Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Propan-2-ol from Acetone-2-13C
2.2. Effect of Fermentation Parameters and Initial Amount of LAB Inoculation on Isopropyl alcohol, Acetaldehyde, Lactic Acid and Acetic Acid Content in Fermented Mashes
3. Materials and Methods
3.1. Materials
- —
- dry distillery Ethanol Red yeast (Saccharomyces cerevisiae) (Fermentis Division of S.I. Lesaffre, France),
- —
- lactic acid bacteria strains: Lactobacillus acidophilus Ł0842, Lactobacillus delbrueckii Ł0854, Lactobacillus casei Ł0901, Lactobacillus fermentum T53 Ł0954 and Lactococcus lactis Ł0877, obtained from the Pure Cultures Collection of Industrial Microorganisms at the Institute of Fermentation Technology and Microbiology (ŁOCK 105, Lodz University of Technology, Poland).
3.2. Preparation of Lactic Acid Bacteria Inoculum
3.3. Preparation of Yeast Inoculum
3.4. Sweet Mash Processing
3.5. Fermentation
3.5.1. Synthesis of Propan-2-ol from Acetone-2-13C
3.5.2. Effect of LAB Count in Sweet Mash on Propan-2-ol Content
3.6. Distillation
3.7. Analysis of Sweet and Fermented Mash
3.7.1. Chemical Composition
3.7.2. Microbial Analysis of Mashes
3.7.3. HS-GC-MS Analysis of Sweet and Fermented Mashes
3.8. Analysis of Distillate
3.8.1. NMR Analysis
3.8.2. GC×GC-TOF MS Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Parliament, Council of the European Union. Regulation (EC) No 110/2008 of the European Parliament and of the Council of 15 January 2008 on the definition, description, presentation, labelling and the protection of geographical indications of spirit drinks and repealing Council Regulation (EEC) No 1576/89. Off. J. Eur. Union 2008, L39, 16–54. [Google Scholar]
- Agricultural Distillate; PN-A-79523:2002; Polish Committee for Standardization: Warsaw, Poland, 2002; pp. 1–7.
- Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Patelski, P.; Różański, M. Effect of starch liberation method and initial pH of sweet mashes on higher alcohols content in distillates obtained from different starchy raw materials. Process Biochem. 2018, 73, 29–37. [Google Scholar] [CrossRef]
- Walther, T.; François, J.M. Microbial production of propanol. Biotechnol. Adv. 2016, 34, 984–996. [Google Scholar] [CrossRef]
- Davídek, T.; Devaud, S.; Robert, F.; Blank, I. The effect of reaction conditions on the origin and yields of acetic acid generated by the Maillard reaction. Ann. N. Y. Acad. Sci. 2005, 1043, 73–79. [Google Scholar] [CrossRef]
- Green, D.W.; Suns, H.W.; Plapp, B.V. Inversion of the substrate specificity of yeast alcohol dehydrogenase. J. Biol. Chem. 1993, 268, 7792–7798. [Google Scholar]
- Raj, S.B.; Ramaswamy, S.; Plapp, B.V. Yeast alcohol dehydrogenase structure and catalysis. Biochemistry 2014, 53, 5791–5803. [Google Scholar] [CrossRef]
- Broda, M.; Leja, K. The microbiological situation of distilleries in Poland. Pol. J. Environ. Stud. 2010, 19, 901–906. [Google Scholar]
- Skinner, K.A.; Leathers, T.D. Bacterial contaminants of fuel ethanol production. J. Ind. Microbiol. Biotechnol. 2004, 31, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Hynes, S.H.; Thomas, K.C.; Ingledew, W.M. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl. Environ. Microbiol. 1997, 63, 4158–4163. [Google Scholar] [PubMed]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.C.; Hynes, S.H.; Ingledew, W.M. Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J. Appl. Microbiol. 2001, 90, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Graves, T.; Narendranath, N.V.; Dawson, K.; Power, R. Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J. Ind. Microbiol. Biotechnol. 2006, 33, 469–474. [Google Scholar] [CrossRef]
- Pampulha, M.E.; Loureiro-Dias, M.C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31, 547–550. [Google Scholar] [CrossRef]
- Dombek, K.M.; Ingram, L.O. Ethanol production during batch fermentation with Saccharomyces cerevisiae: Changes in glycolytic enzymes and internal pH. Appl. Environ. Microbiol. 1987, 53, 1286–1291. [Google Scholar] [PubMed]
- Graves, T.; Narendranath, N.V.; Dawson, K.; Power, R. Interaction effects of lactic acid and acetic acid at different temperatures on ethanol production by Saccharomyces cerevisiae in corn mash. Appl. Microbiol. Biotechnol. 2007, 73, 1190–1196. [Google Scholar] [CrossRef]
- Pampulha, M.E.; Loureiro-Dias, M.C. Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl. Microbiol. Biotechnol. 1990, 34, 375–380. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł.; Patelski, P. The role of Saccharomyces cerevisiae yeast and lactic acid bacteria in the formation of 2-propanol from acetone during fermentation of rye mashes obtained using thermal-pressure method of starch liberation. Molecules 2019, 24, 610. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.; Jang, Y.S.; Lee, S.Y. Metabolic engineering of microorganisms for the production of higher alcohols. MBio 2014, 5, e01524-14. [Google Scholar] [CrossRef]
- Hoshino, K. Studies on the microorganism producing isopropanol from acetone. Part 2. Enzyme investigation on the oxidation-reduction of Lactobacillus brevis var. hofuensis. J. Gen. Appl. Microbiol. 1960, 6, 151–164. Available online: https://www.jstage.jst.go.jp/article/jgam1955/6/3/6_3_151/_pdf (accessed on 4 March 2019). [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Ahmed, T.; Kanwal, R.; Ayub, N. Influence of temperature on growth pattern of Lactococcus lactis, Streptococcus cremoris and Lactobacillus acidophilus isolated from camel milk. Biotechnology 2006, 5, 481–488. [Google Scholar] [CrossRef]
- Garro, M.S.; Valdez, G.F.; de Giori, G.S. Temperature effect on the biological activity of Bifidobacterium longum CRL 849 and Lactobacillus fermentum CRL 251 in pure and mixed cultures grown in soymilk. Food Microbiol. 2004, 21, 511–518. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The lactic acid bacteria: A literature survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef] [PubMed]
- Skory, C.D. Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. J. Ind. Microbiol. Biotechnol. 2003, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. 16 years research on lactic acid production with yeast—Ready for the market? Biotechnol. Genet. Eng. Rev. 2010, 27, 229–256. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W.; Nannen, N.L. pH homeostasis in lactic acid bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Yalcin, S.K.; Ozbas, Z.Y. Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Braz. J. Microbiol. 2008, 39, 325–332. [Google Scholar] [CrossRef]
- Garai-Ibabe, G.; Ibarburu, I.; Berregi, I.; Claisse, O.; Lonvaud-Funel, A.; Irastorza, A.; Dueñas, M.T. Glycerol metabolism and bitterness producing lactic acid bacteria in cidermaking. Int. J. Food Microbiol. 2008, 121, 253–261. [Google Scholar] [CrossRef]
- Pronk, J.T.; Yde Steensma, H.; Van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Shang, Y.S.; Zeng, Y.J.; Zhu, P.; Zhong, Q.P. Acetate metabolism of Saccharomyces cerevisiae at different temperatures during lychee wine fermentation. Biotechnol. Biotechnol. Equip. 2016, 30, 512–520. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Rolbiecka, A.; Czupryński, B. Changes in the concentration of carbonyl compounds during the alcoholic fermentation process carried out with Saccharomyces cerevisiae yeast. Pol. J. Microbiol. 2017, 66, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Osborne, J.P.; Mira de Orduña, R.; Pilone, G.J.; Liu, S.-Q. Acetaldehyde metabolism by wine lactic acid bacteria. FEMS Microbiol. Lett. 2000, 191, 51–55. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Niu, C.; Zhenga, F.; Zhao, Y. Simultaneous determination of diethylacetal and acetaldehyde during beer fermentation and storage process. J. Sci. Food Agric. 2018, 98, 4733–4741. [Google Scholar] [CrossRef]
- Kłosowski, G.; Czupryński, B. Kinetics of acetals and esters formation during alcoholic fermentation of various starchy raw materials with application of yeasts Saccharomyces cerevisiae. J. Food Eng. 2006, 72, 242–246. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Acetic acid and lactic acid inhibition of growth of Saccharomyces cerevisiae by different mechanisms. J. Am. Soc. Brew. Chem. 2001, 59, 187–194. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Wojtczak, M.; Czyżowska, A.; Dziekońska-Kubczak, U.; Patelski, P. The effect of different starch liberation and saccharification methods on the microbial contaminations of distillery mashes, fermentation efficiency, and spirits quality. Molecules 2017, 22, 1647. [Google Scholar] [CrossRef] [PubMed]
- Balcerek, M.; Pielech-Przybylska, K.; Strąk, E.; Patelski, P.; Dziekońska, U. Comparison of fermentation results and quality of the agricultural distillates obtained by application of commercial amylolytic preparations and cereal malts. Eur. Food Res. Technol. 2016, 242, 321–335. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; ISO 21527-1:2008; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Microorganisms. Colony-Count Technique at 30 Degrees C; ISO 4833:2004; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- International Organization for Standardization. Microbiology of Food and Animal Feeding Stuffs-Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination; ISO 6887-1:1999; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
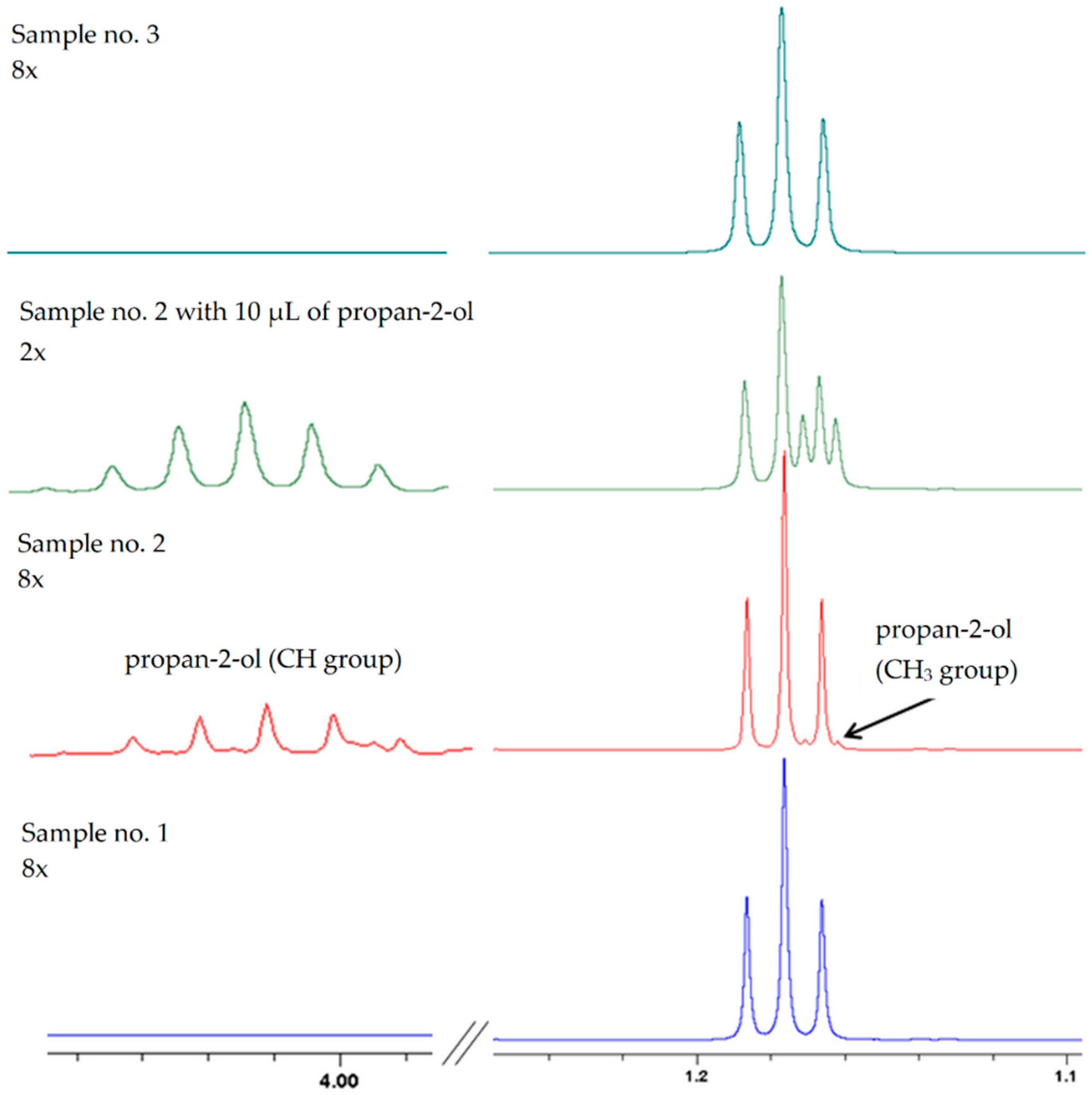
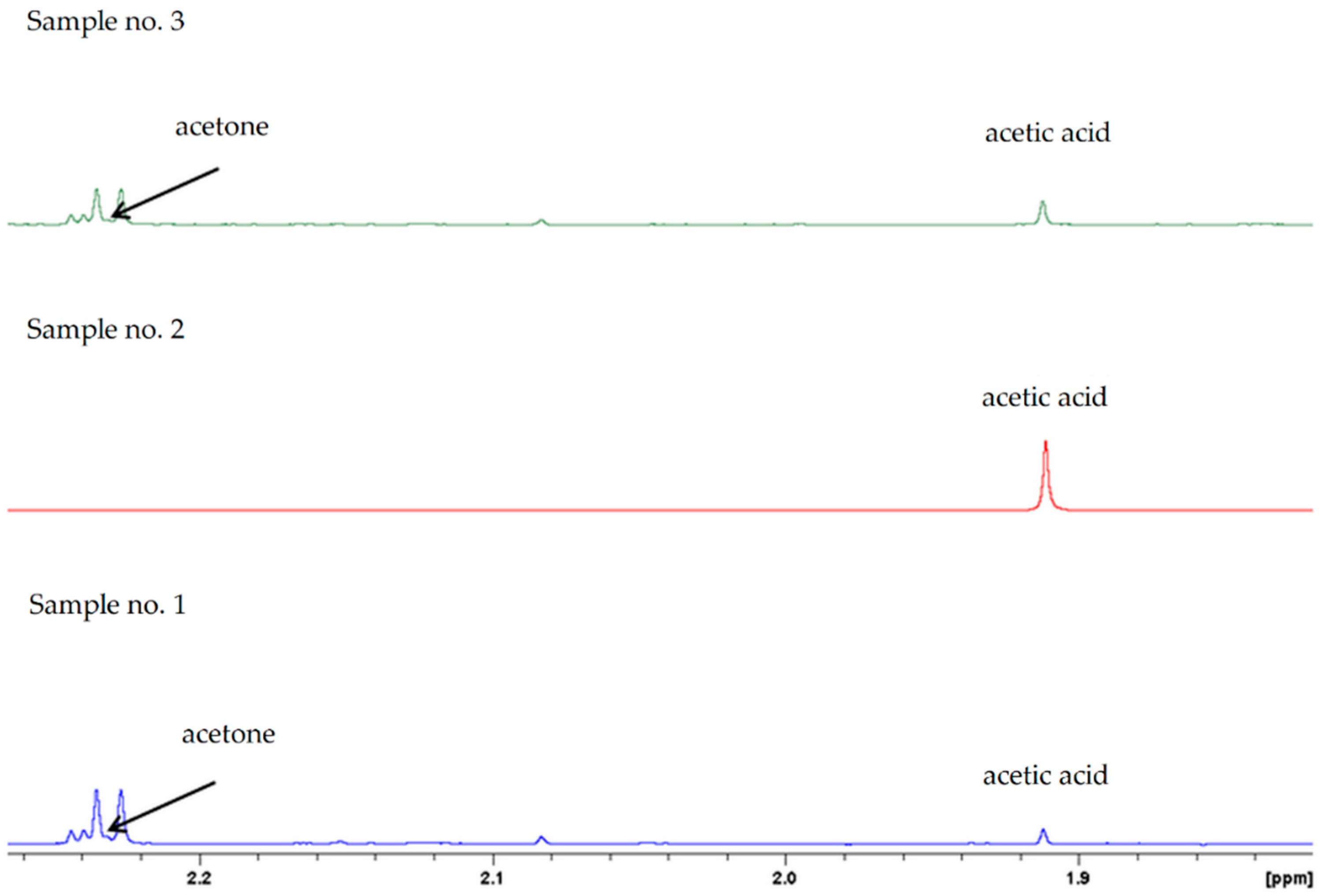
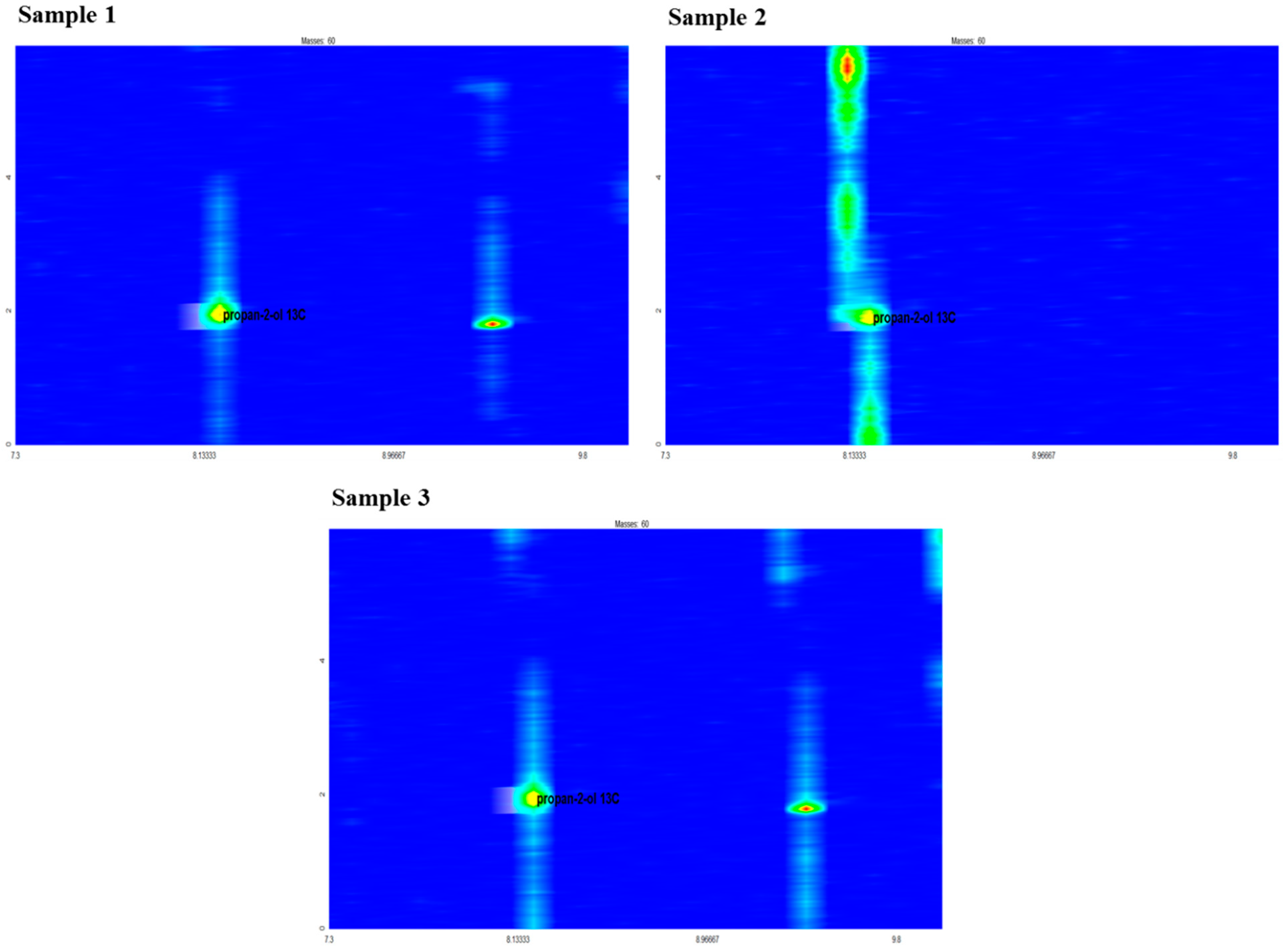
| LAB Count in Sweet Mash log cfu/mL | Temperature (°C) | Initial pH | Fermented Mash | |
|---|---|---|---|---|
| LAB Count log cfu/mL | Yeast * Count log cfu/mL | |||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Control sample (no inoculation with LAB) | 27 | 4.5 | <1.00 a | 8.18 ± 0.38 a |
| 5.0 | <1.00 a | 8.21 ± 0.38 a | ||
| 5.5 | <1.00 a | 8.14 ± 0.38 a | ||
| 35 | 4.5 | <1.00 a | 8.01 ± 0.37 a | |
| 5.0 | <1.00 a | 7.93 ± 0.37 a | ||
| 5.5 | <1.00 a | 8.00 ± 0.37 a | ||
| 3.34 ± 0.18 | 27 | 4.5 | 5.64 ± 0.30 c | 8.17 ± 0.43 a |
| 5.0 | 6.36 ± 0.34 de | 8.21 ± 0.44 a | ||
| 5.5 | 6.90 ± 0.37 efg | 8.24 ± 0.44 a | ||
| 35 | 4.5 | 4.30 ± 0.23 b | 8.10 ± 0.43 a | |
| 5.0 | 4.30 ± 0.23 b | 7.97 ± 0.42 a | ||
| 5.5 | 6.20 ± 0.33 cd | 7.87 ± 0.42 a | ||
| 4.34 ± 0.21 | 27 | 4.5 | 6.42 ± 0.31 def | 8.21 ± 0.40 a |
| 5.0 | 7.08 ± 0.34 fgh | 8.22 ± 0.40 a | ||
| 5.5 | 7.80 ± 0.38 ij | 8.08 ± 0.39 a | ||
| 35 | 4.5 | 4.00 ± 0.19 b | 8.01 ± 0.39 a | |
| 5.0 | 5.74 ± 0.28 cd | 8.11 ± 0.41 a | ||
| 5.5 | 7.06 ± 0.34 fgh | 8.06 ± 0.37 a | ||
| 5.34 ± 0.23 | 27 | 4.5 | 7.58 ± 0.33 ghij | 8.10 ± 0.35 a |
| 5.0 | 8.20 ± 0.36 jk | 8.12 ± 0.35 a | ||
| 5.5 | 8.24 ± 0.36 jk | 8.14 ± 0.35 a | ||
| 35 | 4.5 | 6.00 ± 0.26 cd | 7.96 ± 0.34 a | |
| 5.0 | 7.42 ± 0.32 ghi | 7.84 ± 0.34 a | ||
| 5.5 | 7.79 ± 0.34 ij | 7.72 ± 0.33 a | ||
| 6.34 ± 0.32 | 27 | 4.5 | 8.16 ± 0.41 jk | 8.19 ± 0.41 a |
| 5.0 | 8.48 ± 0.43 k | 8.20 ± 0.41 a | ||
| 5.5 | 8.62 ± 0.43 k | 8.15 ± 0.41 a | ||
| 35 | 4.5 | 7.61 ± 0.38 hij | 7.83 ± 0.39 a | |
| 5.0 | 8.06 ± 0.41 jk | 7.64 ± 0.38 a | ||
| 5.5 | 8.19 ± 0.41 jk | 7.53 ± 0.38 a | ||
| LAB Count in Sweet Mash log cfu/mL | Temperature (°C) | Initial pH | Fermented Mash * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone ** (mg/L) | Isopropyl Alcohol (mg/L) | Acetaldehyde (mg/L) | Ethanol (g/L) | Residual Sugars (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | Glycerol (g/L) | pH | |||
| Control (no inoculation with LAB) | 27 | 4.5 | 0.62 ± 0.06 ijklm | 0.46 ± 0.05 ab | 66.54 ± 2.57 mn | 53.51 ± 1.47 ab | 1.43 ± 0.10 no | nd | 0.15 ± 0.00 ab | 3.02 ± 0.08 a | 3.9 ± 0.1 bc |
| 5.0 | 0.51 ± 0.04 b | 0.52 ± 0.05 fgh | 54.68 ± 1.95 k | 53.83 ± 1.18 a | 1.24 ± 0.09 ij | nd | 0.17 ± 0.00 cd | 3.32 ± 0.07 bc | 4.2 ± 0.1 de | ||
| 5.5 | 0.50 ± 0.05 b | 0.59 ± 0.04 k | 48.81 ± 1.29 i | 58.86 ± 1.06 bc | 1.23 ± 0.09 hi | nd | 0.23 ± 0.00 f | 3.71 ± 0.07 fgh | 4.4 ± 0.1 e | ||
| 35 | 4.5 | 0.53 ± 0.05 bcd | 0.55 ± 0.03 hij | 69.85 ± 3.72 no | 58.19 ± 1.02 bc | 0.88 ± 0.07 f | nd | 0.17 ± 0.00 cd | 3.54 ± 0.06 cdef | 4.0 ± 0.1 cd | |
| 5.0 | 0.55 ± 0.02 cde | 0.55 ± 0.04 hij | 50.49 ± 1.62 ij | 56.71 ± 1.27 abc | 0.89 ± 0.08 fg | nd | 0.20 ± 0.00 e | 3.62 ± 0.08 efg | 4.3 ± 0.1 de | ||
| 5.5 | 0.51 ± 0.04 b | 0.66 ± 0.05 m | 40.31 ± 1.38 fg | 57.98 ± 1.31 bc | 0.79 ± 0.07 de | nd | 0.24 ± 0.01 f | 3.99 ± 0.09 ijk | 4.4 ± 0.1 e | ||
| 3.34 ± 0.18 | 27 | 4.5 | 0.71 ± 0.03 p | 0.48 ± 0.05 abcd | 29.47 ± 1.60 bc | 58.22 ± 1.93 bc | 1.33 ± 0.09 lm | 0.11 ± 0.00 ab | 0.14 ± 0.00 a | 3.32 ± 0.11 bc | 4.0 ± 0.1 cd |
| 5.0 | 0.68 ± 0.06 op | 0.46 ± 0.04 abc | 23.61 ± 1.19 a | 55.28 ± 1.59 ab | 1.17 ± 0.08 h | 0.15 ± 0.00 b | 0.14 ± 0.00 a | 3.56 ± 0.10 cdef | 4.2 ± 0.1 de | ||
| 5.5 | 0.61 ± 0.05 ghijk | 0.53 ± 0.02 fghi | 30.81 ± 1.73 c | 58.05 ± 2.00 bc | 1.19 ± 0.09 hi | 0.30 ± 0.01 c | 0.23 ± 0.01 f | 3.75 ± 0.13 fgh | 4.5 ± 0.2 e | ||
| 35 | 4.5 | 0.64 ± 0.05 klmno | 0.48 ± 0.03 abcde | 31.16 ± 1.73 c | 56.14 ± 1.93 abc | 0.80 ± 0.07 de | 0.08 ± 0.00 ab | 0.16 ± 0.01 bc | 3.39 ± 0.12 bcd | 4.0 ± 0.1 cd | |
| 5.0 | 0.56 ± 0.03 def | 0.53 ± 0.03 fghi | 43.62 ± 1.66 gh | 55.35 ± 1.40 ab | 0.90 ± 0.08 fg | 0.10 ± 0.00 ab | 0.24 ± 0.01 f | 3.51 ± 0.09 cdef | 4.3 ± 0.1 de | ||
| 5.5 | 0.50 ± 0.04 b | 0.63 ± 0.04 l | 40.02 ± 2.74 ef | 55.55 ± 1.57 ab | 0.74 ± 0.06 cd | 0.28 ± 0.01 c | 0.33 ± 0.01 jk | 3.71 ± 0.11 fgh | 4.5 ± 0.1 e | ||
| 4.34 ± 0.21 | 27 | 4.5 | 0.71 ± 0.07 p | 0.47 ± 0.02 abc | 53.77 ± 2.20 k | 57.04 ± 1.61 abc | 1.38 ± 0.07 mn | 0.20 ± 0.01 bc | 0.14 ± 0.00 ab | 3.23 ± 0.09 ab | 4.0 ± 0.1 cd |
| 5.0 | 0.65 ± 0.06 mno | 0.47 ± 0.04 abc | 41.14 ± 1.80 fg | 56.37 ± 1.63 abc | 1.26 ± 0.08 ijk | 0.52 ± 0.02 d | 0.17 ± 0.00 cd | 3.52 ± 0.10 cdef | 4.3 ± 0.1 de | ||
| 5.5 | 0.59 ± 0.05 efghi | 0.57 ± 0.02 jk | 71.16 ± 2.44 o | 54.68 ± 1.32 ab | 1.16 ± 0.07 h | 0.69 ± 0.02 f | 0.27 ± 0.01 gh | 3.62 ± 0.09 defg | 4.4 ± 0.1 e | ||
| 35 | 4.5 | 0.53 ± 0.02 bcd | 0.54 ± 0.02 ghij | 63.74 ± 2.44 m | 59.53 ± 1.60 c | 0.95 ± 0.09 g | 0.12 ± 0.00 ab | 0.17 ± 0.00 d | 3.72 ± 0.10 fgh | 4.0 ± 0.1 cd | |
| 5.0 | 0.56 ± 0.06 def | 0.54 ± 0.05 ghij | 36.35 ± 1.86 de | 56.10 ± 1.86 abc | 0.78 ± 0.08 cde | 0.27 ± 0.01 c | 0.26 ± 0.01 g | 3.60 ± 0.12 defg | 4.3 ± 0.1 de | ||
| 5.5 | 0.44 ± 0.04 a | 0.67 ± 0.04 m | 52.86 ± 1.54 jk | 58.08 ± 1.16 bc | 0.72 ± 0.06 c | 0.71 ± 0.01 f | 0.42 ± 0.01 m | 4.00 ± 0.08 ijk | 4.5 ± 0.1 e | ||
| 5.34 ± 0.23 | 27 | 4.5 | 0.67 ± 0.03 no | 0.49 ± 0.03 bcdef | 35.43 ± 1.98 d | 58.12 ± 2.09 bc | 1.47 ± 0.11 o | 0.66 ± 0.02 ef | 0.16 ± 0.01 cd | 3.38 ± 0.12 bcde | 4.0 ± 0.1 cd |
| 5.0 | 0.62 ± 0.02 hijklm | 0.50 ± 0.04 cdef | 38.66 ± 1.46 def | 57.58 ± 1.41 bc | 1.32 ± 0.10 klm | 1.44 ± 0.04 g | 0.23 ± 0.01 f | 3.74 ± 0.09 fgh | 4.0 ± 0.1 cd | ||
| 5.5 | 0.58 ± 0.05 efg | 0.51 ± 0.04 dfg | 48.79 ± 1.09 i | 54.13 ± 1.24 ab | 1.31 ± 0.09 jkl | 1.66 ± 0.04 h | 0.29 ± 0.01 i | 3.85 ± 0.09 ghij | 4.1 ± 0.1 cd | ||
| 35 | 4.5 | 0.52 ± 0.05 bc | 0.53 ± 0.03 ghi | 30.87 ± 1.97 bc | 58.72 ± 1.38 bc | 0.84 ± 0.08 ef | 0.56 ± 0.01 de | 0.16 ± 0.00 cd | 3.59 ± 0.08 defg | 4.0 ± 0.1 cd | |
| 5.0 | 0.59 ± 0.05 fghij | 0.54 ± 0.04 ghij | 27.15 ± 1.66 b | 57.94 ± 2.11 bc | 0.61 ± 0.07 b | 1.56 ± 0.06 h | 0.32 ± 0.01 jk | 4.05 ± 0.15 jk | 4.0 ± 0.1 cd | ||
| 5.5 | 0.63 ± 0.06 jklmn | 0.52 ± 0.07 efgh | 53.41 ± 1.08 jk | 58.48 ± 1.53 bc | 0.50 ± 0.07 a | 2.73 ± 0.07 j | 0.38 ± 0.01 l | 4.33 ± 0.11 l | 4.0 ± 0.1 cd | ||
| 6.34 ± 0.32 | 27 | 4.5 | 0.59 ± 0.04 efghi | 0.47 ± 0.02 abc | 45.78 ± 2.02 h | 57.01 ± 1.73 bc | 1.33 ± 0.10 lm | 1.83 ± 0.06 i | 0.23 ± 0.01 f | 3.73 ± 0.11 fghi | 3.7 ± 0.1 a |
| 5.0 | 0.62 ± 0.06 ijklm | 0.47 ± 0.03 abc | 97.06 ± 5.82 p | 56.59 ± 2.52 abc | 1.40 ± 0.09 n | 3.05 ± 0.14 k | 0.28 ± 0.01 hi | 4.90 ± 0.22 m | 3.7 ± 0.2 a | ||
| 5.5 | 0.65 ± 0.06 lmno | 0.52 ± 0.06 defgh | 120.68 ± 5.77 s | 56.07 ± 1.33 abc | 1.30 ± 0.09 jkl | 4.46 ± 0.11 m | 0.33 ± 0.01 k | 3.80 ± 0.09 ghij | 3.7 ± 0.1 a | ||
| 35 | 4.5 | 0.58 ± 0.05 efghi | 0.53 ± 0.06 ghi | 30.55 ± 1.69 bc | 58.47 ± 1.98 bc | 0.77 ± 0.07 cd | 1.80 ± 0.06 i | 0.26 ± 0.01 g | 3.93 ± 0.13 hij | 3.8 ± 0.1 ab | |
| 5.0 | 0.59 ± 0.04 efghi | 0.46 ± 0.05 a | 58.59 ± 3.17 l | 59.03 ± 1.52 bc | 0.53 ± 0.08 a | 3.70 ± 0.10 l | 0.32 ± 0.01 j | 4.21 ± 0.11 kl | 3.8 ± 0.1 ab | ||
| 5.5 | 0.61 ± 0.05 ghijkl | 0.45 ± 0.02 a | 114.03 ± 6.04 r | 53.35 ± 1.43 a | 0.48 ± 0.06 a | 4.64 ± 0.12 n | 0.37 ± 0.01 l | 3.90 ± 0.10 hij | 3.8 ± 0.1 ab | ||
| Factors | Fermented Mash | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | Isopropyl Alcohol | Acetaldehyde | Residual Sugars | Lactic Acid | Acetic Acid | Glycerol | Ethanol | pH | LAB | Yeast | |
| LABC | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** | ns |
| T | *** | *** | *** | *** | *** | *** | *** | * | ns | *** | ** |
| IpH | *** | *** | *** | *** | *** | *** | *** | * | *** | *** | ns |
| LABC * T | *** | *** | *** | *** | *** | *** | *** | * | ns | *** | ns |
| LABC * IpH | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | ns |
| T * IpH | *** | ns | *** | * | *** | *** | *** | ns | ns | ** | ns |
| LABC * T * IpH | *** | *** | *** | *** | *** | *** | *** | * | ns | * | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659. https://doi.org/10.3390/ijms20071659
Pielech-Przybylska K, Balcerek M, Ciepielowski G, Pacholczyk-Sienicka B, Albrecht Ł, Dziekońska-Kubczak U, Bonikowski R, Patelski P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. International Journal of Molecular Sciences. 2019; 20(7):1659. https://doi.org/10.3390/ijms20071659
Chicago/Turabian StylePielech-Przybylska, Katarzyna, Maria Balcerek, Grzegorz Ciepielowski, Barbara Pacholczyk-Sienicka, Łukasz Albrecht, Urszula Dziekońska-Kubczak, Radosław Bonikowski, and Piotr Patelski. 2019. "Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes" International Journal of Molecular Sciences 20, no. 7: 1659. https://doi.org/10.3390/ijms20071659
APA StylePielech-Przybylska, K., Balcerek, M., Ciepielowski, G., Pacholczyk-Sienicka, B., Albrecht, Ł., Dziekońska-Kubczak, U., Bonikowski, R., & Patelski, P. (2019). Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. International Journal of Molecular Sciences, 20(7), 1659. https://doi.org/10.3390/ijms20071659






