TRPM7, Magnesium, and Signaling
Abstract
:1. Introduction
2. Transient Receptor Potential Melastatin 7 Cation Channel (TRPM7)
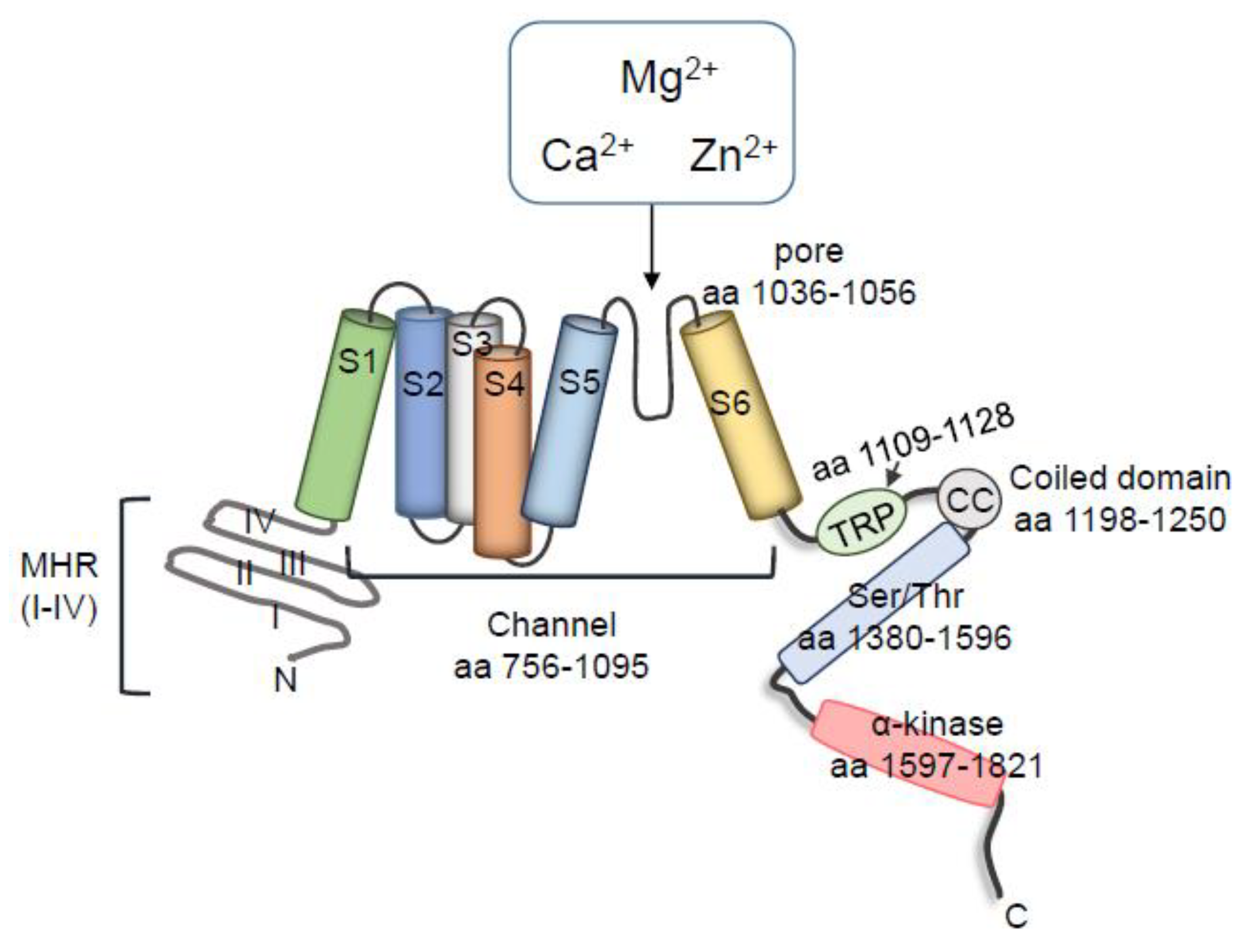
2.1. Characteristics and Regulation of TRPM7
2.2. Interactions between TRPM7 Kinase and TRPM7 Channel Domains
2.3. Relationship between TRPM6 and TRPM7
2.4. TRPM7 and Cell Function
3. Receptor Tyrosine Kinase Signaling and Mg2+
3.1. Characteristics and Regulation of Receptor Tyrosine Kinases
3.2. Growth Factors and Receptor Tyrosine Kinase Signaling
4. Cross-Talk between TRPM6/7, Receptor Tyrosine Kinases and Signaling Kinases
4.1. Regulation of TRPM6/7 by Receptor Tyrosine Kinases
4.2. Regulation of Signaling Kinases by TRPM7
5. TRPM7and Kinase Signaling—Pathophysiological Implications
6. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.B.; Cibulsky, S.M. Biochemistry. TRP ion channels--two proteins in one. Science 2001, 293, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Z.; Li, J.; Hulse, R.E.; Santa-Cruz, A.; Valinsky, W.C.; Abiria, S.A.; Krapivinsky, G.; Zhang, J.; Clapham, D.E. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl. Acad. Sci. USA 2018, 115, E8201–E8210. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hong, C.; Kim, B.J.; So, I. The Pathophysiologic Roles of TRPM7 Channel. Korean J. Physiol. Pharmacol. 2014, 18, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadolni, W.; Zierler, S. The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis. Cells 2018, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, G.; Savoia, C.; Touyz, R.M. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: Role of angiotensin II. Circ. Res. 2005, 96, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Gwanyanya, A.; Amuzescu, B.; Zakharov, S.I.; Macianskiene, R.; Sipido, K.R.; Bolotina, V.M.; Vereecke, J.; Mubagwa, K. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: Permeation of divalent cations and pH-mediated regulation. J. Physiol. 2004, 559, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, S.; Xiao, C.; Jia, Y.; Guo, J.; Jiang, J.; Liu, P. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Trapani, V.; Cappadone, C.; Farruggia, G.; Merolle, L.; Wolf, F.I.; Iotti, S.; Maier, J.A. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015, 5, 16538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stritt, S.; Nurden, P.; Favier, R.; Favier, M.; Ferioli, S.; Gotru, S.K.; van Eeuwijk, J.M.; Schulze, H.; Nurden, A.T.; Lambert, M.P.; et al. Defects in TRPM7 channel function deregulate thrombopoiesis through altered cellular Mg(2+) homeostasis and cytoskeletal architecture. Nat. Commun. 2016, 7, 11097. [Google Scholar] [CrossRef]
- Tashiro, M.; Inoue, H.; Konishi, M. Physiological pathway of magnesium influx in rat ventricular myocytes. Biophys. J. 2014, 107, 2049–2058. [Google Scholar] [CrossRef]
- Abed, E.; Moreau, R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007, 40, 849–865. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Desai, B.N.; Navarro, B.; Donovan, A.; Andrews, N.C.; Clapham, D.E. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 2008, 322, 756–760. [Google Scholar] [CrossRef]
- Romagnani, A.; Vettore, V.; Rezzonico-Jost, T.; Hampe, S.; Rottoli, E.; Nadolni, W.; Perotti, M.; Meier, M.A.; Hermanns, C.; Geiger, S.; et al. TRPM7 kinase activity is essential for T cell colonization and alloreactivity in the gut. Nat. Commun. 2017, 8, 1917. [Google Scholar] [CrossRef] [Green Version]
- Krapivinsky, G.; Krapivinsky, L.; Manasian, Y.; Clapham, D.E. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 2014, 157, 1061–1072. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Rondon, L.J.; Zierler, S.; Hu, Z.; Galli, J.; Yamaguchi, T.P.; Mazur, A.; Fleig, A.; Ryazanov, A.G. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 2010, 1, 109. [Google Scholar] [CrossRef]
- Antunes, T.T.; Callera, G.E.; He, Y.; Yogi, A.; Ryazanov, A.G.; Ryazanova, L.V.; Zhai, A.; Stewart, D.J.; Shrier, A.; Touyz, R.M. Transient Receptor Potential Melastatin 7 Cation Channel Kinase: New Player in Angiotensin II-Induced Hypertension. Hypertension 2016, 67, 763–773. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Katagiri, C.; Beesetty, P.; Nakamura, K.; Hourani, S.; Tomizawa, K.; Kozak, J.A.; Matsushita, M. Inactivation of TRPM7 kinase activity does not impair its channel function in mice. Sci. Rep. 2014, 4, 5718. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Massullo, P.; Sumoza-Toledo, A.; Bhagat, H.; Partida-Sanchez, S. TRPM channels, calcium and redox sensors during innate immune responses. Semin. Cell Dev. Biol. 2006, 17, 654–666. [Google Scholar] [CrossRef]
- Clark, K.; Langeslag, M.; van Leeuwen, B.; Ran, L.; Ryazanov, A.G.; Figdor, C.G.; Moolenaar, W.H.; Jalink, K.; van Leeuwen, F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006, 25, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Schappe, M.S.; Szteyn, K.; Stremska, M.E.; Mendu, S.K.; Downs, T.K.; Seegren, P.V.; Mahoney, M.A.; Dixit, S.; Krupa, J.K.; Stipes, E.J.; et al. Chanzyme TRPM7 Mediates the Ca(2+) Influx Essential for Lipopolysaccharide-Induced Toll-Like Receptor 4 Endocytosis and Macrophage Activation. Immunity 2018, 48, 59–74. [Google Scholar] [CrossRef]
- Faouzi, M.; Kilch, T.; Horgen, F.D.; Fleig, A.; Penner, R. The TRPM7 channel kinase regulates store-operated calcium entry. J. Physiol. 2017, 595, 3165–3180. [Google Scholar] [CrossRef]
- Beesetty, P.; Wieczerzak, K.B.; Gibson, J.N.; Kaitsuka, T.; Luu, C.T.; Matsushita, M.; Kozak, J.A. Inactivation of TRPM7 kinase in mice results in enlarged spleens, reduced T-cell proliferation and diminished store-operated calcium entry. Sci. Rep. 2018, 8, 3023. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Branigan, D.; Xiong, Z.G. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J. Biol. Chem. 2010, 285, 7430–7439. [Google Scholar] [CrossRef]
- Abiria, S.A.; Krapivinsky, G.; Sah, R.; Santa-Cruz, A.G.; Chaudhuri, D.; Zhang, J.; Adstamongkonkul, P.; DeCaen, P.G.; Clapham, D.E. TRPM7 senses oxidative stress to release Zn(2+) from unique intracellular vesicles. Proc. Natl. Acad. Sci. USA 2017, 114, E6079–E6088. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Q.; Zhang, L.H.; Lee, H.C.; Zhang, L.; Yue, J. A cell permeable NPE caged ADP-ribose for studying TRPM2. PLoS ONE 2012, 7, e51028. [Google Scholar] [CrossRef]
- Wagner, T.F.; Drews, A.; Loch, S.; Mohr, F.; Philipp, S.E.; Lambert, S.; Oberwinkler, J. TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch. 2010, 460, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Tominaga, M. Extracellular zinc ion regulates transient receptor potential melastatin 5 (TRPM5) channel activation through its interaction with a pore loop domain. J. Biol. Chem. 2013, 288, 25950–25955. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Tellez-Gabriel, M.; Heymann, M.F.; Heymann, D. Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. J. Bone Oncol. 2015, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Thebault, S.; Alexander, R.T.; Tiel Groenestege, W.M.; Hoenderop, J.G.; Bindels, R.J. EGF increases TRPM6 activity and surface expression. J. Am. Soc. Nephrol. 2009, 20, 78–85. [Google Scholar] [CrossRef]
- Groenestege, W.M.; Thebault, S.; van der Wijst, J.; van den Berg, D.; Janssen, R.; Tejpar, S.; van den Heuvel, L.P.; van Cutsem, E.; Hoenderop, J.G.; Knoers, N.V.; et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J. Clin. Investig. 2007, 117, 2260–2267. [Google Scholar] [CrossRef] [Green Version]
- Dimke, H.; van der Wijst, J.; Alexander, T.R.; Meijer, I.M.; Mulder, G.M.; van Goor, H.; Tejpar, S.; Hoenderop, J.G.; Bindels, R.J. Effects of the EGFR Inhibitor Erlotinib on Magnesium Handling. J. Am. Soc. Nephrol. 2010, 21, 1309–1316. [Google Scholar] [CrossRef] [Green Version]
- Trapani, V.; Arduini, D.; Luongo, F.; Wolf, F.I. EGF stimulates Mg(2+) influx in mammary epithelial cells. Biochem. Biophys. Res. Commun. 2014, 454, 572–575. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Segaert, S.; Safont, M.J.; Demonty, G.; Prenen, H. Management of adverse events during treatment of gastrointestinal cancers with epidermal growth factor inhibitors. Crit. Rev. Oncol. Hematol. 2017, 114, 102–113. [Google Scholar] [CrossRef]
- Middelbeek, J.; Clark, K.; Venselaar, H.; Huynen, M.A.; van Leeuwen, F.N. The alpha-kinase family: An exceptional branch on the protein kinase tree. Cell Mol. Life Sci. 2010, 67, 875–890. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Matsushita, M.; Nairn, A.C.; Kuriyan, J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol. Cell 2001, 7, 1047–1057. [Google Scholar] [CrossRef]
- Kim, T.Y.; Shin, S.K.; Song, M.Y.; Lee, J.E.; Park, K.S. Identification of the phosphorylation sites on intact TRPM7 channels from mammalian cells. Biochem. Biophys. Res. Commun. 2012, 417, 1030–1034. [Google Scholar] [CrossRef]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS ONE 2008, 3, e1876. [Google Scholar] [CrossRef] [PubMed]
- Ryazanova, L.V.; Dorovkov, M.V.; Ansari, A.; Ryazanov, A.G. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J. Biol. Chem. 2004, 279, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Middelbeek, J.; Lasonder, E.; Dulyaninova, N.G.; Morrice, N.A.; Ryazanov, A.G.; Bresnick, A.R.; Figdor, C.G.; van Leeuwen, F.N. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008, 378, 790–803. [Google Scholar] [CrossRef]
- Perraud, A.L.; Zhao, X.; Ryazanov, A.G.; Schmitz, C. The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k. Cell Signal 2011, 23, 586–593. [Google Scholar] [CrossRef]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C gamma2 (PLCgamma2) using TRPM7-kinase. Cell Signal 2012, 24, 2070–2075. [Google Scholar] [CrossRef]
- Desai, B.N.; Krapivinsky, G.; Navarro, B.; Krapivinsky, L.; Carter, B.C.; Febvay, S.; Delling, M.; Penumaka, A.; Ramsey, I.S.; Manasian, Y.; et al. Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev. Cell 2012, 22, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Krapivinsky, G.; Krapivinsky, L.; Renthal, N.E.; Santa-Cruz, A.; Manasian, Y.; Clapham, D.E. Histone phosphorylation by TRPM6’s cleaved kinase attenuates adjacent arginine methylation to regulate gene expression. Proc. Natl. Acad. Sci. USA 2017, 114, E7092–E7100. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, M.; Kozak, J.A.; Shimizu, Y.; McLachlin, D.T.; Yamaguchi, H.; Wei, F.Y.; Tomizawa, K.; Matsui, H.; Chait, B.T.; Cahalan, M.D.; et al. Channel function is dissociated from the intrinsic kinase activity and autophosphorylation of TRPM7/ChaK1. J. Biol. Chem. 2005, 280, 20793–20803. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Perraud, A.L.; Johnson, C.O.; Inabe, K.; Smith, M.K.; Penner, R.; Kurosaki, T.; Fleig, A.; Scharenberg, A.M. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 2003, 114, 191–200. [Google Scholar] [CrossRef]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J. Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Lis, A.; Penner, R.; Fleig, A. TRPM7 is regulated by halides through its kinase domain. Cell Mol. Life Sci. 2013, 70, 2757–2771. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, R.; Schmitz, C.; Demeuse, P.; Scharenberg, A.M.; Penner, R.; Fleig, A. Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. USA 2004, 101, 6009–6014. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. Elife 2016, 5, e20914. [Google Scholar] [CrossRef]
- Li, M.; Du, J.; Jiang, J.; Ratzan, W.; Su, L.T.; Runnels, L.W.; Yue, L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007, 282, 25817–25830. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Mochida, S.; Krapivinsky, L.; Cibulsky, S.M.; Clapham, D.E. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 2006, 52, 485–496. [Google Scholar] [CrossRef]
- Hermosura, M.C.; Nayakanti, H.; Dorovkov, M.V.; Calderon, F.R.; Ryazanov, A.G.; Haymer, D.S.; Garruto, R.M. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 11510–11515. [Google Scholar] [CrossRef]
- Crawley, S.W.; Cote, G.P. Identification of dimer interactions required for the catalytic activity of the TRPM7 alpha-kinase domain. Biochem. J. 2009, 420, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Su, L.T.; Agapito, M.A.; Li, M.; Simonson, W.T.; Huttenlocher, A.; Habas, R.; Yue, L.; Runnels, L.W. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J. Biol. Chem. 2006, 281, 11260–11270. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Hu, Z.; Suzuki, S.; Chubanov, V.; Fleig, A.; Ryazanov, A.G. Elucidating the role of the TRPM7 alpha-kinase: TRPM7 kinase inactivation leads to magnesium deprivation resistance phenotype in mice. Sci. Rep. 2014, 4, 7599. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Waldegger, S.; Mederos y Schnitzler, M.; Vitzthum, H.; Sassen, M.C.; Seyberth, H.W.; Konrad, M.; Gudermann, T. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 2004, 101, 2894–2899. [Google Scholar] [CrossRef] [Green Version]
- Cai, N.; Bai, Z.; Nanda, V.; Runnels, L.W. Mass Spectrometric Analysis of TRPM6 and TRPM7 Phosphorylation Reveals Regulatory Mechanisms of the Channel-Kinases. Sci. Rep. 2017, 7, 42739. [Google Scholar] [CrossRef] [Green Version]
- Deason-Towne, F.; Perraud, A.L.; Schmitz, C. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011, 585, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Moscheni, C.; Trapani, V.; Wolf, F.I.; Farruggia, G.; Sargenti, A.; Iotti, S.; Maier, J.A.; Castiglioni, S. The different expression of TRPM7 and MagT1 impacts on the proliferation of colon carcinoma cells sensitive or resistant to doxorubicin. Sci. Rep. 2017, 7, 40538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goytain, A.; Quamme, G.A. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genom. 2005, 6, 48. [Google Scholar] [CrossRef]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Long, S.; Romani, A.M. Role of Cellular Magnesium in Human Diseases. Austin J. Nutr. Food Sci. 2014, 2, 1051. [Google Scholar]
- Ikari, A.; Sawada, H.; Sanada, A.; Tonegawa, C.; Yamazaki, Y.; Sugatani, J. Magnesium deficiency suppresses cell cycle progression mediated by increase in transcriptional activity of p21(Cip1) and p27(Kip1) in renal epithelial NRK-52E cells. J. Cell Biochem. 2011, 112, 3563–3572. [Google Scholar] [CrossRef]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Capiod, T. Cell proliferation, calcium influx and calcium channels. Biochimie 2011, 93, 2075–2079. [Google Scholar] [CrossRef]
- Resende, R.R.; Andrade, L.M.; Oliveira, A.G.; Guimaraes, E.S.; Guatimosim, S.; Leite, M.F. Nucleoplasmic calcium signaling and cell proliferation: Calcium signaling in the nucleus. Cell Commun. Signal 2013, 11, 14. [Google Scholar] [CrossRef]
- Ni, H.M.; Baty, C.J.; Li, N.; Ding, W.X.; Gao, W.; Li, M.; Chen, X.; Ma, J.; Michalopoulos, G.K.; Yin, X.M. Bid agonist regulates murine hepatocyte proliferation by controlling endoplasmic reticulum calcium homeostasis. Hepatology 2010, 52, 338–348. [Google Scholar] [CrossRef]
- Munaron, L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int J. Mol. Med. 2002, 10, 671–676. [Google Scholar] [CrossRef]
- Paxton, R.; Ye, L. Regulation of heart insulin receptor tyrosine kinase activity by magnesium and spermine. Mol. Cell Biochem. 2005, 277, 7–17. [Google Scholar] [CrossRef]
- Grubbs, R.D. Effect of epidermal growth factor on magnesium homeostasis in BC3H1 myocytes. Am. J. Physiol. 1991, 260, C1158–C1164. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Valinsky, W.C.; Jolly, A.; Miquel, P.; Touyz, R.M.; Shrier, A. Aldosterone Upregulates Transient Receptor Potential Melastatin 7 (TRPM7). J. Biol. Chem. 2016, 291, 20163–20172. [Google Scholar] [CrossRef]
- Yogi, A.; Callera, G.E.; O’Connor, S.E.; He, Y.; Correa, J.W.; Tostes, R.C.; Mazur, A.; Touyz, R.M. Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J. Hypertens. 2011, 29, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Callera, G.E.; He, Y.; Yogi, A.; Montezano, A.C.; Paravicini, T.; Yao, G.; Touyz, R.M. Regulation of the novel Mg2+ transporter transient receptor potential melastatin 7 (TRPM7) cation channel by bradykinin in vascular smooth muscle cells. J. Hypertens. 2009, 27, 155–166. [Google Scholar] [CrossRef]
- Touyz, R.M.; He, Y.; Montezano, A.C.; Yao, G.; Chubanov, V.; Gudermann, T.; Callera, G.E. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R73–R78. [Google Scholar] [CrossRef] [Green Version]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ. J. Off. J. Jpn. Circ. Soc. 2011, 75, 237–245. [Google Scholar] [CrossRef]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Yoon, W.; Shim, J.H.; Jung, H.; Lim, J.H.; Choi, H.J.; Seo, M.; Lee, T.H.; Min, S.H. Role of Protein Kinases and Their Inhibitors in Radiation Response of Tumor Cells. Curr. Pharm. Des. 2017, 23, 4259–4280. [Google Scholar] [CrossRef]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioor.g Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef]
- Hubbard, S.R.; Miller, W.T. Receptor tyrosine kinases: Mechanisms of activation and signaling. Curr. Opin. Cell Biol. 2007, 19, 117–123. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Lee, J.; Miyazaki, M.; Romeo, G.R.; Shoelson, S.E. Insulin receptor activation with transmembrane domain ligands. J. Biol. Chem. 2014, 289, 19769–19777. [Google Scholar] [CrossRef]
- Niu, X.L.; Peters, K.G.; Kontos, C.D. Deletion of the carboxyl terminus of Tie2 enhances kinase activity, signaling, and function. Evidence for an autoinhibitory mechanism. J. Biol. Chem. 2002, 277, 31768–31773. [Google Scholar] [CrossRef]
- Hubbard, S.R. Autoinhibitory mechanisms in receptor tyrosine kinases. Front. Biosci. 2002, 7, d330–d340. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Knighton, D.R.; Ten Eyck, L.F.; Karlsson, R.; Xuong, N.; Taylor, S.S.; Sowadski, J.M. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with magnesium-ATP and peptide inhibitor. Biochemistry 1993, 32, 2154–2161. [Google Scholar] [CrossRef]
- Bastidas, A.C.; Deal, M.S.; Steichen, J.M.; Guo, Y.; Wu, J.; Taylor, S.S. Phosphoryl transfer by protein kinase A is captured in a crystal lattice. J. Am. Chem. Soc. 2013, 135, 4788–4798. [Google Scholar] [CrossRef] [Green Version]
- Hadari, Y.R.; Gotoh, N.; Kouhara, H.; Lax, I.; Schlessinger, J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 2001, 98, 8578–8583. [Google Scholar] [CrossRef]
- Fraser, J.; Cabodevilla, A.G.; Simpson, J.; Gammoh, N. Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking. Essays Biochem. 2017, 61, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Arkun, Y.; Yasemi, M. Dynamics and control of the ERK signaling pathway: Sensitivity, bistability, and oscillations. PLoS ONE 2018, 13, e0195513. [Google Scholar] [CrossRef]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Annenkov, A. Receptor tyrosine kinase (RTK) signalling in the control of neural stem and progenitor cell (NSPC) development. Mol. Neurobiol. 2014, 49, 440–471. [Google Scholar] [CrossRef]
- Papadimitrakopoulou, V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, 1315–1326. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Pause, A. mTOR Pathways in Cancer and Autophagy. Cancers (Basel) 2018, 10, 18. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Ni, J.; Hao, J.; Bucci, J.; Cozzi, P.J.; Li, Y. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 507–517. [Google Scholar] [CrossRef]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef]
- Yang, Y.R.; Follo, M.Y.; Cocco, L.; Suh, P.G. The physiological roles of primary phospholipase C. Adv. Biol. Regul. 2013, 53, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Putney, J.W.; Tomita, T. Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 2012, 52, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Mikoshiba, K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J. Neurochem. 2007, 102, 1426–1446. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Inanobe, S.; Kanemaru, K.; Nakamura, Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res. 2010, 49, 429–437. [Google Scholar] [CrossRef]
- Zhu, M.; Leung, C.Y.; Shahbazi, M.N.; Zernicka-Goetz, M. Actomyosin polarisation through PLC-PKC triggers symmetry breaking of the mouse embryo. Nat. Commun. 2017, 8, 921. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zheng, D.; Cui, H.; Liu, S.; Zhang, L.; Liu, C. Roles and mechanisms of TRPC3 and the PLCgamma/PKC/CPI-17 signaling pathway in regulating parturition. Mol. Med. Rep. 2018, 17, 898–910. [Google Scholar]
- Yu, M.; Chen, Y.; Zeng, H.; Zheng, Y.; Fu, G.; Zhu, W.; Broeckel, U.; Aggarwal, P.; Turner, A.; Neale, G.; et al. PLCgamma-dependent mTOR signalling controls IL-7-mediated early B cell development. Nat. Commun. 2017, 8, 1457. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011205. [Google Scholar] [CrossRef]
- Izzedine, H.; Perazella, M.A. Adverse kidney effects of epidermal growth factor receptor inhibitors. Nephrol. Dial. Transpl. 2017, 32, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.S.; Khankin, E.V.; Karumanchi, S.A.; Humphreys, B.D. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: Mechanisms and potential use as a biomarker. Semin. Nephrol. 2010, 30, 591–601. [Google Scholar] [CrossRef]
- Mouhayar, E.; Salahudeen, A. Hypertension in cancer patients. Tex. Heart Inst. J. 2011, 38, 263–265. [Google Scholar]
- Fakih, M.; Vincent, M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr. Oncol. 2010, 17 (Suppl. 1), S18–S30. [Google Scholar] [CrossRef]
- Cosmai, L.; Gallieni, M.; Porta, C. Renal toxicity of anticancer agents targeting HER2 and EGFR. J. Nephrol. 2015, 28, 647–657. [Google Scholar] [CrossRef]
- Hong, B.Z.; Kang, H.S.; So, J.N.; Kim, H.N.; Park, S.A.; Kim, S.J.; Kim, K.R.; Kwak, Y.G. Vascular endothelial growth factor increases the intracellular magnesium. Biochem. Biophys. Res. Commun. 2006, 347, 496–501. [Google Scholar] [CrossRef]
- Jalkanen, R.; Pronicka, E.; Tyynismaa, H.; Hanauer, A.; Walder, R.; Alitalo, T. Genetic background of HSH in three Polish families and a patient with an X;9 translocation. Eur. J. Hum. Genet. 2006, 14, 55–62. [Google Scholar] [CrossRef]
- Runnels, L.W.; Yue, L.; Clapham, D.E. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat. Cell Biol. 2002, 4, 329–336. [Google Scholar] [CrossRef]
- Gao, H.; Chen, X.; Du, X.; Guan, B.; Liu, Y.; Zhang, H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 2011, 50, 559–568. [Google Scholar] [CrossRef]
- Tian, S.L.; Jiang, H.; Zeng, Y.; Li, L.L.; Shi, J. NGF-induced reduction of an outward-rectifying TRPM7-like current in rat CA1 hippocampal neurons. Neurosci. Lett. 2007, 419, 93–98. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, S.L.; Zeng, Y.; Li, L.L.; Shi, J. TrkA pathway(s) is involved in regulation of TRPM7 expression in hippocampal neurons subjected to ischemic-reperfusion and oxygen-glucose deprivation. Brain Res. Bull. 2008, 76, 124–130. [Google Scholar] [CrossRef]
- Fang, L.; Zhan, S.; Huang, C.; Cheng, X.; Lv, X.; Si, H.; Li, J. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol. Appl. Pharmacol. 2013, 272, 713–725. [Google Scholar] [CrossRef]
- Langeslag, M.; Clark, K.; Moolenaar, W.H.; van Leeuwen, F.N.; Jalink, K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J. Biol. Chem. 2007, 282, 232–239. [Google Scholar] [CrossRef]
- Sun, Y.; Sukumaran, P.; Varma, A.; Derry, S.; Sahmoun, A.E.; Singh, B.B. Cholesterol-induced activation of TRPM7 regulates cell proliferation, migration, and viability of human prostate cells. Biochim. Biophys. Acta 2014, 1843, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, M.; Fan, X.H.; Chen, J.H.; Guan, Y.Y.; Tang, Y.B. Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ. Res. 2012, 111, 1137–1146. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Feng, W.; Chen, R.; Chen, J.; Touyz, R.M.; Wang, J.; Huang, H. Interleukin-18 Enhances Vascular Calcification and Osteogenic Differentiation of Vascular Smooth Muscle Cells Through TRPM7 Activation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1933–1943. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, F.; Wang, J.; Zhao, Y.; Luo, Z.; Gao, Y.; Shi, J. Regulation of TRPM7 Function by IL-6 through the JAK2-STAT3 Signaling Pathway. PLoS ONE 2016, 11, e0152120. [Google Scholar] [CrossRef]
- Inoue, K.; Xiong, Z.G. Silencing TRPM7 promotes growth/proliferation and nitric oxide production of vascular endothelial cells via the ERK pathway. Cardiovasc. Res. 2009, 83, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Su, L.T.; Chen, H.C.; Gonzalez-Pagan, O.; Overton, J.D.; Xie, J.; Yue, L.; Runnels, L.W. TRPM7 activates m-calpain by stress-dependent stimulation of p38 MAPK and c-Jun N-terminal kinase. J. Mol. Biol. 2010, 396, 858–869. [Google Scholar] [CrossRef]
- Zeng, Z.; Leng, T.; Feng, X.; Sun, H.; Inoue, K.; Zhu, L.; Xiong, Z.G. Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways. PLoS ONE 2015, 10, e0119912. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, S.; Zhao, T.; Ju, T.; Zhang, L. TRPM7 channel regulates ox-LDL-induced proliferation and migration of vascular smooth muscle cells via MEK-ERK pathways. FEBS Lett. 2016, 590, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Cai, C.; Wu, J.; Cai, S.; Ye, C.; Chen, H.; Yang, Z.; Zeng, H.; Shen, Q.; Zou, F. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013, 333, 96–102. [Google Scholar] [CrossRef]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W., Jr.; Goodhill, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef]
- Wong, R.; Turlova, E.; Feng, Z.P.; Rutka, J.T.; Sun, H.S. Activation of TRPM7 by naltriben enhances migration and invasion of glioblastoma cells. Oncotarget 2017, 8, 11239–11248. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321–16340. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wu, J.Y.; Lu, M.H.; Shi, Z.; Na, N.; Di, J.M. Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways. Oxid. Med. Cell. Longev. 2016, 2016, 1469693. [Google Scholar] [CrossRef]
- Lu, D.; Qu, J.; Sun, L.; Li, Q.; Ling, H.; Yang, N.; Ma, T.; Wang, Q.; Li, M.; Zhang, K.; et al. Ca2+/Mg2+ homeostasisrelated TRPM7 channel mediates chondrocyte hypertrophy via regulation of the PI3KAkt signaling pathway. Mol. Med. Rep. 2017, 16, 5699–5705. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Guo, S.; Xiong, Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell Signal 2014, 26, 2773–2781. [Google Scholar] [CrossRef] [Green Version]
- Saltarella, I.; Frassanito, M.A.; Lamanuzzi, A.; Brevi, A.; Leone, P.; Desantis, V.; Di Marzo, L.; Bellone, M.; Derudas, D.; Ribatti, D.; et al. Homotypic and Heterotypic Activation of the Notch Pathway in Multiple Myeloma-Enhanced Angiogenesis: A Novel Therapeutic Target? Neoplasia 2019, 21, 93–105. [Google Scholar] [CrossRef]
- Wang, W.M.; Zhao, Z.L.; Ma, S.R.; Yu, G.T.; Liu, B.; Zhang, L.; Zhang, W.F.; Kulkarni, A.B.; Sun, Z.J.; Zhao, Y.F. Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1alpha and Notch1 in head neck squamous cell carcinoma. PLoS ONE 2015, 10, e0119723. [Google Scholar]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Jin, J.; Wu, L.J.; Jun, J.; Cheng, X.; Xu, H.; Andrews, N.C.; Clapham, D.E. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA 2012, 109, E225–E233. [Google Scholar] [CrossRef]
- Liu, Y.S.; Liu, Y.A.; Huang, C.J.; Yen, M.H.; Tseng, C.T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015, 5, 16522. [Google Scholar] [CrossRef] [Green Version]
- Ogunrinde, A.; Pereira, R.D.; Beaton, N.; Lam, D.H.; Whetstone, C.; Hill, C.E. Hepatocellular differentiation status is characterized by distinct subnuclear localization and form of the chanzyme TRPM7. Differentiation 2017, 96, 15–25. [Google Scholar] [CrossRef]
- Yu, M.; Huang, C.; Huang, Y.; Wu, X.; Li, X.; Li, J. Inhibition of TRPM7 channels prevents proliferation and differentiation of human lung fibroblasts. Inflamm. Res. 2013, 62, 961–970. [Google Scholar] [CrossRef]
- Cui, L.; Xu, S.M.; Ma, D.D.; Wu, B.L. The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int. Endod. J. 2014, 47, 583–593. [Google Scholar] [CrossRef]
- Zhang, X.; Zu, H.; Zhao, D.; Yang, K.; Tian, S.; Yu, X.; Lu, F.; Liu, B.; Yu, X.; Wang, B.; et al. Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: In vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater. 2017, 63, 369–382. [Google Scholar] [CrossRef]
- Takahashi, K.; Umebayashi, C.; Numata, T.; Honda, A.; Ichikawa, J.; Hu, Y.; Yamaura, K.; Inoue, R. TRPM7-mediated spontaneous Ca(2+) entry regulates the proliferation and differentiation of human leukemia cell line K562. Physiol. Rep. 2018, 6, e13796. [Google Scholar] [CrossRef]
- Yang, M.; Fang, J.; Liu, Q.; Wang, Y.; Zhang, Z. Role of ROS-TRPM7-ERK1/2 axis in high concentration glucose-mediated proliferation and phenotype switching of rat aortic vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2017, 494, 526–533. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Liang, I.C. Transient receptor potential ion channel Trpm7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Model. Mech. 2011, 4, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Liu, K.; Xu, S.H.; Chen, Z.; Zeng, Q.X.; Li, Z.J.; Chen, Z.M. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90alpha/uPA/MMP2 signaling pathway. BMC Cancer 2018, 18, 1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, N.; Wang, Y.; Zhang, X.; Xia, B.; Tang, J.; Cai, J.; Zhao, Z.; Liao, Q.; Wang, J. TRPM7 promotes the epithelial-mesenchymal transition in ovarian cancer through the calcium-related PI3K / AKT oncogenic signaling. J. Exp. Clin. Cancer Res. 2019, 38, 106. [Google Scholar] [CrossRef]
- Xiao, E.; Yang, H.Q.; Gan, Y.H.; Duan, D.H.; He, L.H.; Guo, Y.; Wang, S.Q.; Zhang, Y. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 2015, 33, 615–621. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, J.M.; Figueiredo, M.L.; Zhang, H.; Nelson, P.L.; Marigo, V.; Beck, A. Transient receptor potential melastatin type 7 channel is critical for the survival of bone marrow derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 1393–1403. [Google Scholar] [CrossRef]
- Diaz-Tocados, J.M.; Herencia, C.; Martinez-Moreno, J.M.; Montes de Oca, A.; Rodriguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almaden, Y.; Rodriguez, M.; et al. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, N.S. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals (Basel) 2017, 10, 39. [Google Scholar] [CrossRef]
- Yang, H.; Kim, T.H.; Lee, G.S.; Hong, E.J.; Jeung, E.B. Comparing the expression patterns of placental magnesium/phosphorus-transporting channels between healthy and preeclamptic pregnancies. Mol. Reprod. Dev. 2014, 81, 851–860. [Google Scholar] [CrossRef]
- Weintraub, A.Y.; Amash, A.; Eshkoli, T.; Piltcher Haber, E.; Bronfenmacher, B.; Sheiner, E.; Holcberg, G.; Huleihel, M. The effects of magnesium sulfate on placental vascular endothelial growth factor expression in preeclampsia. Hypertens. Pregnancy 2013, 32, 178–188. [Google Scholar] [CrossRef]
- Costa, A.; Tejpar, S.; Prenen, H.; Van Cutsem, E. Hypomagnesaemia and targeted anti-epidermal growth factor receptor (EGFR) agents. Target. Oncol. 2011, 6, 227–233. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Z.-G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. https://doi.org/10.3390/ijms20081877
Zou Z-G, Rios FJ, Montezano AC, Touyz RM. TRPM7, Magnesium, and Signaling. International Journal of Molecular Sciences. 2019; 20(8):1877. https://doi.org/10.3390/ijms20081877
Chicago/Turabian StyleZou, Zhi-Guo, Francisco J. Rios, Augusto C. Montezano, and Rhian M. Touyz. 2019. "TRPM7, Magnesium, and Signaling" International Journal of Molecular Sciences 20, no. 8: 1877. https://doi.org/10.3390/ijms20081877




