Sphingosine-1-phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts In Vivo
Abstract
:1. Introduction
2. Results
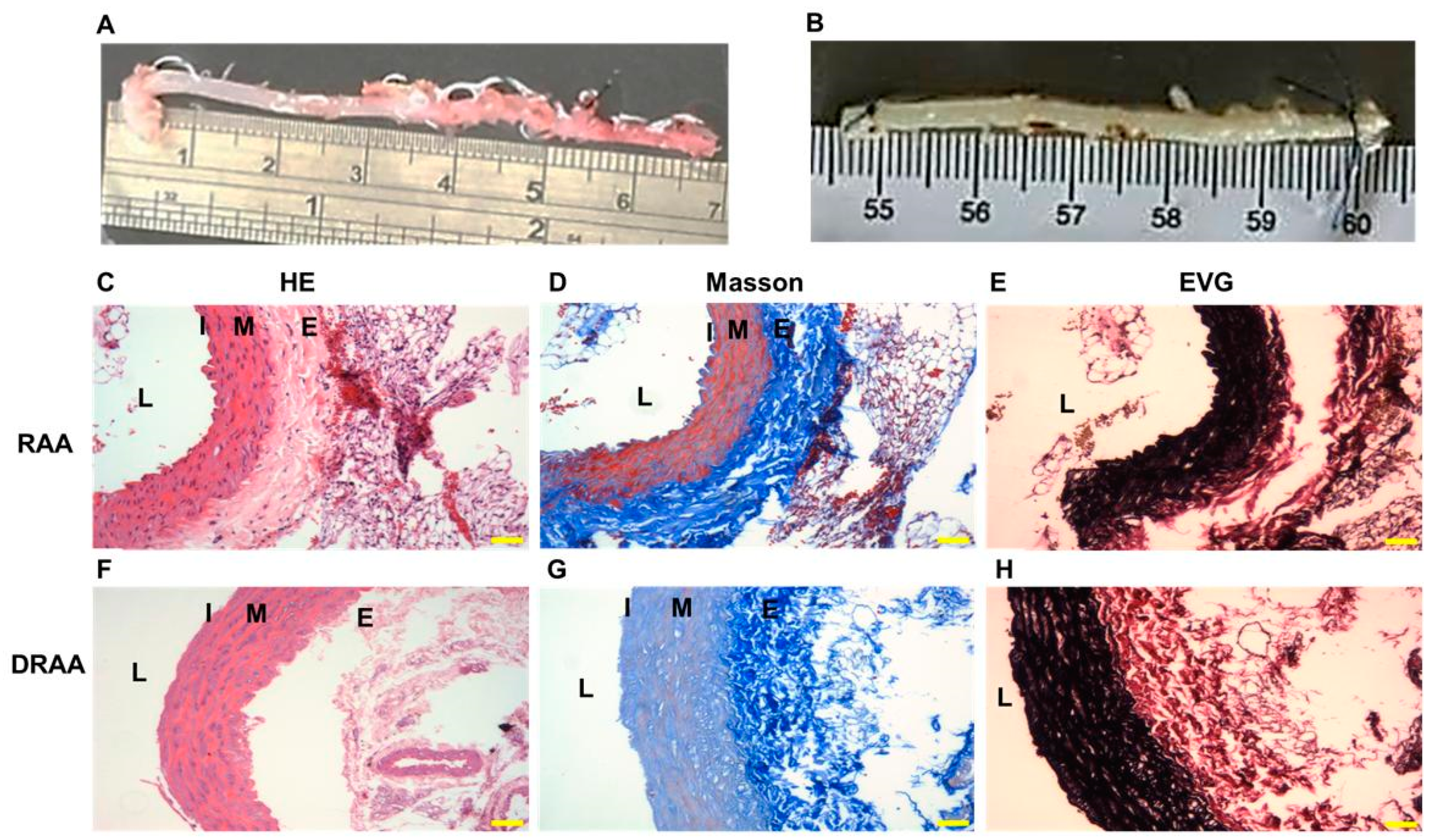
2.1. Characteristics of DRAA
2.2. Characteristics of Rat ECs
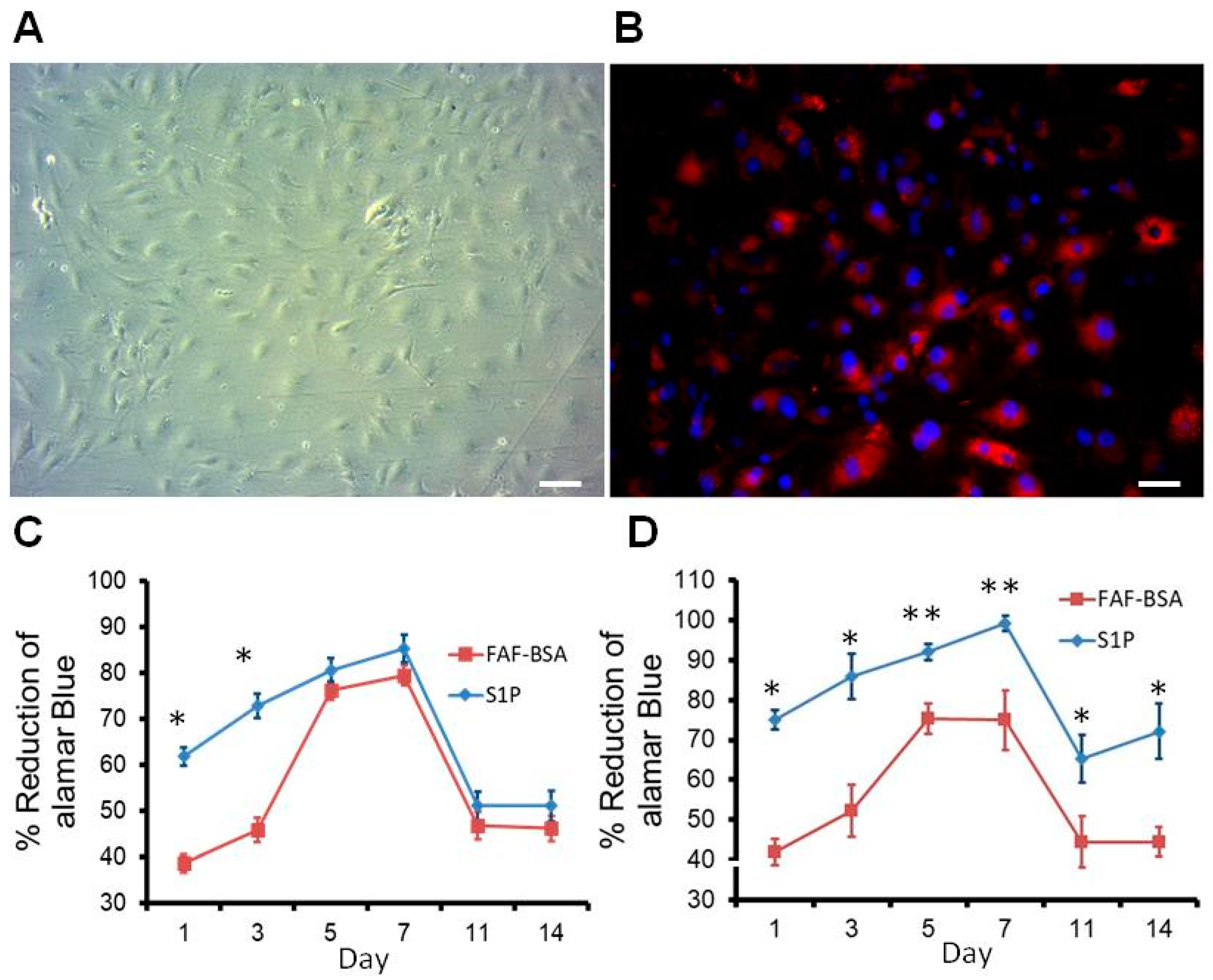
2.3. Proliferation Effect of S1P on Rat ECs on DRAA
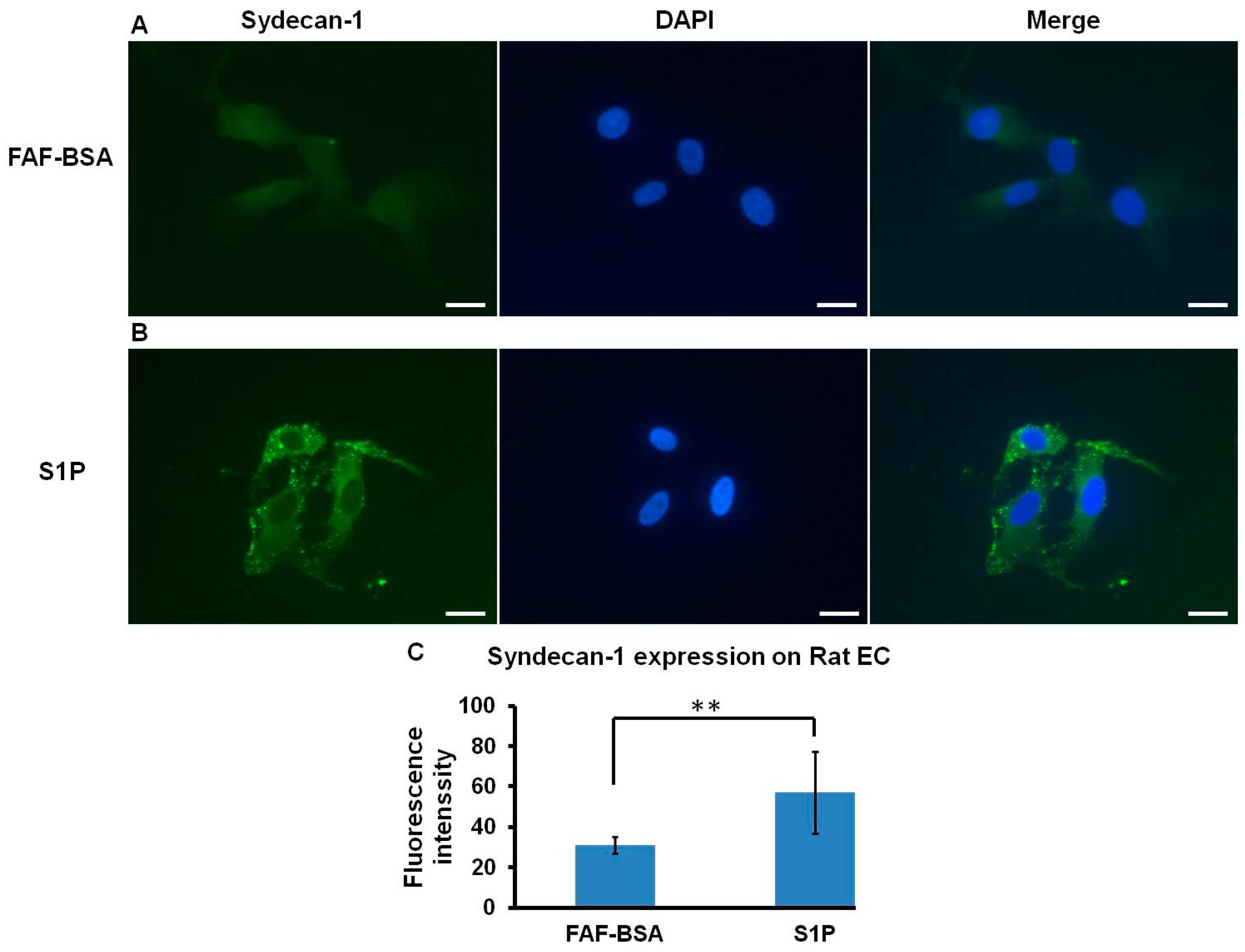
2.4. S1P Promotes SDC-1 Expression on Rat ECs
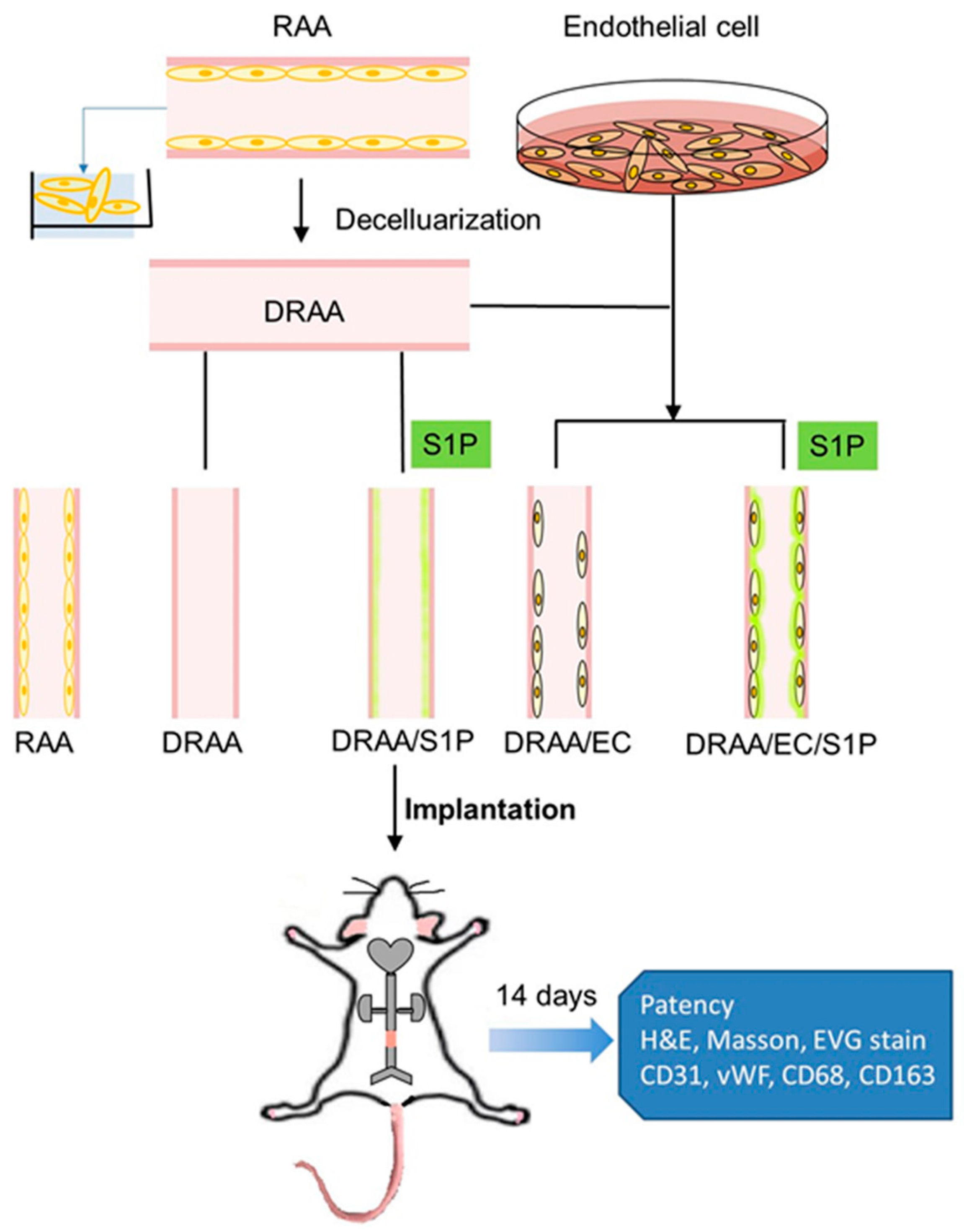
2.5. TEVG Implantation
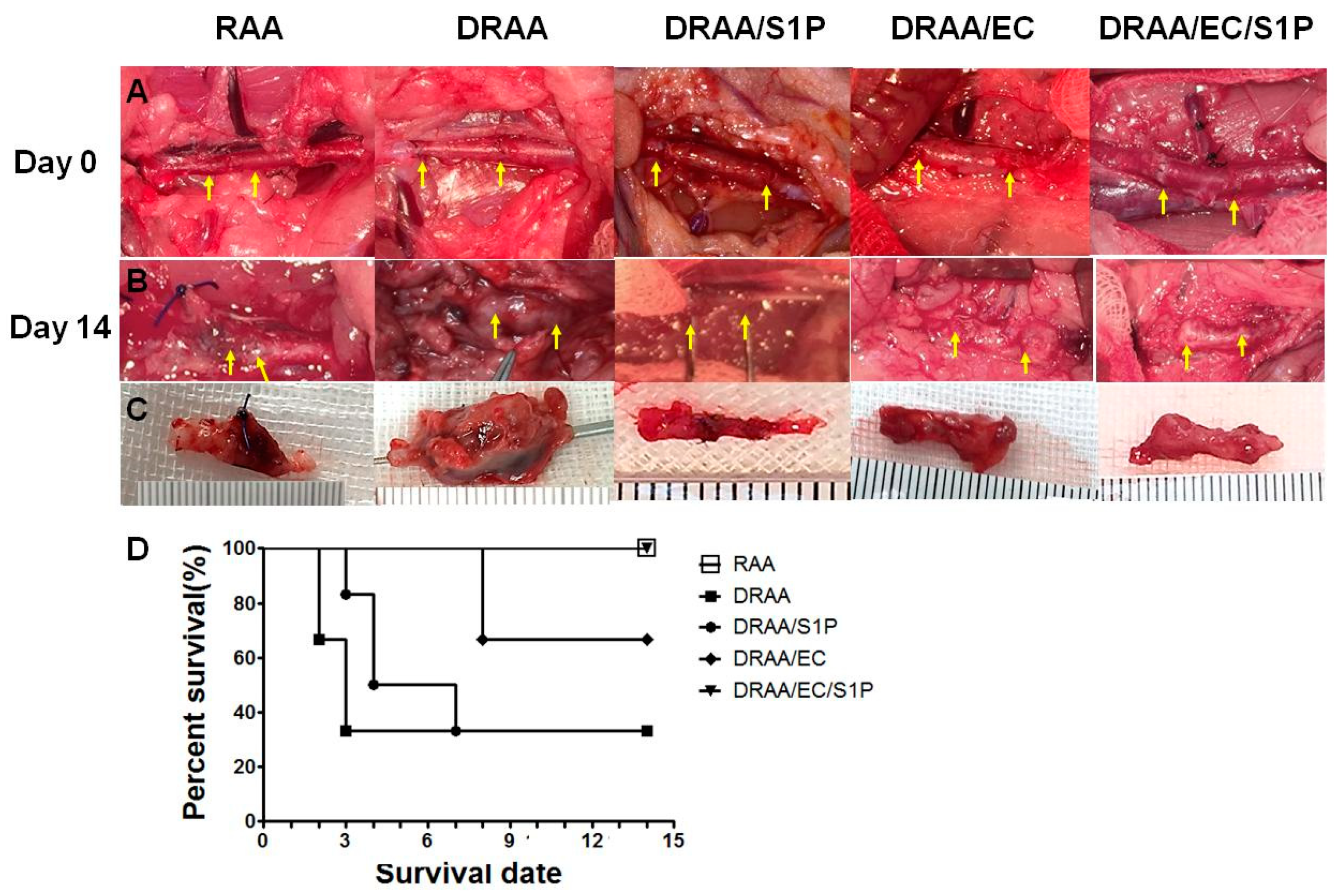
2.5.1. Patency Rate and Animal Survival
2.5.2. Histomorphology of Explanted Vessels
2.5.3. Endothelialization of Implanted Vessels
2.5.4. Macrophage Infiltration of Implanted Vessels
3. Materials and Methods
3.1. Preparation and Storage of Native and Decellularized Rat Aortas
3.2. Primary Culture, Maintenance and Characterization of Rat EC
3.3. Proliferation Effect of S1P on Rat ECs
- O1 = Molar Extinction Coefficient of OXIDIZED alamarBlue at 570 nm is 80586
- O2 = Molar Extinction Coefficient of OXIDIZED alamarBlue at 600 nm is 117216
- R1 = Molar Extinction Coefficient of REDUCED alamarBlue at 570 nm is 155677
- R2 = Molar Extinction Coefficient of REDUCED alamarBlue at 600 nm is 80586
- A1 = Absorbance value of test wells at 570 nm
- A2 = Absorbance value of test wells at 600 nm
- N1 = Absorbance value of Negative Control well at 570 nm
- N2 = Absorbance value of Negative Control well at 600 nm
3.4. Effect of S1P on SDC1 Expression in Rat ECs
3.5. Construction of TEVGs
3.6. Rat Abdominal Aorta Interposition Graft Model
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, K.W.; Gade, P.S.; Dong, L.; Zhang, Z.; Aral, A.M.; Gao, J.; Ding, X.; Stowell, C.E.T.; Nisar, M.U.; Kim, K.; et al. A biodegradable synthetic graft for small arteries matches the performance of autologous vein in rat carotid arteries. Biomaterials 2018, 181, 67–80. [Google Scholar] [CrossRef]
- Courtman, D.W.; Errett, B.F.; Wilson, G.J. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J. Biomed. Mater. Res. 2001, 55, 576–586. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Fukunishi, T.; Drews, J.; Khosravi, R.; Hor, K.; Mahler, N.; Yi, T.; Humphrey, J.D.; Johnson, J.; Breuer, C.K.; et al. Oversized Biodegradable Arterial Grafts Promote Enhanced Neointimal Tissue Formation. Tissue Eng. Part A 2018, 24, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2015, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Reing, J.E.; Zhang, L.; Myers-Irvin, J.; Cordero, K.E.; Freytes, D.O.; Heber-Katz, E.; Bedelbaeva, K.; McIntosh, D.; Dewilde, A.; Braunhut, S.J.; et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng. Part A 2009, 15, 605–614. [Google Scholar] [CrossRef]
- Cho, S.W.; Lim, S.H.; Kim, I.K.; Hong, Y.S.; Kim, S.S.; Yoo, K.J.; Park, H.Y.; Jang, Y.; Chang, B.C.; Choi, C.Y.; et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann. Surg. 2005, 241, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Conklin, B.S.; Richter, E.R.; Kreutziger, K.L.; Zhong, D.S.; Chen, C. Development and evaluation of a novel decellularized vascular xenograft. Med. Eng. Phys. 2002, 24, 173–183. [Google Scholar] [CrossRef]
- Hsia, K.; Yang, M.J.; Chen, W.M.; Yao, C.L.; Lin, C.H.; Loong, C.C.; Huang, Y.L.; Lin, Y.T.; Lander, A.D.; Lee, H.; et al. Sphingosine-1-phosphate improves endothelialization with reduction of thrombosis in recellularized human umbilical vein graft by inhibiting syndecan-1 shedding in vitro. Acta Biomater. 2017, 51, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Fields, R.; Lancaster, M. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 1993, 11, 48–50. [Google Scholar]
- Quint, C.; Kondo, Y.; Manson, R.J.; Lawson, J.H.; Dardik, A.; Niklason, L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyligers, J.; Arts, C.; Verhagen, H.; De Groot, P.G.; Moll, F. Improving small-diameter vascular grafts: From the application of an endothelial cell lining to the construction of atissue-engineered blood vessel. Ann. Vasc. Surg. 2005, 19, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.S.; Joung, Y.K.; Lee, Y.; Bae, J.W.; Park, H.K.; Park, Y.H.; Park, J.C.; Park, K.D. Enhanced Patency and Endothelialization of Small-Caliber Vascular Grafts Fabricated by Coimmobilization of Heparin and Cell-Adhesive Peptides. ACS Appl. Mater. Interfaces 2016, 8, 4336–4346. [Google Scholar] [CrossRef] [PubMed]
- Row, S.; Santandreu, A.; Swartz, D.D.; Andreadis, S.T. Cell-free vascular grafts: Recent developments and clinical potential. Technology 2017, 5, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.J., Jr.; Koobatian, M.T.; Shahini, A.; Swartz, D.D.; Andreadis, S.T. Capture of endothelial cells under flow using immobilized vascular endothelial growth factor. Biomaterials 2015, 51, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.W.; Lim, J.E.; Chu, H.S.; Hyun, H.J.; Choi, C.Y.; Hwang, K.C.; Yoo, K.J.; Kim, D.I.; Kim, B.S. Enhancement of in vivo endothelialization of tissue-engineered vascular grafts by granulocyte colony-stimulating factor. J. Biomed. Mater. Res. Part A 2006, 76, 252–263. [Google Scholar] [CrossRef]
- Zeng, W.; Wen, C.; Wu, Y.; Li, L.; Zhou, Z.; Mi, J.; Chen, W.; Yang, M.; Hou, C.; Sun, J.; et al. The use of BDNF to enhance the patency rate of small-diameter tissue-engineered blood vessels through stem cell homing mechanisms. Biomaterials 2012, 33, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, A.; Tang, Z.; Henry, J.; Li-Ping Lee, B.; Zhu, Y.; Yuan, F.; Huang, F.; Li, S. The effect of stromal cell-derived factor-1alpha/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 2012, 33, 8062–8074. [Google Scholar] [CrossRef] [PubMed]
- Boer, U.; Spengler, C.; Jonigk, D.; Klingenberg, M.; Schrimpf, C.; Lutzner, S.; Harder, M.; Kreipe, H.H.; Haverich, A.; Wilhelmi, M. Coating decellularized equine carotid arteries with CCN1 improves cellular repopulation, local biocompatibility, and immune response in sheep. Tissue Eng. Part A 2013, 19, 1829–1842. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Bracaglia, L.G.; Kimerer, L.K.; Hibino, N.; Fisher, J.P. In Vitro Endothelialization of Biodegradable Vascular Grafts Via Endothelial Progenitor Cell Seeding and Maturation in a Tubular Perfusion System Bioreactor. Tissue Eng. Part C Methods 2016, 22, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; He, Z.; Li, L.; Liu, G.; Li, Q.; Yang, D.; Zhang, Y.; Li, N. Development and in vivo validation of tissue-engineered, small-diameter vascular grafts from decellularized aortae of fetal pigs and canine vascular endothelial cells. J. Cardiothorac. Surg. 2017, 12, 101. [Google Scholar] [CrossRef] [Green Version]
- Hibino, N.; Duncan, D.R.; Nalbandian, A.; Yi, T.; Qyang, Y.; Shinoka, T.; Breuer, C.K. Evaluation of the use of an induced puripotent stem cell sheet for the construction of tissue-engineered vascular grafts. J. Thorac. Cardiovasc. Surg. 2012, 143, 696–703. [Google Scholar] [CrossRef]
- Thomas, L.V.; Lekshmi, V.; Nair, P.D. Tissue engineered vascular grafts—Preclinical aspects. Int. J. Cardiol. 2013, 167, 1091–1100. [Google Scholar] [CrossRef]
- Wacker, B.K.; Scott, E.A.; Kaneda, M.M.; Alford, S.K.; Elbert, D.L. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules 2006, 7, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.H.; Kim, Y.M.; Lee, Y.M.; Moon, E.J.; Lee, D.J.; Kim, J.H.; Kim, K.W.; Kwon, Y.G. Sphingosine 1-phosphate induces angiogenesis: Its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 1999, 264, 743–750. [Google Scholar] [CrossRef]
- Lin, C.I.; Chen, C.N.; Lin, P.W.; Lee, H. Sphingosine 1-phosphate regulates inflammation-related genes in human endothelial cells through S1P1 and S1P3. Biochem. Biophys. Res. Commun. 2007, 355, 895–901. [Google Scholar] [CrossRef]
- Alford, S.K.; Kaneda, M.M.; Wacker, B.K.; Elbert, D.L. Endothelial cell migration in human plasma is enhanced by a narrow range of added sphingosine 1-phosphate: Implications for biomaterials design. J. Biomed. Mater. Res. Part A 2009, 88, 205–212. [Google Scholar] [CrossRef]
- DiMilla, P.A.; Stone, J.A.; Quinn, J.A.; Albelda, S.M.; Lauffenburger, D.A. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 1993, 122, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Palecek, S.P.; Loftus, J.C.; Ginsberg, M.H.; Lauffenburger, D.A.; Horwitz, A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 1997, 385, 537–540. [Google Scholar] [CrossRef]
- Ushiyama, A.; Kataoka, H.; Iijima, T. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensive Care 2016, 4, 59. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, M.; Fan, J.; Tarbell, J.M.; Curry, F.R.; Fu, B.M. Sphingosine-1-phosphate Maintains Normal Vascular Permeability by Preserving Endothelial Surface Glycocalyx in Intact Microvessels. Microcirculation 2016, 23, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Adamson, R.H.; Curry, F.R.; Tarbell, J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H363–H372. [Google Scholar] [CrossRef]
- Popova, T.G.; Millis, B.; Bailey, C.; Popov, S.G. Platelets, inflammatory cells, von Willebrand factor, syndecan-1, fibrin, fibronectin, and bacteria co-localize in the liver thrombi of Bacillus anthracis-infected mice. Microb. Pathog. 2012, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Teebken, O.E.; Bader, A.; Steinhoff, G.; Haverich, A. Tissue engineering of vascular grafts: Human cell seeding of decellularised porcine matrix. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2000, 19, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Pasic, M.; Muller-Glauser, W.; Odermatt, B.; Lachat, M.; Seifert, B.; Turina, M. Seeding with omental cells prevents late neointimal hyperplasia in small-diameter Dacron grafts. Circulation 1995, 92, 2605–2616. [Google Scholar] [CrossRef] [PubMed]
- Teebken, O.E.; Pichlmaier, A.M.; Haverich, A. Cell seeded decellularised allogeneic matrix grafts and biodegradable polydioxanone-prostheses compared with arterial autografts in a porcine model. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2001, 22, 139–145. [Google Scholar] [CrossRef]
- Noishiki, Y.; Yamane, Y.; Okoshi, T.; Tomizawa, Y.; Satoh, S. Choice, isolation, and preparation of cells for bioartificial vascular grafts. Artif. Organs 1998, 22, 50–62. [Google Scholar] [CrossRef]
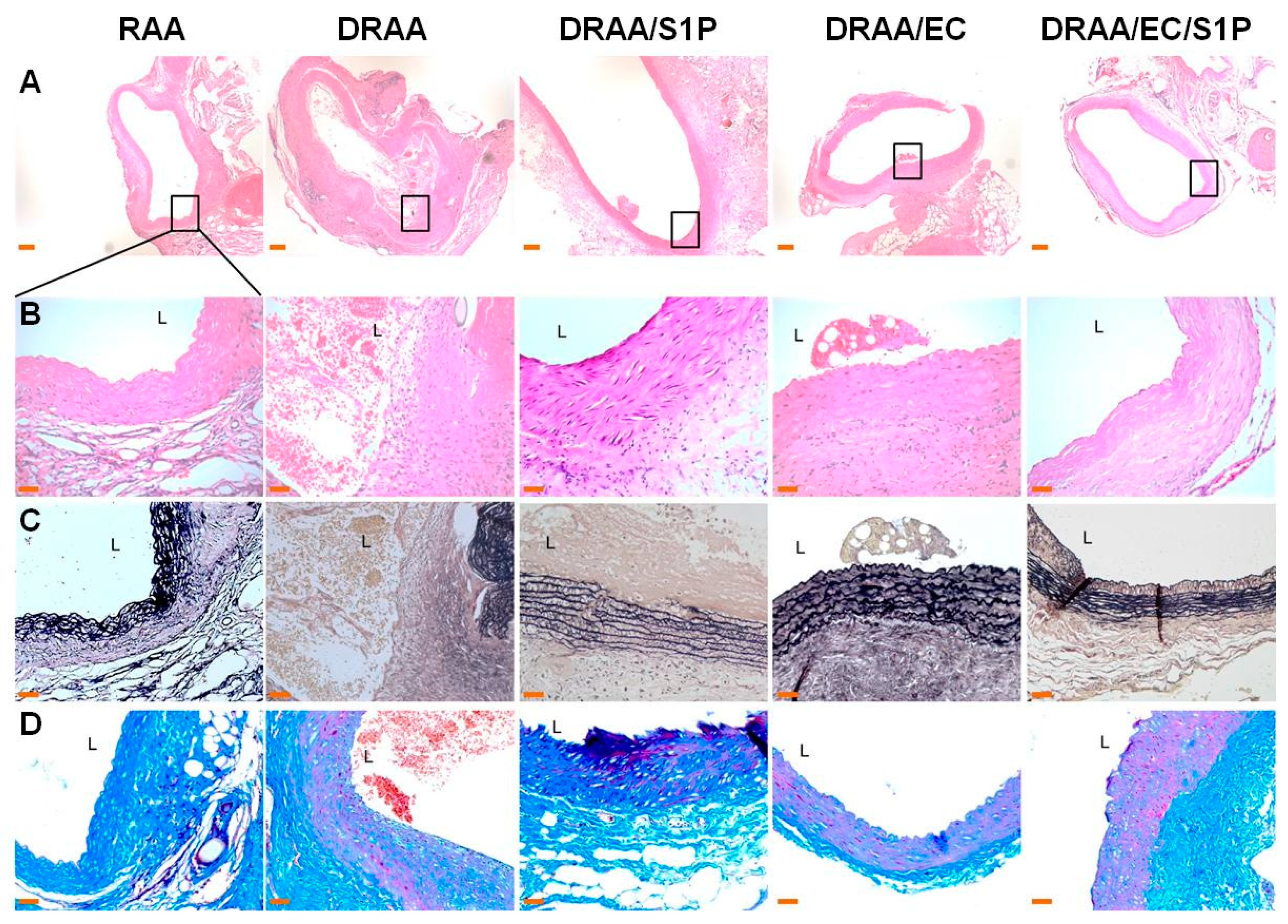
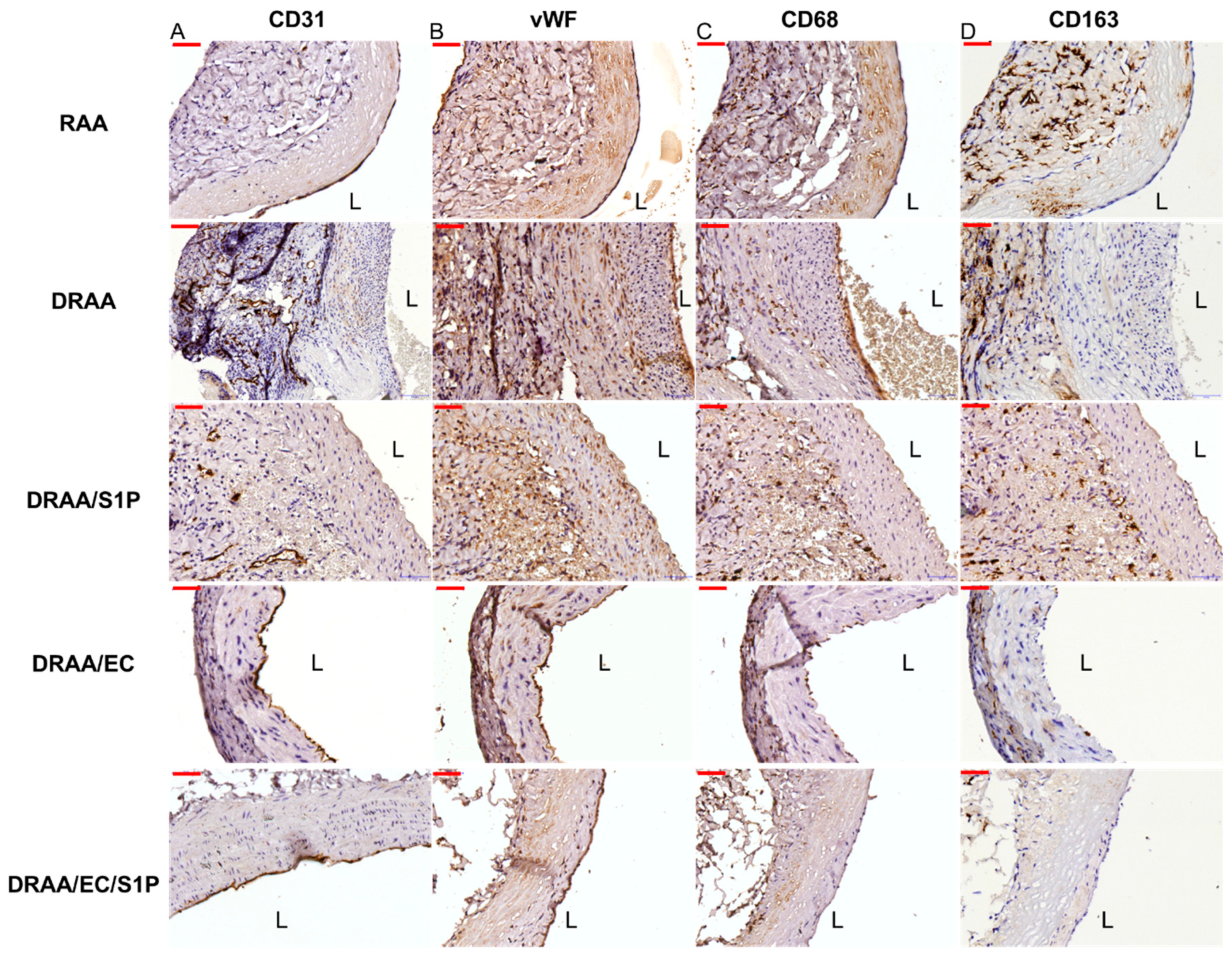
| No | RAA | DRAA | DRAA/S1P | DRAA/EC | DRAA/EC/S1P |
|---|---|---|---|---|---|
| 1 | 14 | 2 | 7 | 14 | 14 |
| 2 | 14 | 14 | 3 | 14 | 14 |
| 3 | 14 | 3 | 14 | 14 | 14 |
| 4 | 14 | 0 | 4 | 8 | 14 |
| 5 | 14 | 0 | 14 | 8 | 14 |
| 6 | 14 | 0 | 4 | 14 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, K.; Lin, C.-H.; Lee, H.-Y.; Chen, W.-M.; Yao, C.-L.; Chen, C.-C.; Ma, H.; Wang, S.-J.; Lu, J.-H. Sphingosine-1-phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts In Vivo. Int. J. Mol. Sci. 2019, 20, 1641. https://doi.org/10.3390/ijms20071641
Hsia K, Lin C-H, Lee H-Y, Chen W-M, Yao C-L, Chen C-C, Ma H, Wang S-J, Lu J-H. Sphingosine-1-phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts In Vivo. International Journal of Molecular Sciences. 2019; 20(7):1641. https://doi.org/10.3390/ijms20071641
Chicago/Turabian StyleHsia, Kai, Chih-Hsun Lin, Hsin-Yu Lee, Wei-Min Chen, Chao-Ling Yao, Chien-Chin Chen, Hsu Ma, Shyh-Jen Wang, and Jen-Her Lu. 2019. "Sphingosine-1-phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts In Vivo" International Journal of Molecular Sciences 20, no. 7: 1641. https://doi.org/10.3390/ijms20071641
APA StyleHsia, K., Lin, C.-H., Lee, H.-Y., Chen, W.-M., Yao, C.-L., Chen, C.-C., Ma, H., Wang, S.-J., & Lu, J.-H. (2019). Sphingosine-1-phosphate in Endothelial Cell Recellularization Improves Patency and Endothelialization of Decellularized Vascular Grafts In Vivo. International Journal of Molecular Sciences, 20(7), 1641. https://doi.org/10.3390/ijms20071641









