Comparison of Proton and Photon Beam Irradiation in Radiation-Induced Intestinal Injury Using a Mouse Model
Abstract
:1. Introduction
2. Results
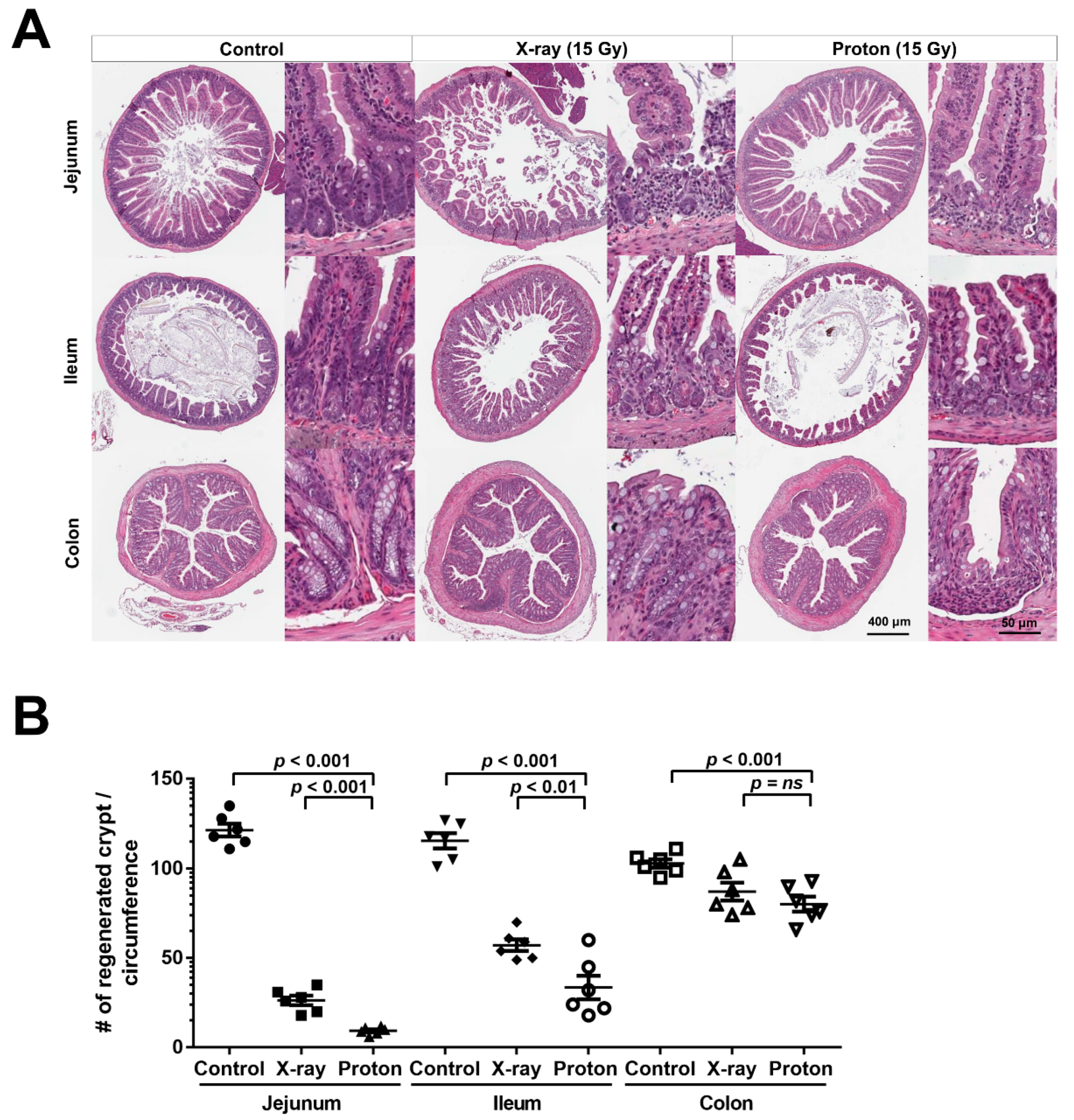
2.1. High Doses of Therapeutic Radiation Reduce the Number of Crypts in the Mouse Intestine
2.2. Proton Irradiation Leads to a Higher Decrease in the Numbers of Surviving Crypts in the Jejunum versus X-Ray Irradiation
2.3. Proton Irradiation Leads to a Higher Decrease in Quiescent Stem Cells and Progenitor Cells in the Jejunum versus X-Ray Irradiation
2.4. Proton Irradiation Leads to a Higher Rate of Apoptotic Cell Death in the Jejunum Crypts versus X-Ray Irradiation
3. Discussion
4. Materials and Methods
4.1. Mice and Irradiation Procedures
4.2. Crypt Microcolony Assay
4.3. Edu Assay
4.4. Immunohistochemistry
4.5. TUNEL Assay
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EdU | 5-ethynyl-2′-deoxyuridine |
| GI | gastrointestinal |
| H&E | haematoxylin and eosin |
| ISC | intestinal stem cell |
| IHC | immunohistochemistry |
| OLFM4 | Olfactomedin 4 |
| PBT | proton beam therapy |
| RBE | relative biological effectiveness |
| RT | radiation therapy |
| SOBP | spread-out-Bragg-peak |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling |
References
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-particle therapy in cancer: Clinical uses and future perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Tian, X.; Liu, K.; Hou, Y.; Cheng, J.; Zhang, J. The evolution of proton beam therapy: Current and future status. Mol. Clin. Oncol. 2018, 8, 15–21. [Google Scholar] [CrossRef]
- Gueulette, J.; Octave-Prignot, M.; De Costera, B.M.; Wambersie, A.; Gregoire, V. Intestinal crypt regeneration in mice: A biological system for quality assurance in non-conventional radiation therapy. Radiother. Oncol. 2004, 7, S148–S154. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willers, H.; Allen, A.; Grosshans, D.; McMahon, S.J.; von Neubeck, C.; Wiese, C.; Vikram, B. Toward A variable RBE for proton beam therapy. Radiother. Oncol. 2018, 128, 68–75. [Google Scholar] [CrossRef]
- Paganetti, H.; Giantsoudi, D. Relative Biological Effectiveness Uncertainties and Implications for Beam Arrangements and Dose Constraints in Proton Therapy. Semin. Radiat. Oncol. 2018, 28, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Luhr, A.; von Neubeck, C.; Pawelke, J.; Seidlitz, A.; Peitzsch, C.; Bentzen, S.M.; Bortfeld, T.; Debus, J.; Deutsch, E.; Langendijk, J.A.; et al. Radiobiology of Proton Therapy: Results of an international expert workshop. Radiother. Oncol. 2018, 128, 56–67. [Google Scholar] [CrossRef]
- Kim, C.K.; Yang, V.W.; Bialkowska, A.B. The Role of Intestinal Stem Cells in Epithelial Regeneration Following Radiation-Induced Gut Injury. Curr. Stem Cell Rep. 2017, 3, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Beumer, J.; Clevers, H. Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 2016, 143, 3639–3649. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, M.; Li, L.; Lengner, C.J. Hierarchy and Plasticity in the Intestinal Stem Cell Compartment. Trends Cell Biol. 2017, 27, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Choo, D.W.; Shin, D.; Baek, H.J.; Kim, T.H.; Motoyama, N.; De Coster, B.M.; Gueulette, J.; Furusawa, Y.; Ando, K.; et al. In vivo radiobiological characterization of proton beam at the National Cancer Center in Korea: Effect of the Chk2 mutation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.A.; Gillin, M.T.; Mohan, R.; Cox, J.D. Preclinical biologic assessment of proton beam relative biologic effectiveness at Proton Therapy Center Houston. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 968–970. [Google Scholar] [CrossRef]
- Gueulette, J.; Gregoire, V.; Octave-Prignot, M.; Wambersie, A. Measurements of radiobiological effectiveness in the 85 MeV proton beam produced at the cyclotron CYCLONE of Louvain-la-Neuve, Belgium. Radiat. Res. 1996, 145, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Gueulette, J.; Blattmann, H.; Pedroni, E.; Coray, A.; De Coster, B.M.; Mahy, P.; Wambersie, A.; Goitein, G. Relative biologic effectiveness determination in mouse intestine for scanning proton beam at Paul Scherrer Institute, Switzerland. Influence of motion. J. Radiat. Oncol. Biol. Phys. 2005, 62, 838–845. [Google Scholar] [CrossRef]
- Hua, G.; Wang, C.; Pan, Y.; Zeng, Z.; Lee, S.G.; Martin, M.L.; Haimovitz-Friedman, A.; Fuks, Z.; Paty, P.B.; Kolesnick, R. Distinct Levels of Radioresistance in Lgr5(+) Colonic Epithelial Stem Cells versus Lgr5(+) Small Intestinal Stem Cells. Cancer Res. 2017, 77, 2124–2133. [Google Scholar] [CrossRef]
- Qiu, W.; Carson-Walter, E.B.; Liu, H.; Epperly, M.; Greenberger, J.S.; Zambetti, G.P.; Zhang, L.; Yu, J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008, 2, 576–583. [Google Scholar] [CrossRef]
- Ilicic, K.; Combs, S.E.; Schmid, T.E. New insights in the relative radiobiological effectiveness of proton irradiation. Radiat. Oncol. 2018, 13, 6. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.; McMahon, S.J.; Prise, K.M. The Radiobiology of Proton Therapy: Challenges and Opportunities Around Relative Biological Effectiveness. Clin. Oncol. (R Coll Radiol) 2018, 30, 285–292. [Google Scholar] [CrossRef]
- Mohan, R.; Peeler, C.R.; Guan, F.; Bronk, L.; Cao, W.; Grosshans, D.R. Radiobiological issues in proton therapy. Acta Oncol. 2017, 56, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Underwood, T.S.; McMahon, S.J. Proton relative biological effectiveness (RBE): A multiscale problem. Br. J. Radiol. 2018, 92, 20180004. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Matsuura, T.; Wada, M.; Egashira, Y.; Nishio, T.; Furusawa, Y. Enhanced radiobiological effects at the distal end of a clinical proton beam: In vitro study. J. Radiat. Res. 2014, 55, 816–822. [Google Scholar] [CrossRef]
- Paganetti, H. Proton Relative Biological Effectiveness–Uncertainties and Opportunities. Int. J. Part. Ther. 2018, 5, 2–14. [Google Scholar] [CrossRef]
- McNamara, A.L.; Schuemann, J.; Paganetti, H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys. Med. Boil. 2015, 60, 8399–8416. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.I.; Choi, C.; Shin, S.W.; Son, A.; Lee, G.H.; Kim, S.Y.; Park, H.C. Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation. Sci. Rep. 2017, 7, 14986. [Google Scholar] [CrossRef]
- Zlobinskaya, O.; Siebenwirth, C.; Greubel, C.; Hable, V.; Hertenberger, R.; Humble, N.; Reinhardt, S.; Michalski, D.; Roper, B.; Multhoff, G.; et al. The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiat. Res. 2014, 181, 177–183. [Google Scholar] [CrossRef]
- Urano, M.; Verhey, L.J.; Goitein, M.; Tepper, J.E.; Suit, H.D.; Mendiondo, O.; Gragoudas, E.S.; Koehler, A. Relative biological effectiveness of modulated proton beams in various murine tissues. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 509–514. [Google Scholar] [CrossRef]
- Nemoto, K.; Pickles, T.; Minchinton, A.I.; Lam, G.K. The relative biological effectiveness of the modulated proton beam at TRIUMF. Radiat. Med. 1998, 16, 43–46. [Google Scholar]
- Tatsuzaki, H.; Inada, T.; Shimizu, T.; Arimoto, T.; Satoh, S.; Akisada, M. Early skin reaction following 250 MeV proton peak irradiation. J. Radiat. Res. 1987, 28, 150–155. [Google Scholar] [CrossRef]
- Tepper, J.; Verhey, L.; Goitein, M.; Suit, H.D. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 1115–1122. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Bassler, N.; Nielsen, S.; Horsman, M.R.; Grzanka, L.; Spejlborg, H.; Swakon, J.; Olko, P.; Overgaard, J. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol. 2017, 56, 1387–1391. [Google Scholar] [CrossRef]
- Gueulette, J.; Slabbert, J.P.; Bohm, L.; De Coster, B.M.; Rosier, J.F.; Octave-Prignot, M.; Ruifrok, A.; Schreuder, A.N.; Wambersie, A.; Scalliet, P.; et al. Proton RBE for early intestinal tolerance in mice after fractionated irradiation. Radiother. Oncol. 2001, 61, 177–184. [Google Scholar] [CrossRef]
- Wei, L.; Leibowitz, B.J.; Wang, X.; Epperly, M.; Greenberger, J.; Zhang, L.; Yu, J. Inhibition of CDK4/6 protects against radiation-induced intestinal injury in mice. J. Clin. Investig. 2016, 126, 4076–4087. [Google Scholar] [CrossRef] [Green Version]
- Medina, V.A.; Croci, M.; Mohamad, N.A.; Massari, N.; Garbarino, G.; Cricco, G.P.; Nunez, M.A.; Martin, G.A.; Crescenti, E.J.; Bergoc, R.M.; et al. Mechanisms underlying the radioprotective effect of histamine on small intestine. Int. J. Radiat. Biol. 2007, 83, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, B.J.; Qiu, W.; Liu, H.; Cheng, T.; Zhang, L.; Yu, J. Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21. Mol. Cancer Res. 2011, 9, 616–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Tian, H.; Jiang, J.; Yang, Y.; Tan, S.; Lin, X.; Liu, H.; Wu, B. beta-Arrestin-2 modulates radiation-induced intestinal crypt progenitor/stem cell injury. Cell Death Differ. 2016, 23, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.H.; Otsuka, K. The role of gene mutations and gene products in intestinal tissue reactions from ionising radiation. Mutat. Res. 2016, 770, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Tudor, G.; Tudor, J.; Katz, B.P.; MacVittie, T.J. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012, 103, 383–399. [Google Scholar] [CrossRef]
- Hua, G.; Thin, T.H.; Feldman, R.; Haimovitz-Friedman, A.; Clevers, H.; Fuks, Z.; Kolesnick, R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012, 143, 1266–1276. [Google Scholar] [CrossRef]
- Barriga, F.M.; Montagni, E.; Mana, M.; Mendez-Lago, M.; Hernando-Momblona, X.; Sevillano, M.; Guillaumet-Adkins, A.; Rodriguez-Esteban, G.; Buczacki, S.J.A.; Gut, M.; et al. Mex3a Marks a Slowly Dividing Subpopulation of Lgr5+ Intestinal Stem Cells. Cell Stem Cell 2017, 20, 801–816.e7. [Google Scholar] [CrossRef]
- Kim, T.H.; Saadatpour, A.; Guo, G.; Saxena, M.; Cavazza, A.; Desai, N.; Jadhav, U.; Jiang, L.; Rivera, M.N.; Orkin, S.H.; et al. Single-Cell Transcript Profiles Reveal Multilineage Priming in Early Progenitors Derived from Lgr5(+) Intestinal Stem Cells. Cell Rep. 2016, 16, 2053–2060. [Google Scholar] [CrossRef]
- Asfaha, S.; Hayakawa, Y.; Muley, A.; Stokes, S.; Graham, T.A.; Ericksen, R.E.; Westphalen, C.B.; von Burstin, J.; Mastracci, T.L.; Worthley, D.L.; et al. Krt19(+)/Lgr5(-) Cells Are Radioresistant Cancer-Initiating Stem Cells in the Colon and Intestine. Cell Stem Cell 2015, 16, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.G.; Kim, C.K.; Ghaleb, A.M.; Bialkowska, A.B.; Kuo, C.J.; Yang, V.W. Kruppel-like Factor 4 Modulates Development of BMI1(+) Intestinal Stem Cell-Derived Lineage Following gamma-Radiation-Induced Gut Injury in Mice. Stem Cell Rep. 2016, 6, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, L.; Cramer, J.M.; Leibowitz, B.J.; Judge, C.; Epperly, M.; Greenberger, J.; Wang, F.; Li, L.; Stelzner, M.G.; et al. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci. Rep. 2015, 5, 8566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alan Mitteer, R.; Wang, Y.; Shah, J.; Gordon, S.; Fager, M.; Butter, P.P.; Jun Kim, H.; Guardiola-Salmeron, C.; Carabe-Fernandez, A.; Fan, Y. Proton beam radiation induces DNA damage and cell apoptosis in glioma stem cells through reactive oxygen species. Sci. Rep. 2015, 5, 13961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pietro, C.; Piro, S.; Tabbi, G.; Ragusa, M.; Di Pietro, V.; Zimmitti, V.; Cuda, F.; Anello, M.; Consoli, U.; Salinaro, E.T.; et al. Cellular and molecular effects of protons: Apoptosis induction and potential implications for cancer therapy. Apoptosis 2006, 11, 57–66. [Google Scholar] [CrossRef]
- Lee, K.B.; Lee, J.S.; Park, J.W.; Huh, T.L.; Lee, Y.M. Low energy proton beam induces tumor cell apoptosis through reactive oxygen species and activation of caspases. Exp. Mol. Med. 2008, 40, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonai, S.; Kanematsu, N.; Komori, M.; Kanai, T.; Takei, Y.; Takahashi, O.; Isobe, Y.; Tashiro, M.; Koikegami, H.; Tomita, H. Evaluation of beam wobbling methods for heavy-ion radiotherapy. Med. Phys. 2008, 35, 927–938. [Google Scholar] [CrossRef]
- Yilmaz, O.H.; Katajisto, P.; Lamming, D.W.; Gultekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef]
- Vidrich, A.; Buzan, J.M.; Barnes, S.; Reuter, B.K.; Skaar, K.; Ilo, C.; Cominelli, F.; Pizarro, T.; Cohn, S.M. Altered epithelial cell lineage allocation and global expansion of the crypt epithelial stem cell population are associated with ileitis in SAMP1/YitFc mice. Am. J. Pathol. 2005, 166, 1055–1067. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, C.; Lee, C.; Shin, S.-W.; Kim, S.-Y.; Hong, S.N.; Park, H.C. Comparison of Proton and Photon Beam Irradiation in Radiation-Induced Intestinal Injury Using a Mouse Model. Int. J. Mol. Sci. 2019, 20, 1894. https://doi.org/10.3390/ijms20081894
Choi C, Lee C, Shin S-W, Kim S-Y, Hong SN, Park HC. Comparison of Proton and Photon Beam Irradiation in Radiation-Induced Intestinal Injury Using a Mouse Model. International Journal of Molecular Sciences. 2019; 20(8):1894. https://doi.org/10.3390/ijms20081894
Chicago/Turabian StyleChoi, Changhoon, Chansu Lee, Sung-Won Shin, Shin-Yeong Kim, Sung Noh Hong, and Hee Chul Park. 2019. "Comparison of Proton and Photon Beam Irradiation in Radiation-Induced Intestinal Injury Using a Mouse Model" International Journal of Molecular Sciences 20, no. 8: 1894. https://doi.org/10.3390/ijms20081894




