The Origin and Evolution of Release Factors: Implications for Translation Termination, Ribosome Rescue, and Quality Control Pathways
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Prior State of Understanding of the Evolution of Peptidyl Hydrolase Release Factors
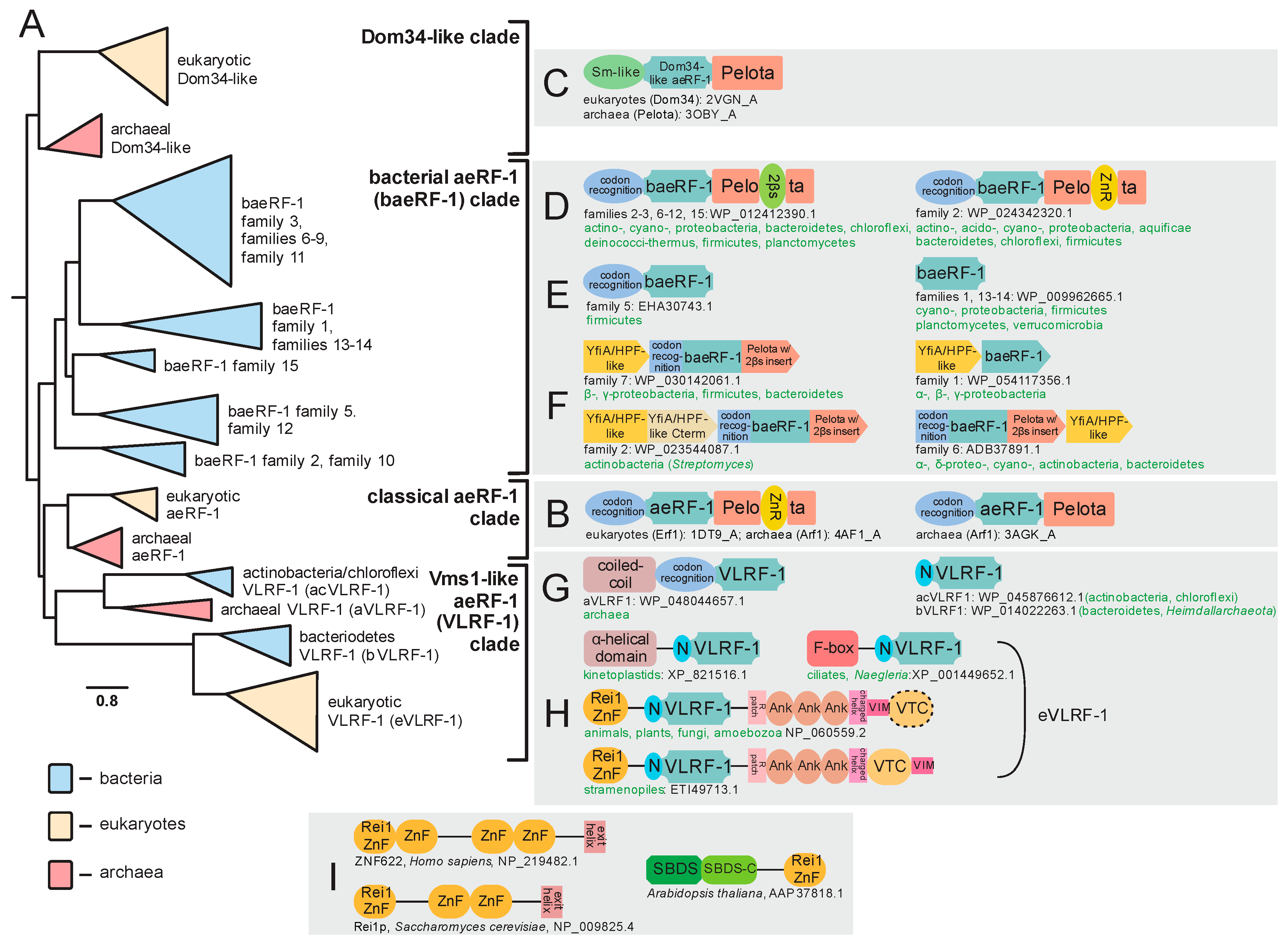
2.2. Novel Members of the aeRF-1 Superfamily: Their Domain Architectures and Conserved Gene Neighborhood Associations
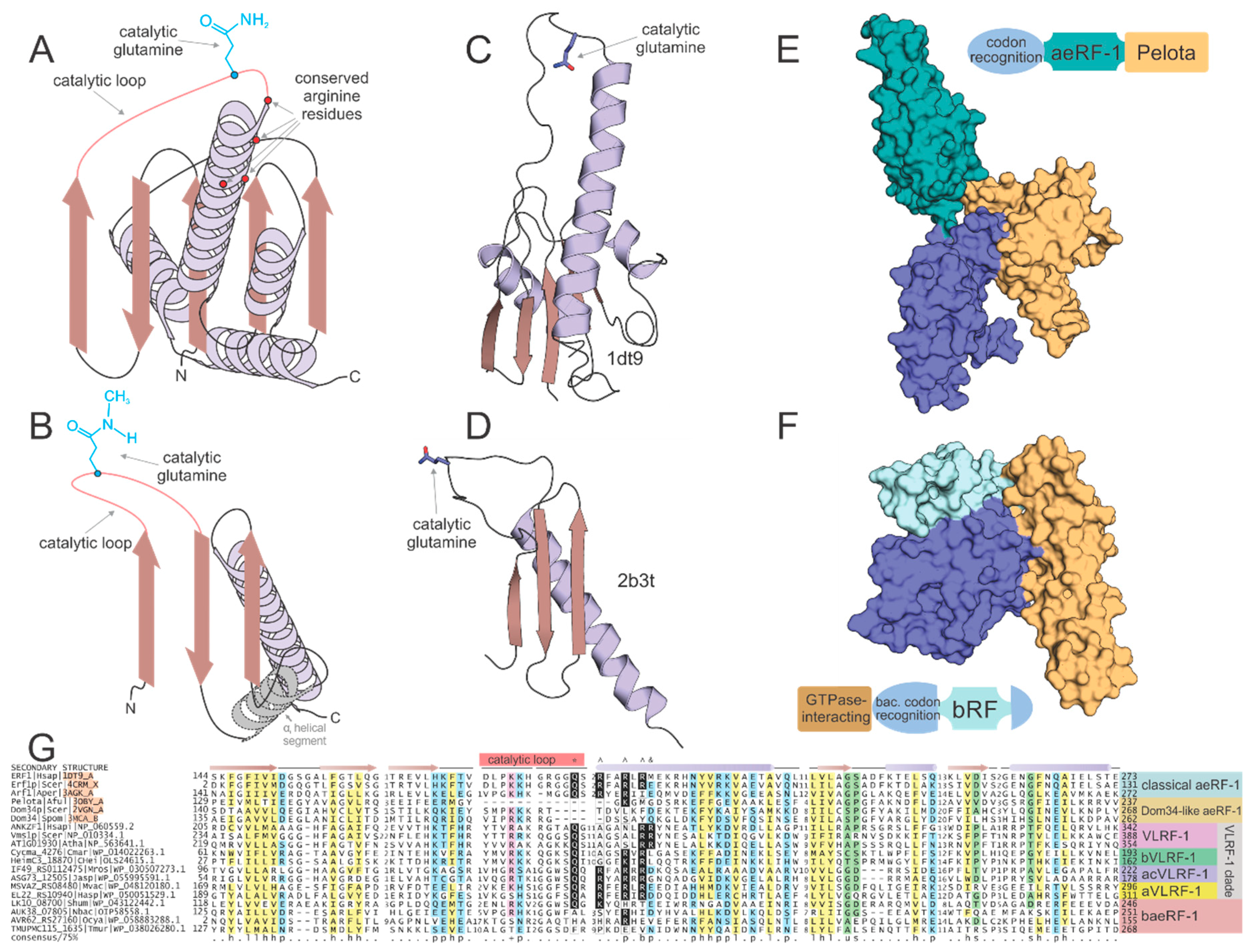
2.3. Members of the aeRF-1 Superfamily Display Sequence and Structure Diversity in Their Core RNase H Fold Domain
2.4. Diversity and Phylogeny of bRF-PHs in the Bacteria
2.5. bRF-PH Domains Transferred to Eukaryotes
3. Functional and Evolutionary Implications
3.1. The State of Translation Termination in the LUCA and the Early History of RF-PH Domains
3.2. Multiple Early Recruitments of Paralogous RF-PHs to Distinct Ribosome Rescue Pathways
3.3. Origin of the Eukaryotic Ribosome Quality Control System
3.4. Alternative Catalytic Mechanisms for Releasing Peptides from Peptidyl-tRNA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RF-PH | release factor-peptidyl hydrolase |
| LUCA | Last Universal Common Ancestor |
| AA | amino acylated |
| bRF-PH | bacterial release factor-peptidyl hydrolase |
| aeRF-1 | archaeo-eukaryotic release factor |
| RQC | ribosome quality control |
| ZnR | zinc ribbon |
| VLRF-1 | Vms1-like archaeo-eukaryotic release factor |
| aVLRF-1 | archaeal Vms1-like archaeo-eukaryotic release factor |
| eVLRF-1 | eukaryotic Vms1-like archaeo-eukaryotic release factor |
| acVLRF-1 | actinobacterial/chloroflexi Vms1-like archaeo-eukaryotic release factor |
| bVLRF-1 | bacteroidetes Vms1-like archaeo-eukaryotic release factor |
| baeRF-1 | bacterial archaeo-eukaryotic release factor |
| VTC | Vms1 treble clef |
| SBDS | Shwachman-Bodian-Diamond syndrome |
| PSU | pseudouridine |
| PSYN | pseudouridine synthase |
| NTase | nucleotidyltransferase |
| Pth | peptidyl-tRNA hydrolase |
| aatRs | aminoacyl-tRNA synthetases |
References
- Leipe, D.D.; Wolf, Y.I.; Koonin, E.V.; Aravind, L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 2002, 317, 41–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berchtold, H.; Reshetnikova, L.; Reiser, C.O.; Schirmer, N.K.; Sprinzl, M.; Hilgenfeld, R. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature 1993, 365, 126–132. [Google Scholar] [CrossRef]
- Kavaliauskas, D.; Nissen, P.; Knudsen, C.R. The busiest of all ribosomal assistants: Elongation factor Tu. Biochemistry 2012, 51, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Qin, Y.; Achenbach, J.; Li, C.; Kijek, J.; Spahn, C.M.; Nierhaus, K.H. EF-G and EF4: Translocation and back-translocation on the bacterial ribosome. Nat. Rev. Microbiol. 2014, 12, 89–100. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Wintermeyer, W. The ribosome as a molecular machine: The mechanism of tRNA-mRNA movement in translocation. Biochem. Soc. Trans. 2011, 39, 658–662. [Google Scholar] [CrossRef]
- Szkaradkiewicz, K.; Zuleeg, T.; Limmer, S.; Sprinzl, M. Interaction of fMet-tRNAfMet and fMet-AMP with the C-terminal domain of Thermus thermophilus translation initiation factor 2. Eur. J. Biochem. 2000, 267, 4290–4299. [Google Scholar] [CrossRef] [Green Version]
- Pestova, T.V.; Lomakin, I.B.; Lee, J.H.; Choi, S.K.; Dever, T.E.; Hellen, C.U. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 2000, 403, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Merrick, W.C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 1992, 56, 291–315. [Google Scholar]
- Inagaki, Y.; Blouin, C.; Susko, E.; Roger, A.J. Assessing functional divergence in EF-1alpha and its paralogs in eukaryotes and archaebacteria. Nucleic Acids Res. 2003, 31, 4227–4237. [Google Scholar] [CrossRef]
- Kobayashi, K.; Saito, K.; Ishitani, R.; Ito, K.; Nureki, O. Structural basis for translation termination by archaeal RF1 and GTP-bound EF1alpha complex. Nucleic Acids Res. 2012, 40, 9319–9328. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ito, K.; Isaksson, L.A. Emerging understanding of translation termination. Cell 1996, 87, 147–150. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ito, K. Making sense of mimic in translation termination. Trends Biochem. Sci. 2003, 28, 99–105. [Google Scholar] [CrossRef]
- Frolova, L.Y.; Tsivkovskii, R.Y.; Sivolobova, G.F.; Oparina, N.Y.; Serpinsky, O.I.; Blinov, V.M.; Tatkov, S.I.; Kisselev, L.L. Mutations in the highly conserved GGQ motif of class 1 polypeptide release factors abolish ability of human eRF1 to trigger peptidyl-tRNA hydrolysis. RNA 1999, 5, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, L.; Heurgue-Hamard, V.; Champ, S.; Ehrenberg, M.; Kisselev, L.L.; Buckingham, R.H. The essential role of the invariant GGQ motif in the function and stability in vivo of bacterial release factors RF1 and RF2. Mol. Microbiol. 2003, 47, 267–275. [Google Scholar] [CrossRef]
- Shaw, J.J.; Green, R. Two distinct components of release factor function uncovered by nucleophile partitioning analysis. Mol. Cell 2007, 28, 458–467. [Google Scholar] [CrossRef]
- Weixlbaumer, A.; Jin, H.; Neubauer, C.; Voorhees, R.M.; Petry, S.; Kelley, A.C.; Ramakrishnan, V. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 2008, 322, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Korostelev, A.; Asahara, H.; Lancaster, L.; Laurberg, M.; Hirschi, A.; Zhu, J.; Trakhanov, S.; Scott, W.G.; Noller, H.F. Crystal structure of a translation termination complex formed with release factor RF2. Proc. Natl. Acad. Sci. USA 2008, 105, 19684–19689. [Google Scholar] [CrossRef]
- Laurberg, M.; Asahara, H.; Korostelev, A.; Zhu, J.; Trakhanov, S.; Noller, H.F. Structural basis for translation termination on the 70S ribosome. Nature 2008, 454, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Ford Doolittle, W. Evolution of the eukaryotic translation termination system: Origins of release factors. Mol. Biol. Evol. 2000, 17, 882–889. [Google Scholar] [CrossRef]
- Vestergaard, B.; Van, L.B.; Andersen, G.R.; Nyborg, J.; Buckingham, R.H.; Kjeldgaard, M. Bacterial polypeptide release factor RF2 is structurally distinct from eukaryotic eRF1. Mol. Cell 2001, 8, 1375–1382. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Skabkin, M.A.; Pisareva, V.P.; Skabkina, O.V.; Rakotondrafara, A.M.; Hentze, M.W.; Hellen, C.U.; Pestova, T.V. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 2010, 37, 196–210. [Google Scholar] [CrossRef]
- Shoemaker, C.J.; Green, R. Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc. Natl. Acad. Sci. USA 2011, 108, E1392–E1398. [Google Scholar] [CrossRef] [Green Version]
- Zavialov, A.V.; Hauryliuk, V.V.; Ehrenberg, M. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol. Cell 2005, 18, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Mugnier, P.; Das, A.K.; Webb, H.M.; Evans, D.R.; Tuite, M.F.; Hemmings, B.A.; Barford, D. The crystal structure of human eukaryotic release factor eRF1--mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell 2000, 100, 311–321. [Google Scholar] [CrossRef]
- Cheng, Z.; Saito, K.; Pisarev, A.V.; Wada, M.; Pisareva, V.P.; Pestova, T.V.; Gajda, M.; Round, A.; Kong, C.; Lim, M.; et al. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev. 2009, 23, 1106–1118. [Google Scholar] [CrossRef] [Green Version]
- Preis, A.; Heuer, A.; Barrio-Garcia, C.; Hauser, A.; Eyler, D.E.; Berninghausen, O.; Green, R.; Becker, T.; Beckmann, R. Cryoelectron microscopic structures of eukaryotic translation termination complexes containing eRF1-eRF3 or eRF1-ABCE1. Cell Rep. 2014, 8, 59–65. [Google Scholar] [CrossRef]
- Anantharaman, V.; Koonin, E.V.; Aravind, L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002, 30, 1427–1464. [Google Scholar] [CrossRef] [Green Version]
- Caban, K.; Kinzy, S.A.; Copeland, P.R. The L7Ae RNA binding motif is a multifunctional domain required for the ribosome-dependent Sec incorporation activity of Sec insertion sequence binding protein 2. Mol. Cell Biol. 2007, 27, 6350–6360. [Google Scholar] [CrossRef]
- Mora, L.; Zavialov, A.; Ehrenberg, M.; Buckingham, R.H. Stop codon recognition and interactions with peptide release factor RF3 of truncated and chimeric RF1 and RF2 from Escherichia coli. Mol. Microbiol. 2003, 50, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Pallesen, J.; Hashem, Y.; Korkmaz, G.; Koripella, R.K.; Huang, C.; Ehrenberg, M.; Sanyal, S.; Frank, J. Cryo-EM visualization of the ribosome in termination complex with apo-RF3 and RF1. eLife 2013, 2, e00411. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, O.; Laurberg, M.; Liljas, A.; Selmer, M. Is tRNA binding or tRNA mimicry mandatory for translation factors? Curr. Protein Pept. Sci. 2002, 3, 133–141. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ito, K. tRNA mimicry in translation termination and beyond. Wiley Interdiscip. Rev. RNA 2011, 2, 647–668. [Google Scholar] [CrossRef]
- Passos, D.O.; Doma, M.K.; Shoemaker, C.J.; Muhlrad, D.; Green, R.; Weissman, J.; Hollien, J.; Parker, R. Analysis of Dom34 and its function in no-go decay. Mol. Biol. Cell 2009, 20, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kikuno, I.; Kuroha, K.; Saito, K.; Ito, K.; Ishitani, R.; Inada, T.; Nureki, O. Structural basis for mRNA surveillance by archaeal Pelota and GTP-bound EF1alpha complex. Proc. Natl. Acad. Sci. USA 2010, 107, 17575–17579. [Google Scholar] [CrossRef]
- Saito, S.; Hosoda, N.; Hoshino, S. The Hbs1-Dom34 protein complex functions in non-stop mRNA decay in mammalian cells. J. Biol. Chem. 2013, 288, 17832–17843. [Google Scholar] [CrossRef] [PubMed]
- Doma, M.K.; Parker, R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 2006, 440, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Guydosh, N.R.; Green, R. Dom34 rescues ribosomes in 3’ untranslated regions. Cell 2014, 156, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Graille, M.; Chaillet, M.; van Tilbeurgh, H. Structure of yeast Dom34: A protein related to translation termination factor Erf1 and involved in No-Go decay. J. Biol. Chem. 2008, 283, 7145–7154. [Google Scholar] [CrossRef]
- Hilal, T.; Yamamoto, H.; Loerke, J.; Burger, J.; Mielke, T.; Spahn, C.M. Structural insights into ribosomal rescue by Dom34 and Hbs1 at near-atomic resolution. Nat. Commun. 2016, 7, 13521. [Google Scholar] [CrossRef] [Green Version]
- Carr-Schmid, A.; Pfund, C.; Craig, E.A.; Kinzy, T.G. Novel G-protein complex whose requirement is linked to the translational status of the cell. Mol. Cell Biol. 2002, 22, 2564–2574. [Google Scholar] [CrossRef]
- Scolnick, E.; Tompkins, R.; Caskey, T.; Nirenberg, M. Release factors differing in specificity for terminator codons. Proc. Natl. Acad. Sci. USA 1968, 61, 768–774. [Google Scholar] [CrossRef]
- Duarte, I.; Nabuurs, S.B.; Magno, R.; Huynen, M. Evolution and diversification of the organellar release factor family. Mol. Biol. Evol. 2012, 29, 3497–3512. [Google Scholar] [CrossRef]
- Zaremba-Niedzwiedzka, K.; Caceres, E.F.; Saw, J.H.; Backstrom, D.; Juzokaite, L.; Vancaester, E.; Seitz, K.W.; Anantharaman, K.; Starnawski, P.; Kjeldsen, K.U.; et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 2017, 541, 353–358. [Google Scholar] [CrossRef]
- Aravind, L. Guilt by association: Contextual information in genome analysis. Genome Res. 2000, 10, 1074–1077. [Google Scholar] [CrossRef]
- Huynen, M.; Snel, B.; Lathe, W., 3rd; Bork, P. Predicting protein function by genomic context: Quantitative evaluation and qualitative inferences. Genome Res. 2000, 10, 1204–1210. [Google Scholar] [CrossRef]
- Heo, J.M.; Livnat-Levanon, N.; Taylor, E.B.; Jones, K.T.; Dephoure, N.; Ring, J.; Xie, J.; Brodsky, J.L.; Madeo, F.; Gygi, S.P.; et al. A stress-responsive system for mitochondrial protein degradation. Mol Cell 2010, 40, 465–480. [Google Scholar] [CrossRef]
- Tran, J.R.; Brodsky, J.L. The Cdc48-Vms1 complex maintains 26S proteasome architecture. Biochem. J. 2014, 458, 459–467. [Google Scholar] [CrossRef]
- Stapf, C.; Cartwright, E.; Bycroft, M.; Hofmann, K.; Buchberger, A. The general definition of the p97/valosin-containing protein (VCP)-interacting motif (VIM) delineates a new family of p97 cofactors. J. Biol. Chem. 2011, 286, 38670–38678. [Google Scholar] [CrossRef]
- Greber, B.J.; Gerhardy, S.; Leitner, A.; Leibundgut, M.; Salem, M.; Boehringer, D.; Leulliot, N.; Aebersold, R.; Panse, V.G.; Ban, N. Insertion of the Biogenesis Factor Rei1 Probes the Ribosomal Tunnel during 60S Maturation. Cell 2016, 164, 91–102. [Google Scholar] [CrossRef]
- Finch, A.J.; Hilcenko, C.; Basse, N.; Drynan, L.F.; Goyenechea, B.; Menne, T.F.; Gonzalez Fernandez, A.; Simpson, P.; D’Santos, C.S.; Arends, M.J.; et al. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes Dev. 2011, 25, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Wessels, D.; Srikantha, T.; Yi, S.; Kuhl, S.; Aravind, L.; Soll, D.R. The Shwachman-Bodian-Diamond syndrome gene encodes an RNA-binding protein that localizes to the pseudopod of Dictyostelium amoebae during chemotaxis. J. Cell Sci. 2006, 119, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, A.; Howorth, D.; Chandonia, J.M.; Brenner, S.E.; Hubbard, T.J.; Chothia, C.; Murzin, A.G. Data growth and its impact on the SCOP database: New developments. Nucleic Acids Res. 2008, 36, D419–D425. [Google Scholar] [CrossRef]
- Dyer, D.H.; Rubio, L.M.; Thoden, J.B.; Holden, H.M.; Ludden, P.W.; Rayment, I. The three-dimensional structure of the core domain of Naf Y from Azotobacter vinelandii determined at 1.8-A resolution. J. Biol. Chem. 2003, 278, 32150–32156. [Google Scholar] [CrossRef]
- Padmanabhan, B.; Paehler, A.; Horikoshi, M. Structure of creatine amidinohydrolase from Actinobacillus. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Wibley, J.E.; Pegg, A.E.; Moody, P.C. Crystal structure of the human O(6)-alkylguanine-DNA alkyltransferase. Nucleic Acids Res. 2000, 28, 393–401. [Google Scholar] [CrossRef]
- Katayanagi, K.; Miyagawa, M.; Matsushima, M.; Ishikawa, M.; Kanaya, S.; Ikehara, M.; Matsuzaki, T.; Morikawa, K. Three-dimensional structure of ribonuclease H from E. coli. Nature 1990, 347, 306–309. [Google Scholar] [CrossRef]
- Yang, W.; Hendrickson, W.A.; Crouch, R.J.; Satow, Y. Structure of ribonuclease H phased at 2 A resolution by MAD analysis of the selenomethionyl protein. Science 1990, 249, 1398–1405. [Google Scholar] [CrossRef]
- Majorek, K.A.; Dunin-Horkawicz, S.; Steczkiewicz, K.; Muszewska, A.; Nowotny, M.; Ginalski, K.; Bujnicki, J.M. The RNase H-like superfamily: New members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 2014, 42, 4160–4179. [Google Scholar] [CrossRef]
- Seit-Nebi, A.; Frolova, L.; Justesen, J.; Kisselev, L. Class-1 translation termination factors: Invariant GGQ minidomain is essential for release activity and ribosome binding but not for stop codon recognition. Nucleic Acids Res. 2001, 29, 3982–3987. [Google Scholar] [CrossRef]
- Verma, R.; Reichermeier, K.M.; Burroughs, A.M.; Oania, R.S.; Reitsma, J.M.; Aravind, L.; Deshaies, R.J. Vms1 and ANKZF1 peptidyl-tRNA hydrolases release nascent chains from stalled ribosomes. Nature 2018, 557, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Kuroha, K.; Zinoviev, A.; Hellen, C.U.T.; Pestova, T.V. Release of Ubiquitinated and Non-ubiquitinated Nascent Chains from Stalled Mammalian Ribosomal Complexes by ANKZF1 and Ptrh1. Mol. Cell 2018, 72, 286–302.e288. [Google Scholar] [CrossRef]
- Trobro, S.; Aqvist, J. A model for how ribosomal release factors induce peptidyl-tRNA cleavage in termination of protein synthesis. Mol. Cell 2007, 27, 758–766. [Google Scholar] [CrossRef]
- Handa, Y.; Inaho, N.; Nameki, N. YaeJ is a novel ribosome-associated protein in Escherichia coli that can hydrolyze peptidyl-tRNA on stalled ribosomes. Nucleic Acids Res. 2011, 39, 1739–1748. [Google Scholar] [CrossRef]
- Chadani, Y.; Ono, K.; Kutsukake, K.; Abo, T. Escherichia coli YaeJ protein mediates a novel ribosome-rescue pathway distinct from SsrA- and ArfA-mediated pathways. Mol. Microbiol. 2011, 80, 772–785. [Google Scholar] [CrossRef] [Green Version]
- Huter, P.; Muller, C.; Arenz, S.; Beckert, B.; Wilson, D.N. Structural Basis for Ribosome Rescue in Bacteria. Trends Biochem. Sci. 2017, 42, 669–680. [Google Scholar] [CrossRef]
- Buskirk, A.R.; Green, R. Ribosome pausing, arrest and rescue in bacteria and eukaryotes. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Huang, L.; Ku, J.; Pookanjanatavip, M.; Gu, X.; Wang, D.; Greene, P.J.; Santi, D.V. Identification of two Escherichia coli pseudouridine synthases that show multisite specificity for 23S RNA. Biochemistry 1998, 37, 15951–15957. [Google Scholar] [CrossRef]
- Hoernes, T.P.; Clementi, N.; Faserl, K.; Glasner, H.; Breuker, K.; Lindner, H.; Huttenhofer, A.; Erlacher, M.D. Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code. Nucleic Acids Res. 2016, 44, 852–862. [Google Scholar] [CrossRef]
- Baranov, P.V.; Vestergaard, B.; Hamelryck, T.; Gesteland, R.F.; Nyborg, J.; Atkins, J.F. Diverse bacterial genomes encode an operon of two genes, one of which is an unusual class-I release factor that potentially recognizes atypical mRNA signals other than normal stop codons. Biol. Direct. 2006, 1, 28. [Google Scholar] [CrossRef]
- Englert, M.; Sheppard, K.; Aslanian, A.; Yates, J.R., 3rd; Soll, D. Archaeal 3’-phosphate RNA splicing ligase characterization identifies the missing component in tRNA maturation. Proc. Natl. Acad. Sci. USA 2011, 108, 1290–1295. [Google Scholar] [CrossRef]
- Popow, J.; Englert, M.; Weitzer, S.; Schleiffer, A.; Mierzwa, B.; Mechtler, K.; Trowitzsch, S.; Will, C.L.; Luhrmann, R.; Soll, D.; et al. HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science 2011, 331, 760–764. [Google Scholar] [CrossRef]
- Tanaka, N.; Shuman, S. RtcB is the RNA ligase component of an Escherichia coli RNA repair operon. J. Biol. Chem. 2011, 286, 7727–7731. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Aravind, L. RNA damage in biological conflicts and the diversity of responding RNA repair systems. Nucleic Acids Res. 2016, 44, 8525–8555. [Google Scholar] [CrossRef]
- Klaiman, D.; Steinfels-Kohn, E.; Krutkina, E.; Davidov, E.; Kaufmann, G. The wobble nucleotide-excising anticodon nuclease RloC is governed by the zinc-hook and DNA-dependent ATPase of its Rad50-like region. Nucleic Acids Res. 2012, 40, 8568–8578. [Google Scholar] [CrossRef] [Green Version]
- Feaga, H.A.; Quickel, M.D.; Hankey-Giblin, P.A.; Keiler, K.C. Human Cells Require Non-stop Ribosome Rescue Activity in Mitochondria. PLoS Genet. 2016, 12, e1005964. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, M.; Gorman, G.S.; Alston, C.L.; Pajak, A.; Pyle, A.; He, L.; Griffin, H.; Chinnery, P.F.; Miller, J.A.; Schaefer, A.M.; et al. Adult Onset Leigh Syndrome in the Intensive Care Setting: A Novel Presentation of a C12orf65 Related Mitochondrial Disease. J. Neuromuscul. Dis. 2015, 2, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Antonicka, H.; Ostergaard, E.; Sasarman, F.; Weraarpachai, W.; Wibrand, F.; Pedersen, A.M.; Rodenburg, R.J.; van der Knaap, M.S.; Smeitink, J.A.; Chrzanowska-Lightowlers, Z.M.; et al. Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am. J. Hum. Genet. 2010, 87, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.; Ramesh, V.; Bartsakoulia, M.; Boczonadi, V.; Gomez-Duran, A.; Herczegfalvi, A.; Blakely, E.L.; Smertenko, T.; Duff, J.; Eglon, G.; et al. Behr’s Syndrome is Typically Associated with Disturbed Mitochondrial Translation and Mutations in the C12orf65 Gene. J. Neuromuscul. Dis. 2014, 1, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Nishihara, H.; Omoto, M.; Takao, M.; Higuchi, Y.; Koga, M.; Kawai, M.; Kawano, H.; Ikeda, E.; Takashima, H.; Kanda, T. Autopsy case of the C12orf65 mutation in a patient with signs of mitochondrial dysfunction. Neurol. Genet. 2017, 3, e171. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, R.; Iyer, L.M.; Vasudevan, S.; Aravind, L. Detection of novel members, structure-function analysis and evolutionary classification of the 2H phosphoesterase superfamily. Nucleic Acids Res. 2002, 30, 5229–5243. [Google Scholar] [CrossRef] [Green Version]
- Kogure, H.; Handa, Y.; Nagata, M.; Kanai, N.; Guntert, P.; Kubota, K.; Nameki, N. Identification of residues required for stalled-ribosome rescue in the codon-independent release factor YaeJ. Nucleic Acids Res. 2014, 42, 3152–3163. [Google Scholar] [CrossRef]
- Handa, Y.; Hikawa, Y.; Tochio, N.; Kogure, H.; Inoue, M.; Koshiba, S.; Guntert, P.; Inoue, Y.; Kigawa, T.; Yokoyama, S.; et al. Solution structure of the catalytic domain of the mitochondrial protein ICT1 that is essential for cell vitality. J. Mol. Biol. 2010, 404, 260–273. [Google Scholar] [CrossRef]
- Kaushal, P.S.; Sharma, M.R.; Booth, T.M.; Haque, E.M.; Tung, C.S.; Sanbonmatsu, K.Y.; Spremulli, L.L.; Agrawal, R.K. Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc. Natl. Acad. Sci. USA 2014, 111, 7284–7289. [Google Scholar] [CrossRef] [Green Version]
- Greber, B.J.; Bieri, P.; Leibundgut, M.; Leitner, A.; Aebersold, R.; Boehringer, D.; Ban, N. The complete structure of the 55S mammalian mitochondrial ribosome. Science 2015, 348, 303–308. [Google Scholar] [CrossRef]
- Brown, A.; Amunts, A.; Bai, X.C.; Sugimoto, Y.; Edwards, P.C.; Murshudov, G.; Scheres, S.H.W.; Ramakrishnan, V. Structure of the large ribosomal subunit from human mitochondria. Science 2014, 346, 718–722. [Google Scholar] [CrossRef]
- Amunts, A.; Brown, A.; Toots, J.; Scheres, S.H.W.; Ramakrishnan, V. The structure of the human mitochondrial ribosome. Science 2015, 348, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.; Rathore, S.; Kimanius, D.; Aibara, S.; Bai, X.C.; Rorbach, J.; Amunts, A.; Ramakrishnan, V. Structures of the human mitochondrial ribosome in native states of assembly. Nat. Struct. Mol. Biol. 2017, 24, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Noller, H.F. Evolution of protein synthesis from an RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003681. [Google Scholar] [CrossRef]
- Van der Gulik, P.T.; Speijer, D. How amino acids and peptides shaped the RNA world. Life (Basel) 2015, 5, 230–246. [Google Scholar] [CrossRef]
- Caskey, C.T.; Beaudet, A.L.; Scolnick, E.M.; Rosman, M. Hydrolysis of fMet-tRNA by peptidyl transferase. Proc. Natl. Acad. Sci. USA 1971, 68, 3163–3167. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Aravind, L.; Grishin, N.V.; Koonin, E.V. Evolution of aminoacyl-tRNA synthetases--analysis of unique domain architectures and phylogenetic trees reveals a complex history of horizontal gene transfer events. Genome Res. 1999, 9, 689–710. [Google Scholar]
- Yokoyama, T.; Shaikh, T.R.; Iwakura, N.; Kaji, H.; Kaji, A.; Agrawal, R.K. Structural insights into initial and intermediate steps of the ribosome-recycling process. EMBO J. 2012, 31, 1836–1846. [Google Scholar] [CrossRef]
- Gao, N.; Zavialov, A.V.; Li, W.; Sengupta, J.; Valle, M.; Gursky, R.P.; Ehrenberg, M.; Frank, J. Mechanism for the disassembly of the posttermination complex inferred from cryo-EM studies. Mol. Cell 2005, 18, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Iyer, L.M.; Burroughs, A.M.; Aravind, L. The prokaryotic antecedents of the ubiquitin-signaling system and the early evolution of ubiquitin-like beta-grasp domains. Genome Biol. 2006, 7, R60. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.M.; Iyer, L.M.; Aravind, L. Functional diversification of the RING finger and other binuclear treble clef domains in prokaryotes and the early evolution of the ubiquitin system. Mol. Biosyst. 2011, 7, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.M.; Iyer, L.M.; Aravind, L. Structure and evolution of ubiquitin and ubiquitin-related domains. Methods Mol. Biol. 2012, 832, 15–63. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, A.M.; Iyer, L.M.; Aravind, L. The natural history of ubiquitin and ubiquitin-related domains. Front. Biosci. (Landmark Ed.) 2012, 17, 1433–1460. [Google Scholar] [CrossRef]
- Hurst, L.D.; Atlan, A.; Bengtsson, B.O. Genetic conflicts. Q. Rev. Biol. 1996, 71, 317–364. [Google Scholar] [CrossRef]
- Aravind, L.; Anantharaman, V.; Zhang, D.; de Souza, R.F.; Iyer, L.M. Gene flow and biological conflict systems in the origin and evolution of eukaryotes. Front. Cell Infect. Microbiol. 2012, 2, 89. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ishitani, R.; Nureki, O. Recent structural studies on Dom34/aPelota and Hbs1/aEF1alpha: Important factors for solving general problems of ribosomal stall in translation. Biophysics (Nagoya-shi) 2013, 9, 131–140. [Google Scholar] [CrossRef]
- Goralski, T.D.P.; Kirimanjeswara, G.S.; Keiler, K.C. A New Mechanism for Ribosome Rescue Can Recruit RF1 or RF2 to Nonstop Ribosomes. MBio 2018, 9. [Google Scholar] [CrossRef]
- Stacy, R.A.; Aalen, R.B. Identification of sequence homology between the internal hydrophilic repeated motifs of group 1 late-embryogenesis-abundant proteins in plants and hydrophilic repeats of the general stress protein GsiB of Bacillus subtilis. Planta 1998, 206, 476–478. [Google Scholar] [CrossRef]
- Maul, B.; Volker, U.; Riethdorf, S.; Engelmann, S.; Hecker, M. sigma B-dependent regulation of gsiB in response to multiple stimuli in Bacillus subtilis. Mol. Genes Genet. 1995, 248, 114–120. [Google Scholar] [CrossRef]
- Asteri, I.A.; Boutou, E.; Anastasiou, R.; Pot, B.; Vorgias, C.E.; Tsakalidou, E.; Papadimitriou, K. In silico evidence for the horizontal transfer of gsiB, a sigma(B)-regulated gene in gram-positive bacteria, to lactic acid bacteria. Appl. Environ. Microbiol. 2011, 77, 3526–3531. [Google Scholar] [CrossRef]
- Fernandez-Puentes, C.; Vazquez, D. Effects of some proteins that inactivate the eukaryotic ribosome. FEBS Lett. 1977, 78, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Winther, K.S.; Brodersen, D.E.; Brown, A.K.; Gerdes, K. VapC20 of Mycobacterium tuberculosis cleaves the sarcin-ricin loop of 23S rRNA. Nat. Commun. 2013, 4, 2796. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; Gao, Y.G.; Andersen, K.R.; Dunham, C.M.; Kelley, A.C.; Hentschel, J.; Gerdes, K.; Ramakrishnan, V.; Brodersen, D.E. The structural basis for mRNA recognition and cleavage by the ribosome-dependent endonuclease RelE. Cell 2009, 139, 1084–1095. [Google Scholar] [CrossRef]
- Polikanov, Y.S.; Blaha, G.M.; Steitz, T.A. How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 2012, 336, 915–918. [Google Scholar] [CrossRef]
- Ueta, M.; Ohniwa, R.L.; Yoshida, H.; Maki, Y.; Wada, C.; Wada, A. Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J. Biochem. 2008, 143, 425–433. [Google Scholar] [CrossRef]
- Puri, P.; Eckhardt, T.H.; Franken, L.E.; Fusetti, F.; Stuart, M.C.; Boekema, E.J.; Kuipers, O.P.; Kok, J.; Poolman, B. Lactococcus lactis YfiA is necessary and sufficient for ribosome dimerization. Mol. Microbiol. 2014, 91, 394–407. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Wada, A. Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 2010, 15, 43–58. [Google Scholar] [CrossRef]
- Akiyama, T.; Williamson, K.S.; Schaefer, R.; Pratt, S.; Chang, C.B.; Franklin, M.J. Resuscitation of Pseudomonas aeruginosa from dormancy requires hibernation promoting factor (PA4463) for ribosome preservation. Proc. Natl. Acad. Sci. USA 2017, 114, 3204–3209. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Yap, M.N. Ribosome hibernation factor promotes Staphylococcal survival and differentially represses translation. Nucleic Acids Res. 2016, 44, 4881–4893. [Google Scholar] [CrossRef] [Green Version]
- Akanuma, G.; Kazo, Y.; Tagami, K.; Hiraoka, H.; Yano, K.; Suzuki, S.; Hanai, R.; Nanamiya, H.; Kato-Yamada, Y.; Kawamura, F. Ribosome dimerization is essential for the efficient regrowth of Bacillus subtilis. Microbiology 2016, 162, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Yap, M.N. Disassembly of the Staphylococcus aureus hibernating 100S ribosome by an evolutionarily conserved GTPase. Proc. Natl. Acad. Sci. USA 2017, 114, E8165–E8173. [Google Scholar] [CrossRef] [Green Version]
- Jacob, Y.; Seif, E.; Paquet, P.O.; Lang, B.F. Loss of the mRNA-like region in mitochondrial tmRNAs of jakobids. RNA 2004, 10, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Hafez, M.; Burger, G.; Steinberg, S.V.; Lang, B.F. A second eukaryotic group with mitochondrion-encoded tmRNA: In silico identification and experimental confirmation. RNA Biol 2013, 10, 1117–1124. [Google Scholar] [CrossRef]
- Keiler, K.C.; Shapiro, L.; Williams, K.P. tmRNAs that encode proteolysis-inducing tags are found in all known bacterial genomes: A two-piece tmRNA functions in Caulobacter. Proc. Natl. Acad. Sci. USA 2000, 97, 7778–7783. [Google Scholar] [CrossRef] [Green Version]
- Stiller, J.W.; Schreiber, J.; Yue, J.; Guo, H.; Ding, Q.; Huang, J. The evolution of photosynthesis in chromist algae through serial endosymbioses. Nat. Commun. 2014, 5, 5764. [Google Scholar] [CrossRef] [Green Version]
- Archibald, J.M. Genomic perspectives on the birth and spread of plastids. Proc. Natl. Acad. Sci. USA 2015, 112, 10147–10153. [Google Scholar] [CrossRef] [Green Version]
- Horn, M.; Harzenetter, M.D.; Linner, T.; Schmid, E.N.; Muller, K.D.; Michel, R.; Wagner, M. Members of the Cytophaga-Flavobacterium-Bacteroides phylum as intracellular bacteria of acanthamoebae: Proposal of ‘Candidatus Amoebophilus asiaticus’. Environ. Microbiol. 2001, 3, 440–449. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Kaur, G.; Zhang, D.; Aravind, L. Novel clades of the HU/IHF superfamily point to unexpected roles in the eukaryotic centrosome, chromosome partitioning, and biologic conflicts. Cell Cycle 2017, 16, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Izawa, T.; Park, S.H.; Zhao, L.; Hartl, F.U.; Neupert, W. Cytosolic Protein Vms1 Links Ribosome Quality Control to Mitochondrial and Cellular Homeostasis. Cell 2017, 171, 890–903.e818. [Google Scholar] [CrossRef]
- Tran, J.R.; Tomsic, L.R.; Brodsky, J.L. A Cdc48p-associated factor modulates endoplasmic reticulum-associated degradation, cell stress, and ubiquitinated protein homeostasis. J. Biol. Chem. 2011, 286, 5744–5755. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.S.; Park, J.; Qin, Y.; Li, X.; Parsawar, K.; Larson, M.H.; Cox, J.; Cheng, Y.; Lambowitz, A.M.; Weissman, J.S.; et al. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 2015, 347, 75–78. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Aravind, L. A highly conserved family of domains related to the DNA-glycosylase fold helps predict multiple novel pathways for RNA modifications. RNA Biol. 2014, 11, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Menninger, J.R. Accumulation of peptidyl tRNA is lethal to Escherichia coli. J. Bacteriol. 1979, 137, 694–696. [Google Scholar]
- Garcia-Villegas, M.R.; De La Vega, F.M.; Galindo, J.M.; Segura, M.; Buckingham, R.H.; Guarneros, G. Peptidyl-tRNA hydrolase is involved in lambda inhibition of host protein synthesis. EMBO J. 1991, 10, 3549–3555. [Google Scholar] [CrossRef] [PubMed]
- Menez, J.; Buckingham, R.H.; de Zamaroczy, M.; Campelli, C.K. Peptidyl-tRNA hydrolase in Bacillus subtilis, encoded by spoVC, is essential to vegetative growth, whereas the homologous enzyme in Saccharomyces cerevisiae is dispensable. Mol. Microbiol. 2002, 45, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Heurgue-Hamard, V.; Karimi, R.; Mora, L.; MacDougall, J.; Leboeuf, C.; Grentzmann, G.; Ehrenberg, M.; Buckingham, R.H. Ribosome release factor RF4 and termination factor RF3 are involved in dissociation of peptidyl-tRNA from the ribosome. EMBO J. 1998, 17, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Pavlov, M.Y.; Heurgue-Hamard, V.; Buckingham, R.H.; Ehrenberg, M. Initiation factors IF1 and IF2 synergistically remove peptidyl-tRNAs with short polypeptides from the P-site of translating Escherichia coli ribosomes. J. Mol. Biol. 1998, 281, 241–252. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burroughs, A.M.; Aravind, L. The Origin and Evolution of Release Factors: Implications for Translation Termination, Ribosome Rescue, and Quality Control Pathways. Int. J. Mol. Sci. 2019, 20, 1981. https://doi.org/10.3390/ijms20081981
Burroughs AM, Aravind L. The Origin and Evolution of Release Factors: Implications for Translation Termination, Ribosome Rescue, and Quality Control Pathways. International Journal of Molecular Sciences. 2019; 20(8):1981. https://doi.org/10.3390/ijms20081981
Chicago/Turabian StyleBurroughs, A. Maxwell, and L Aravind. 2019. "The Origin and Evolution of Release Factors: Implications for Translation Termination, Ribosome Rescue, and Quality Control Pathways" International Journal of Molecular Sciences 20, no. 8: 1981. https://doi.org/10.3390/ijms20081981
APA StyleBurroughs, A. M., & Aravind, L. (2019). The Origin and Evolution of Release Factors: Implications for Translation Termination, Ribosome Rescue, and Quality Control Pathways. International Journal of Molecular Sciences, 20(8), 1981. https://doi.org/10.3390/ijms20081981




