Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig
Abstract
:1. Introduction
2. Results
2.1. Glucose Serum Level
2.2. Changes in Chemical Coding of the ENS Neurons in the Small Intestine
2.2.1. Myenteric Plexus (MP)
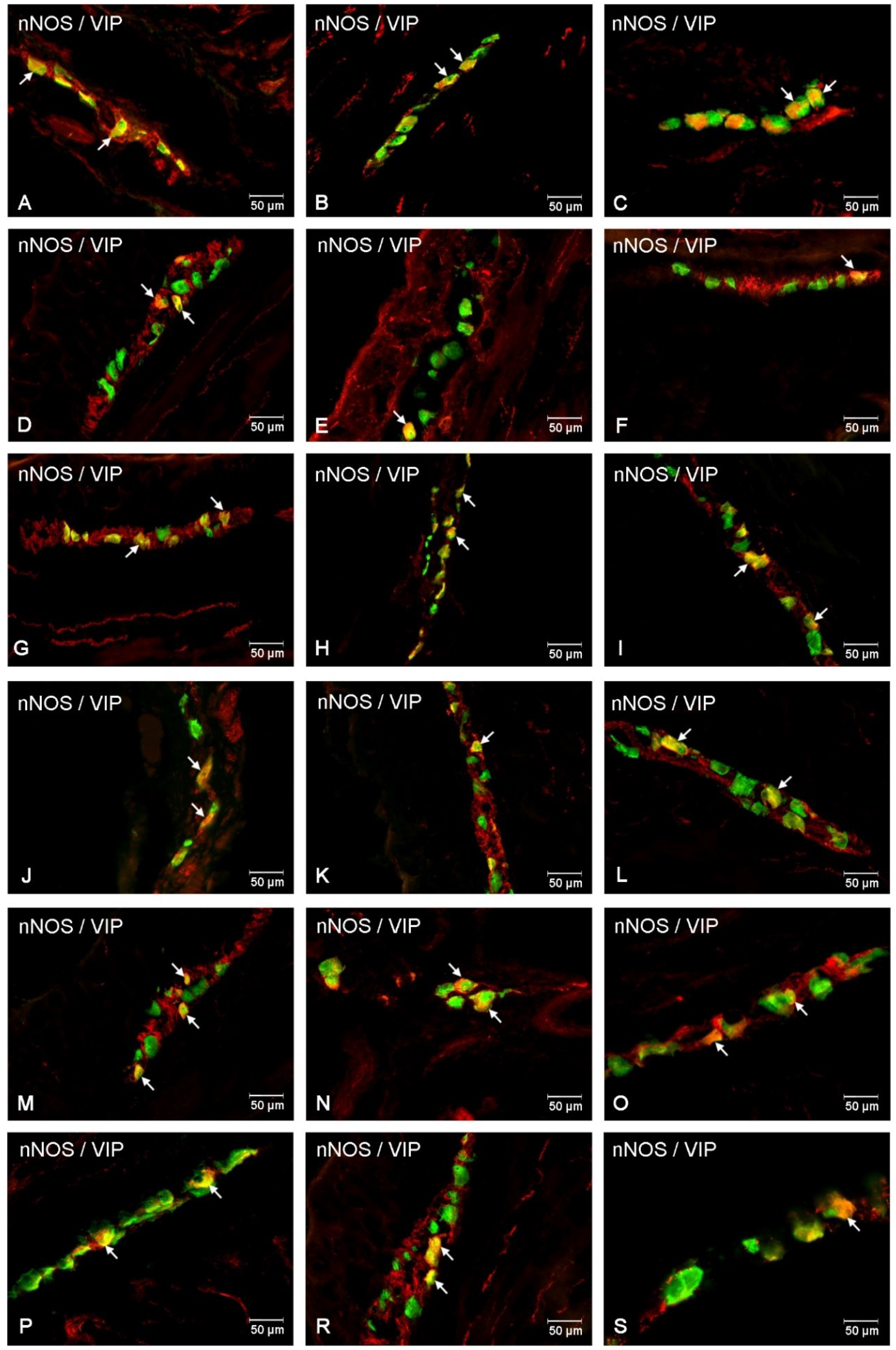
Neuronal Nitric Oxide Synthase (nNOS) Activity
Co-Localization of nNOS with Other Biologically Active Substances in the MP
2.2.2. Outer Submucosal Plexus (OSP)
Neuronal Nitric Oxide Synthase (nNOS) Activity
Co-Localization of nNOS with Other Biologically Active Substances in the OSP
2.2.3. Inner Submucous Plexus (ISP)
Neuronal Nitric Oxide Synthase (nNOS) Activity
Co-Localization of nNOS with Other Biologically Active Substances in the ISP
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takahashi, T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J. Gastroenterol. 2003, 38, 421–430. [Google Scholar] [CrossRef]
- Freeman, R. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2014, 126, 63–79. [Google Scholar]
- Chandrasekharan, B.; Srinivasan, S. Diabetes and the enteric nervous system. Neurogastroenterol. Motil. 2007, 19, 951–960. [Google Scholar] [PubMed] [Green Version]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [PubMed]
- Makowska, K.; Rytel, L.; Lech, P.; Osowski, A.; Kruminis-Kaszkiel, E.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in the enteric nervous system of the porcine esophagus. C. R. Biol. 2018, 341, 325–333. [Google Scholar] [CrossRef]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric. Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Clerc, N.; Furness, J.B. Intrinsic primary afferent neurones of the digestive tract. Neurogastroenterol. Motil. 2004, 16, 24–27. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Handb. Exp. Pharmacol. 2017, 239, 1–16. [Google Scholar]
- Sharkey, K.A.; Beck, P.; McKay, D.M. Neuroimmunophysiology of the gut: Advances and emerging concepts focusing on the epithelium. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 765–784. [Google Scholar] [CrossRef]
- Nezami, B.G.; Srinivasan, S. Enteric Nervous System in the Small Intestine: Pathophysiology and Clinical Implications. Curr. Gastroenterol. Rep. 2010, 12, 358–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, M.; Schlabrakowski, A.; Schrodl, F.; Neuhuber, W.; Brehmer, A. ChAT and NOS in human myenteric neurons: Co-existence and co-absence. Cell Tissue Res. 2009, 338, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M. Nitric oxide as a mediator of nonadrenergic, noncholinergic neurotransmission. Am. J. Physiol. 1992, 262, G379–G392. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Schleiffer, R.; Raul, F. Nitric oxide and the digestive system in mammals and nonmammalian vertebrates. Comp. Biochem. Physiol. 1997, 118A, 965–974. [Google Scholar] [CrossRef]
- Young, H.M.; Furness, J.B.; Shuttleworth, C.W.R.; Bredt, D.S.; Snyder, S.H. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry 1992, 97, 375–378. [Google Scholar] [CrossRef]
- Barbiers, M.; Timmermans, J.P.; Scheuermann, D.W.; Adriaensen, D.; Mayer, B.; De Groodt Lasseel, M.H.A. Nitric oxide synthase-containing neurons in the pig large intestine: Topography, morphology, and viscerofugal projections. Microsc. Res. Tech. 1994, 29, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Schrodl, F.; Neuhuber, W. Morphology of VIP/nNOS-immunoreactive myenteric neurons in the human gut. Histochem. Cell Biol. 2006, 125, 557–565. [Google Scholar] [CrossRef]
- Rivera, L.R.; Poole, D.P.; Thacker, M.; Furness, J.B. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil. 2011, 23, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Srinivasan, S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: Current status and future directions. Neurogastroenterol. Motil. 2014, 26, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 1, 50–54. [Google Scholar] [CrossRef]
- Smith, A.C.; Swindle, M.M. Preparation of swine for the laboratory. ILAR J. 2006, 47, 358–363. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C. Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 1998, 25, 11–21. [Google Scholar]
- Larsen, M.O.; Wilken, M.; Gotfredsen, C.F. Mild streptozotocin diabetes in the Gottingen minipig. A novel model of moderate insulin deficiency and diabetes. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1342–E1351. [Google Scholar] [CrossRef]
- Litten-Brown, J.C.; Corson, A.M.; Clarke, L. Porcine models for the metabolic syndrome, digestive and bone disorders: A general overview. Animal 2010, 4, 899–920. [Google Scholar] [CrossRef]
- Aimi, Y.; Kiura, H.; Kinoshita, T.; Minami, Y.; Fujimura, M.; Vincent, S.R. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience 1993, 53, 553–560. [Google Scholar] [CrossRef]
- Bolekova, A.; Spakovska, T.; Kluchova, D.; Toth, S.; Vesela, J. NADPH-diaphorase expression in the rat jejunum after intestinal ischemia/reperfusion. Eur. J. Histochem. 2011, 55, e23. [Google Scholar] [CrossRef]
- Sang, Q.; Young, H.M. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996, 284, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Altdorfer, K.; Feher, E.; Donath, T.; Feher, J. Nitric oxide synthase-containing nerve elements in the pylorus of the cat. Neurosci. Lett. 1996, 212, 195–198. [Google Scholar] [CrossRef]
- Singaram, C.; Sengupta, A.; Sweet, M.A.; Sugarbaker, D.J.; Goyal, R.K. Nitrergic and peptidergic innervation of the human oesophagus. Gut 1994, 35, 1690–1696. [Google Scholar] [CrossRef]
- LePard, K.J. Choline acetyltransferase and inducible nitric oxide synthase are increased in myenteric plexus of diabetic guinea pig. Auton Neurosci. 2005, 118, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef] [Green Version]
- Vincent, A.M.; Russell, J.W.; Low, P.; Feldman, E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocin. Rev. 2004, 25, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Gatopoulou, A.; Papanas, N.; Maltezos, E. Diabetic gastrointestinal autonomic neuropathy: Current status and new achievements for everyday clinical practice. Eur. J. Intern. Med. 2012, 23, 499–505. [Google Scholar] [CrossRef]
- He, C.L.; Soffer, E.E.; Ferris, C.D.; Walsh, R.M.; Szurszewski, J.H.; Farrugia, G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001, 121, 427–434. [Google Scholar] [CrossRef]
- Cellek, S.; Foxwell, N.A.; Moncada, S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes 2003, 52, 2353–2362. [Google Scholar] [CrossRef]
- Halama, A.; Kahal, H.; Bhagwat, A.M.; Zierer, J.; Sathyapalan, T.; Graumann, J.; Suhre, K.; Atkin, S.L. Metabolic and proteomic signatures of hypoglycaemia in type 2 diabetes. Diabetes Obes. Metab. 2018, 21, 909–919. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.C.; Sawa, A.; Jaffrey, S.; Blackshaw, S.; Barrow, R.K.; Snyder, S.H.; Ferris, C.D. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J. Clin. Investig. 2000, 106, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Franck, H.; Sweeney, K.M.; Sanders, K.M.; Shuttleworth, C.W.R. Effects of a novel guanylate cyclase inhibitor on nitric oxide-dependent inhibitory neurotransmission in canine proximal colon. Br. J. Pharmacol. 1997, 122, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Palus, K.; Rytel, L. Co-localisation of cocaine- and amphetamine-regulated transcript peptide and vasoactive intestinal polypeptide in the myenteric plexus of the porcine transverse colon. Folia Morphol. (Warsz) 2013, 72, 328–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 127, 264–272. [Google Scholar] [CrossRef]
- Wierup, N.; Gunnarsdóttir, A.; Ekblad, E.; Sundler, F. Characterisation of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci. 2007, 11, 8–51. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Sand, E.; Ekblad, E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul. Pept. 2008, 146, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol. Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Foxt-Threlkeld, J.E.T.; McDonald, T.J.; Cipris, S.; Woskowska, Z.; Daniel, E.E. Galanin inhibition of vasoactive intestinal polypeptide release and circular muscle motility in the isolated perfused canine ileum. Gastroenterology 1991, 101, 1471–1476. [Google Scholar] [CrossRef]
- El-Salhy, M. Neuroendocrine peptides of the gastrointestinal tract of an animal model of human type 2 diabetes mellitus. Acta Diabetol. 1998, 35, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Substance P as a neuronal factor in the enteric nervous system of the porcine descending colon in physiological conditions and during selected pathogenic processes. Biofactors 2013, 39, 542–551. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and their functions in the gastrointestinal tract. Cell Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef]
- Maggi, C.A. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 1997, 70, 75–90. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Zielonka, Ł.; Gajęcka, M.; Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin- induced diabetes in the pig. World J. Gastroenterol. 2017, 23, 6088–6099. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Palus, K.; Całka, J.; Zielonka, Ł. Changes in Immunoreactivity of Sensory Substances within the Enteric Nervous System of the Porcine Stomach during Experimentally Induced Diabetes. J. Diabetes Res. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]








| Date of Blood Collection | Control Group (mmol/L) | SEM± | Experimental Group (mmol/L) | SEM± |
|---|---|---|---|---|
| Before streptozotocin injection | 5.01 | 0.10 | 5.03 | 0.10 |
| 1 week after streptozotocin injection | 5.08 | 0.10 | 17.36 | 0.38 |
| 2 weeks after streptozotocin injection | 4.91 | 0.18 | 20.72 | 0.24 |
| 3 weeks after streptozotocin injection | 5.19 | 0.06 | 21.58 | 0.27 |
| 4 weeks after streptozotocin injection | 5.31 | 0.12 | 20.08 | 0.09 |
| 5 weeks after streptozotocin injection | 4.84 | 0.32 | 22.26 | 1.21 |
| 6 weeks after streptozotocin injection | 5.20 | 0.1 | 21.45 | 1.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulc, M.; Palus, K.; Dąbrowski, M.; Całka, J. Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig. Int. J. Mol. Sci. 2019, 20, 1681. https://doi.org/10.3390/ijms20071681
Bulc M, Palus K, Dąbrowski M, Całka J. Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig. International Journal of Molecular Sciences. 2019; 20(7):1681. https://doi.org/10.3390/ijms20071681
Chicago/Turabian StyleBulc, Michał, Katarzyna Palus, Michał Dąbrowski, and Jarosław Całka. 2019. "Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig" International Journal of Molecular Sciences 20, no. 7: 1681. https://doi.org/10.3390/ijms20071681
APA StyleBulc, M., Palus, K., Dąbrowski, M., & Całka, J. (2019). Hyperglycaemia-Induced Downregulation in Expression of nNOS Intramural Neurons of the Small Intestine in the Pig. International Journal of Molecular Sciences, 20(7), 1681. https://doi.org/10.3390/ijms20071681




