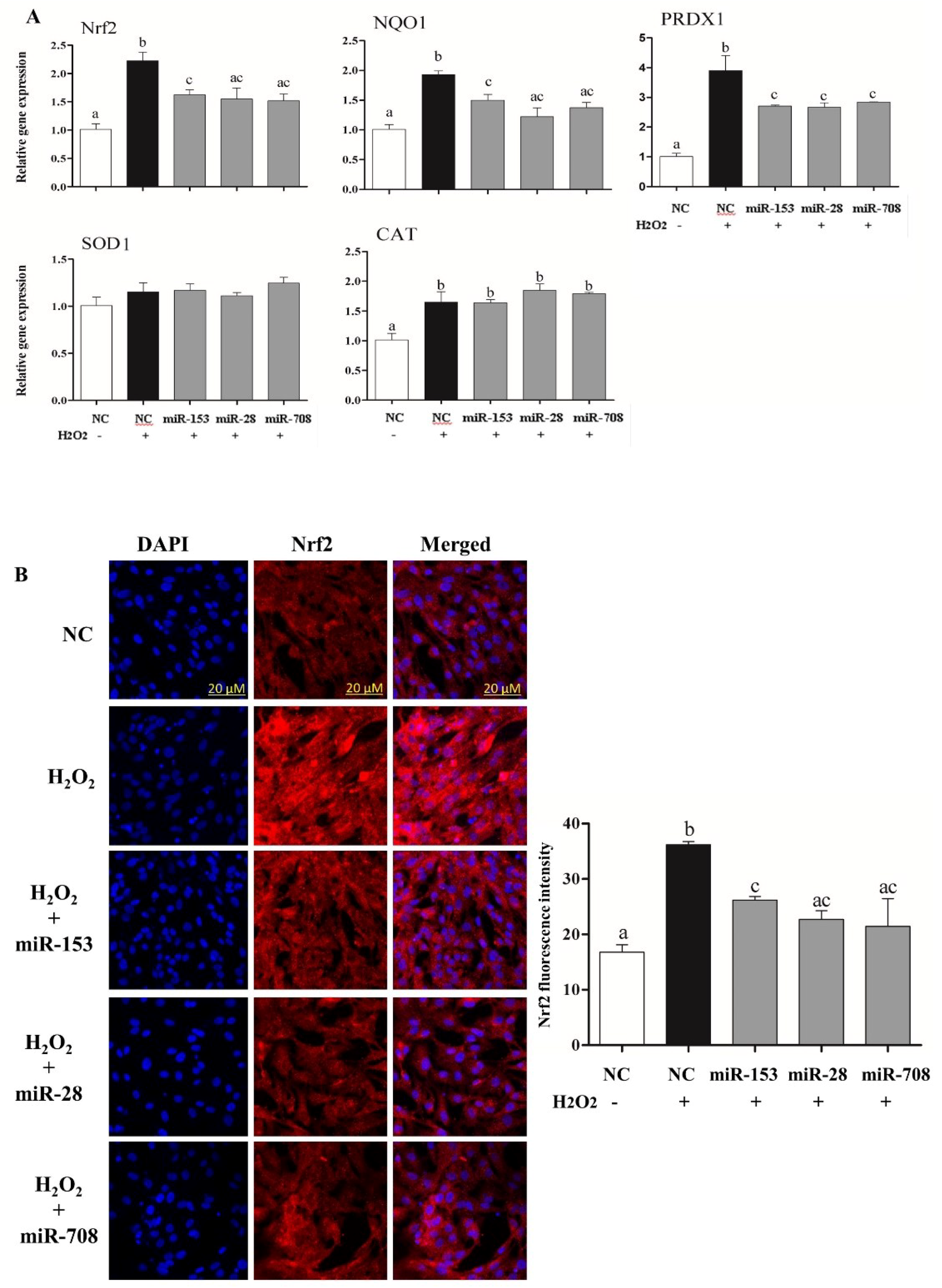
2.6. Overexpression of miR-153, miR-28 and miR-708 Under Oxidative Stress Negatively Impact on Bovine Granulosa Cell Functions
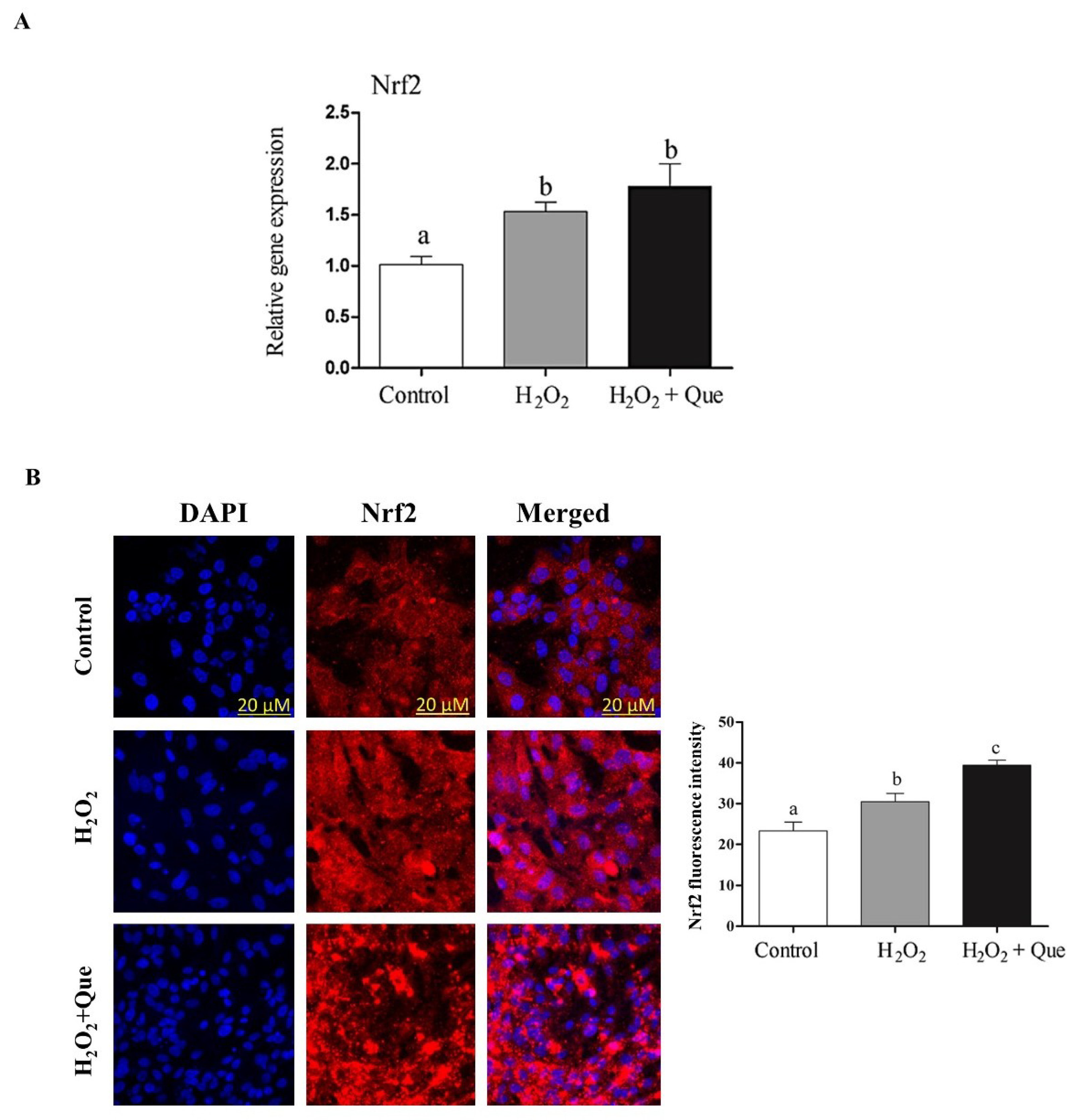
2.7. Quercetin Enhanced Bovine Granulosa Cell Functions under Oxidative Stress Conditions by Inducing the Nrf2 Expression and its Downstream Antioxidants
3. Discussion
4. Materials and Methods
4.1. Bovine Granulosa Cell Culture
4.2. MicroRNA Target Gene Prediction and Luciferase Reporter Assay
4.3. MicroRNA and siRNA Transfection
4.4. Exogenous Induction of Nrf2 by Quercetin
4.5. Induction of Oxidative Stress Using H2O2
4.6. Total RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.7. Cell Proliferation Assay
4.8. Protein Immunofluorescence Detection
4.9. Assessment of Mitochondrial Activity
4.10. Cell Cycle Assay
4.11. Intracellular ROS Detection
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Son, W.Y.; Das, M.; Shalom-Paz, E.; Holzer, H. Mechanisms of follicle selection and development. Minerva Ginecol. 2011, 63, 89–102. [Google Scholar]
- Garratt, M.; Vasilaki, A.; Stockley, P.; McArdle, F.; Jackson, M.; Hurst, J.L. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. Biol. Sci. 2011, 278, 1098–1106. [Google Scholar] [CrossRef]
- Celi, P.; Gabai, G. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front. Vet. Sci. 2015, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, N.B.; Alonso-Alvarez, C. Oxidative stress as a life-history constraint: The role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 2010, 24, 984–996. [Google Scholar] [CrossRef]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative Stress in Alzheimer’s Disease: Why Did Antioxidant Therapy Fail? Oxid. Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Khan, A.U.; Wilson, T. Reactive Oxygen Species as Cellular Messengers. Chem. Biol. 1995, 2, 437–445. [Google Scholar] [CrossRef]
- Finkel, T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998, 10, 248–253. [Google Scholar] [CrossRef]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef]
- Chaube, S.K.; Prasad, P.V.; Thakur, S.C.; Shrivastav, T.G. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristic of apoptosis in rat oocytes cultured in vitro. Apoptosis 2005, 10, 863–874. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. Biomed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S.R.; Nallella, K.P.; George, A.T.; Mascha, E. Correlation of reactive oxygen species levels with the fertilization rate after in vitro fertilization: A qualified meta-analysis. Fertil. Steril. 2005, 84, 228–231. [Google Scholar] [CrossRef]
- Alvarez, J.G. DNA fragmentation in human spermatozoa: Significance in the diagnosis and treatment of infertility. Minerva Ginecol. 2003, 55, 233–239. [Google Scholar] [PubMed]
- Lai, Q.; Xiang, W.; Li, Q.; Zhang, H.; Li, Y.; Zhu, G.; Xiong, C.; Jin, L. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front. Med. 2017, 12, 518–524. [Google Scholar] [CrossRef]
- Liang, L.-F.; Qi, S.-T.; Xian, Y.-X.; Huang, L.; Sun, X.-F.; Wang, W.-H. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci. Rep. 2017, 7, 1434. [Google Scholar] [CrossRef]
- Chanas, S.A.; Jiang, Q.; McMahon, M.; McWalter, G.K.; McLellan, L.I.; Elcombe, C.R.; Henderson, C.J.; Wolf, C.R.; Moffat, G.J.; Itoh, K.; et al. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem. J. 2002, 365, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Wakabayashi, N.; Itoh, K.; Motohashi, H.; Yamamoto, M.; Kensler, T.W. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway—Identification of novel gene clusters for cell survival. J. Biol. Chem. 2003, 278, 8135–8145. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The cap ‘n’ collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Kalayarasan, S.; Prabhu, P.N.; Sriram, N.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 2009, 606, 162–171. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Amin, A.; Gad, A.; Salilew-Wondim, D.; Prastowo, S.; Held, E.; Hoelker, M.; Rings, F.; Tholen, E.; Neuhoff, C.; Looft, C.; et al. Bovine embryo survival under oxidative-stress conditions is associated with activity of the NRF2-mediated oxidative-stress-response pathway. Mol. Reprod. Dev. 2014, 81, 497–513. [Google Scholar] [CrossRef]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.-N.T. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Preusse, M.; Theis, F.J.; Mueller, N.S. miTALOS v2: Analyzing Tissue Specific microRNA Function. PLoS ONE 2016, 11, e0151771. [Google Scholar] [CrossRef] [PubMed]
- Mor, E.; Cabilly, Y.; Goldshmit, Y.; Zalts, H.; Modai, S.; Edry, L.; Elroy-Stein, O.; Shomron, N. Species-specific microRNA roles elucidated following astrocyte activation. Nucleic Acids Res. 2011, 39, 3710–3723. [Google Scholar] [CrossRef] [PubMed]
- Sauer, E.; Extra, A.; Cachée, P.; Courts, C. Identification of organ tissue types and skin from forensic samples by microRNA expression analysis. Forensic Sci. Int. Genet. 2017, 28, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Blenkiron, C.; Miska, E.A. miRNAs in cancer: Approaches, aetiology, diagnostics and therapy. Hum. Mol. Genet. 2007, 16, R106–R113. [Google Scholar] [CrossRef]
- Andreas, E.; Hoelker, M.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D.; Salilew-Wondim, D. MicroRNA 17–92 cluster regulates proliferation and differentiation of bovine granulosa cells by targeting PTEN and BMPR2 genes. Cell Tissue Res. 2016, 366, 219–230. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Schellander, K.; Tesfaye, D. MicroRNA-183-96-182 Cluster Regulates Bovine Granulosa Cell Proliferation and Cell Cycle Transition by Coordinately Targeting FOXO1. Biol. Reprod. 2016, 94, 127. [Google Scholar] [CrossRef]
- Pande, H.O.; Tesfaye, D.; Hoelker, M.; Gebremedhn, S.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Wondim, D.S. MicroRNA-424/503 cluster members regulate bovine granulosa cell proliferation and cell cycle progression by targeting SMAD7 gene through activin signalling pathway. J. Ovarian Res. 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sohel, M.M.H.; Schellander, K.; Tesfaye, D. Characterization and importance of microRNAs in mammalian gonadal functions. Cell Tissue Res. 2012, 349, 679–690. [Google Scholar] [CrossRef]
- Lei, L.; Jin, S.; Gonzalez, G.; Behringer, R.R.; Woodruff, T.K. The regulatory role of Dicer in folliculogenesis in mice. Mol. Cell. Endocrinol. 2010, 315, 63–73. [Google Scholar] [CrossRef]
- Bahrami, A.; Miraie-Ashtiani, S.R.; Sadeghi, M.; Najafi, A. miRNA-mRNA network involved in folliculogenesis interactome: Systems biology approach. Reproduction 2017, 154, 51–65. [Google Scholar] [CrossRef]
- Gilchrist, G.C.; Tscherner, A.; Nalpathamkalam, T.; Merico, D.; LaMarre, J. MicroRNA Expression during Bovine Oocyte Maturation and Fertilization. Int. J. Mol. Sci. 2016, 17, 396. [Google Scholar] [CrossRef]
- Sinha, P.B.; Tesfaye, D.; Rings, F.; Hossien, M.; Hoelker, M.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Salilew-Wondim, D. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J. Ovarian Res. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, S.W.; Smith, C.L.; Pate, J.L. Changes in MicroRNA Expression During Maturation of the Bovine Corpus Luteum: Regulation of Luteal Cell Proliferation and Function by MicroRNA-34a. Biol. Reprod. 2016, 94, 71. [Google Scholar] [CrossRef]
- Jerome, A.; Thirumaran, S.M.K.; Kala, S.N. Repertoire of noncoding RNAs in corpus luteum of early pregnancy in buffalo (Bubalus bubalis). Vet. World 2017, 10, 1129–1134. [Google Scholar] [CrossRef]
- García-López, J.; del Mazo, J. Expression dynamics of microRNA biogenesis during preimplantation mouse development. Biochim. Biophys. Acta 2012, 1819, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Schuster, A.; Tang, C.; Yu, T.; Ortogero, N.; Bao, J.; Zheng, H.; Yan, W. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 2016, 143, 635–647. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, J.; Liu, M.; Li, R.; Tian, B.; Zhang, X.; Xu, B.; Liu, M.; Zhang, X.; Li, Y.; et al. Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Sci. Adv. 2016, 2, e1501482. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res. Treat. 2011, 129, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Teng, Y.; Liu, Q. MicroRNA-153 Regulates NRF2 Expression and is Associated with Breast Carcinogenesis. Clin. Lab. 2016, 62, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of novel microRNAs in post-transcriptional control of Nrf2 expression and redox homeostasis in neuronal, SH-SY5Y cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shen, Y.; Wei, J.; Liu, F. MicroRNA-153/Nrf-2/GPx1 pathway regulates radiosensitivity and stemness of glioma stem cells via reactive oxygen species. Oncotarget 2015, 6, 22006–22027. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Gao, J.; Zheng, Y.; Liu, X.; Zhou, Q.; Shi, C.; Yao, M.; Chen, X. Inhibition of microRNA-153 protects neurons against ischemia/reperfusion injury in an oxygen-glucose deprivation and reoxygenation cellular model by regulating Nrf2/HO-1 signaling. J. Biochem. Mol. Toxicol. 2017, 31, e21905. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Wojtasik, W.; Kostyn, K.; Czuj, T.; Szopa, J.; Kulma, A. Crossbreeding of transgenic flax plants overproducing flavonoids and glucosyltransferase results in progeny with improved antifungal and antioxidative properties. Mol. Breed. 2014, 34, 1917–1932. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharm. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Vasilescu, D.; Girma, R. Quantum molecular modeling of quercetin?: Simulation of the interaction with the free radicalt-BuOO? Int. J. Quantum Chem. 2002, 90, 888–902. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Naderi, G.A.; Asgary, S.; Sarraf-Zadegan, N.; Shirvany, H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol. Cell. Biochem. 2003, 246, 193–196. [Google Scholar] [CrossRef]
- Kim, M.-R.; Lee, J.Y.; Lee, H.-H.; Aryal, D.K.; Kim, Y.G.; Kim, S.K.; Woo, E.-R.; Kang, K.W. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem. Toxicol. 2006, 44, 1299–1307. [Google Scholar] [CrossRef]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells. Molecules 2017, 22, 1122. [Google Scholar] [CrossRef]
- Yang, T.; Kong, B.; Gu, J.-W.; Kuang, Y.-Q.; Cheng, L.; Yang, W.-T.; Xia, X.; Shu, H.-F. Anti-apoptotic and anti-oxidative roles of quercetin after traumatic brain injury. Cell. Mol. Neurobiol. 2014, 34, 797–804. [Google Scholar] [CrossRef]
- Kang, C.-H.; Choi, Y.H.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int. Immunopharmacol. 2013, 17, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef]
- Chun, K.-S.; Kundu, J.; Kundu, J.K.; Surh, Y.-J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol. Lett. 2014, 229, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.-L.; Sheng, Y.-C.; Zheng, Z.-Y.; Shi, L.; Wang, Z.-T. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.N.; Tripathi, A.; Premkumar, K.V.; Shrivastav, T.G.; Chaube, S.K. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J. Cell. Biochem. 2010, 111, 521–528. [Google Scholar] [CrossRef]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.B.C.; Antonelli, E.J.; da Cunha, D.F.; Júnior, A.A.J.; Júnior, V.R.; Vannucchi, H. Oxidative stress and acute-phase response in patients with pressure sores. Nutrition 2005, 21, 901–907. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Martín-Romero, F.J.; Miguel-Lasobras, E.M.; Domínguez-Arroyo, J.A.; González-Carrera, E.; Alvarez, I.S. Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod. Biomed. Online 2008, 17, 652–661. [Google Scholar] [CrossRef]
- Tripathi, A.; Khatun, S.; Pandey, A.N.; Mishra, S.K.; Chaube, R.; Shrivastav, T.G.; Chaube, S.K. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic. Res. 2009, 43, 287–294. [Google Scholar] [CrossRef]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Lai, Z.; Tian, Y.; Fang, L.; Wu, M.; Xiong, J.; Qin, X.; Luo, A.; Wang, S. Long-Term Moderate Oxidative Stress Decreased Ovarian Reproductive Function by Reducing Follicle Quality and Progesterone Production. PLoS ONE 2016, 11, e0162194. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wirth, A.-K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef]
- Homma, S.; Ishii, Y.; Morishima, Y.; Yamadori, T.; Matsuno, Y.; Haraguchi, N.; Kikuchi, N.; Satoh, H.; Sakamoto, T.; Hizawa, N.; et al. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 2009, 15, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.Y.; Hughes, M.; Asnes, A.G.; Leventhal, J.M. Child maltreatment and risk patterns among participants in a child abuse prevention program. Child Abus. Negl. 2015, 44, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef]
- Ayers, D.; Baron, B.; Hunter, T. miRNA Influences in NRF2 Pathway Interactions within Cancer Models. J. Nucleic Acids 2015, 2015, 143636. [Google Scholar] [CrossRef]
- Papp, D.; Lenti, K.; Módos, D.; Fazekas, D.; Dúl, Z.; Türei, D.; Földvári-Nagy, L.; Nussinov, R.; Csermely, P.; Korcsmáros, T. The NRF2-related interactome and regulome contain multifunctional proteins and fine-tuned autoregulatory loops. FEBS Lett. 2012, 586, 1795–1802. [Google Scholar] [CrossRef]
- Singh, B.; Ronghe, A.M.; Chatterjee, A.; Bhat, N.K.; Bhat, H.K. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis 2013, 34, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Fink, R. The protective role of antioxidants in the defence against ROS/RNS-mediated environmental pollution. Oxid. Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar]
- Castillo, C.; Pereira, V.; Abuelo, Á.; Hernández, J. Effect of supplementation with antioxidants on the quality of bovine milk and meat production. Sci. World J. 2013, 2013, 616098. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mishra, S.; Singh, P.; Rath, S.K. Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar. Res. Treat. 2013, 2013, 141734. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-N.; Jeong, S.-M.; Huh, G.H.; Kim, J.-I. Quercetin ameliorates insulin sensitivity and liver steatosis partly by increasing adiponectin expression in ob/ob mice. Food Sci. Biotechnol. 2015, 24, 273–279. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.; Uçan, U.; Boyacioğlu, M.; İpek, E.; Akosy, M. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.-M.; Liang, Y.-L.; Dajas, F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Pallauf, K.; Duckstein, N.; Hasler, M.; Klotz, L.-O.; Rimbach, G. Flavonoids as Putative Inducers of the Transcription Factors Nrf2, FoxO, and PPARγ. Oxid. Med. Cell. Longev. 2017, 2017, 4397340. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.-S.; Joe, Y.; Chung, H.T.; Ha, T.Y.; Yu, R. Quercetin Reduces Tumor Necrosis Factor Alpha-Induced Muscle Atrophy by Upregulation of Heme Oxygenase-1. J. Med. Food 2018, 21, 551–559. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.-H.; Jaquet, V.; Cuadrado, A. Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus. Redox Biol. 2017, 13, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Robaszkiewicz, A.; Balcerczyk, A.; Bartosz, G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007, 31, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- de Marchi, U.; Biasutto, L.; Garbisa, S.; Toninello, A.; Zoratti, M. Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim. Biophys. Acta 2009, 1787, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Long, C.; Junming, T.; Qihuan, L.; Youshun, Z.; Chan, Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol. Biol. Rep. 2012, 39, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Ebermann, R.; Marian, B. Quercetin-induced apoptosis in colorectal tumor cells: Possible role of EGF receptor signaling. Nutr. Cancer 1999, 34, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef]
- Suh, D.K.; Lee, E.J.; Kim, H.C.; Kim, J.H. Induction of G(1)/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch. Pharmacal Res. 2010, 33, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-R.; Du, Y.-J.; Chen, L.; Liu, Z.-G.; Pan, Y.-H.; Liu, J.-F.; Liu, B. Quercetin protects against high glucose-induced damage in bone marrow-derived endothelial progenitor cells. Int. J. Mol. Med. 2014, 34, 1025–1031. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Wang, B.; Wu, D.; Li, H.; Lu, H.; Wu, H.; Chai, Y. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp. Biol. Med. 2014, 239, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Wagner, A.E.; Wolffram, S.; Rimbach, G. Effect of quercetin on inflammatory gene expression in mice liver in vivo - role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 2012, 65, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Q.; Chen, J.; Chen, Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis. Mol. Cells 2015, 38, 638–642. [Google Scholar] [CrossRef]
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer 2013, 65, 494–504. [Google Scholar] [CrossRef]
- Chuammitri, P.; Srikok, S.; Saipinta, D.; Boonyayatra, S. The effects of quercetin on microRNA and inflammatory gene expression in lipopolysaccharide-stimulated bovine neutrophils. Vet. World 2017, 10, 403–410. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [PubMed]
- Poot, M.; Zhang, Y.Z.; Krämer, J.A.; Wells, K.S.; Jones, L.J.; Hanzel, D.K.; Lugade, A.G.; Singer, V.L.; Haugland, R.P. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J. Histochem. Cytochem. 2017, 44, 1363–1372. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadrawy, O.; Gebremedhn, S.; Salilew-Wondim, D.; Taqi, M.O.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function. Int. J. Mol. Sci. 2019, 20, 1635. https://doi.org/10.3390/ijms20071635
Khadrawy O, Gebremedhn S, Salilew-Wondim D, Taqi MO, Neuhoff C, Tholen E, Hoelker M, Schellander K, Tesfaye D. Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function. International Journal of Molecular Sciences. 2019; 20(7):1635. https://doi.org/10.3390/ijms20071635
Chicago/Turabian StyleKhadrawy, Omar, Samuel Gebremedhn, Dessie Salilew-Wondim, Mohamed Omar Taqi, Christiane Neuhoff, Ernst Tholen, Michael Hoelker, Karl Schellander, and Dawit Tesfaye. 2019. "Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function" International Journal of Molecular Sciences 20, no. 7: 1635. https://doi.org/10.3390/ijms20071635
APA StyleKhadrawy, O., Gebremedhn, S., Salilew-Wondim, D., Taqi, M. O., Neuhoff, C., Tholen, E., Hoelker, M., Schellander, K., & Tesfaye, D. (2019). Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function. International Journal of Molecular Sciences, 20(7), 1635. https://doi.org/10.3390/ijms20071635




