Twist and Turn—Topoisomerase Functions in Mitochondrial DNA Maintenance
Abstract
1. Topoisomerase Types and Their Known Functions
Topoisomerase Functions
2. Topoisomerase Requirements in Mitochondrial DNA Maintenance
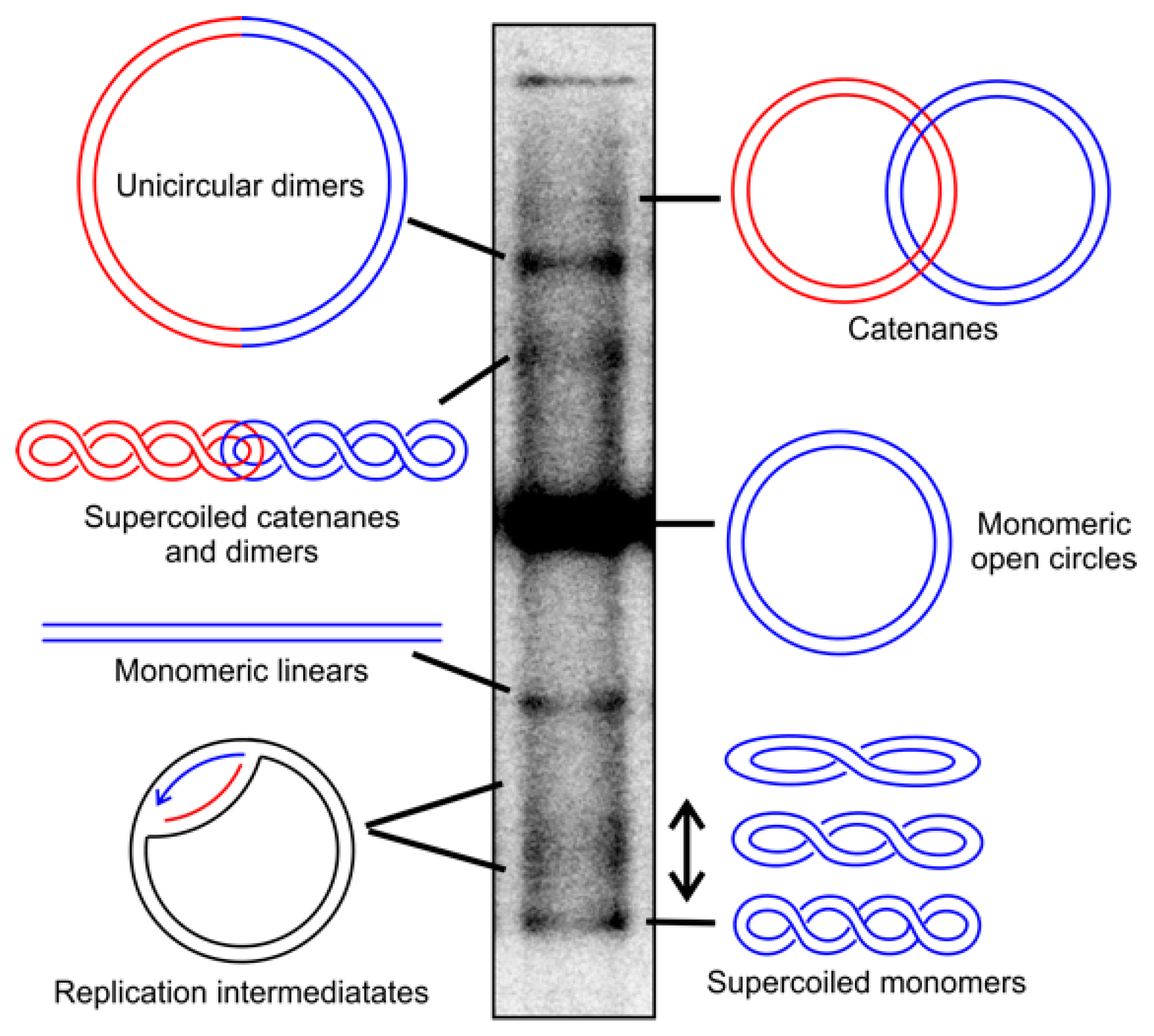
3. Mitochondrial Topoisomerases throughout the Eukaryotic Kingdoms
4. Mitochondrial Topoisomerases in Higher Animals
4.1. Top1mt
4.2. Top2
4.3. Top3α
5. Inhibition of Topoisomerase Function in Mitochondria
6. Open Questions
6.1. Regulatory and Accessory Factors of Mitochondrial Topoisomerases
6.2. Recombination of mtDNA
6.3. Molecular Mechanisms of Delayed Mitotoxicity
6.4. The Role of Mitochondrial Topoisomerases in Cancer Biology
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| mtDNA | Mitochondrial DNA |
References
- Majumder, H.K. Topoisomerases. In Brenner’s Encyclopedia of Genetics; Elsevier: Cambridge, MA, USA, 2013; pp. 78–79. ISBN 9780080961569. [Google Scholar]
- Forterre, P.; Gadelle, D. Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res. 2009, 37, 679–692. [Google Scholar] [CrossRef]
- Garnier, F.; Debat, H.; Nadal, M. Type IA DNA Topoisomerases: A Universal Core and Multiple Activities. In Methods in Molecular Biology; Springer: Berlin, Germany, 2018; pp. 1–20. [Google Scholar]
- Slesarev, A.I.; Stetter, K.O.; Lake, J.A.; Gellert, M.; Krah, R.; Kozyavkin, S.A. DNA topoisomerase V is a relative of eukaryotic topoisomerase I from a hyperthermophilic prokaryote. Nature 1993, 364, 735–737. [Google Scholar] [CrossRef]
- Reed, B.; Yakovleva, L.; Shuman, S.; Ghose, R. Characterization of DNA Binding by the Isolated N-Terminal Domain of Vaccinia Virus DNA Topoisomerase IB. Biochemistry 2017, 56, 3307–3317. [Google Scholar] [CrossRef]
- Dorman, C.J.; Dorman, M.J. DNA supercoiling is a fundamental regulatory principle in the control of bacterial gene expression. Biophys. Rev. 2016, 8, 209–220. [Google Scholar] [CrossRef]
- Gadelle, D.; Filée, J.; Buhler, C.; Forterre, P. Phylogenomics of type II DNA topoisomerases. BioEssays 2003, 25, 232–242. [Google Scholar] [CrossRef]
- Bloomfield, G. Atypical ploidy cycles, Spo11, and the evolution of meiosis. Semin. Cell Dev. Biol. 2016, 54, 158–164. [Google Scholar] [CrossRef]
- Manohar, M.; Choi, H.W.; Manosalva, P.; Austin, C.A.; Peters, J.E.; Klessig, D.F. Plant and Human MORC Proteins Have DNA-Modifying Activities Similar to Type II Topoisomerases, but Require One or More Additional Factors for Full Activity. Mol. Plant-Microbe Interact. 2017, 30, 87–100. [Google Scholar] [CrossRef]
- Koch, A.; Kang, H.-G.; Steinbrenner, J.; Dempsey, D.A.; Klessig, D.F.; Kogel, K.-H. MORC Proteins: Novel Players in Plant and Animal Health. Front. Plant Sci. 2017, 8, 1720. [Google Scholar] [CrossRef]
- Hong, S.; Joo, J.H.; Yun, H.; Kim, K. The nature of meiotic chromosome dynamics and recombination in budding yeast. J. Microbiol. 2019, 57, 221–231. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Lin, S.-W.; Wu, Y.-C.; Chan, N.-L.; Chi, P. Functional characterization of the meiosis-specific DNA double-strand break inducing factor SPO-11 from C. elegans. Sci. Rep. 2017, 7, 2370. [Google Scholar] [CrossRef]
- Canela, A.; Maman, Y.; Jung, S.; Wong, N.; Callen, E.; Day, A.; Kieffer-Kwon, K.-R.; Pekowska, A.; Zhang, H.; Rao, S.S.P.; et al. Genome Organization Drives Chromosome Fragility. Cell 2017, 170, 507–521.e18. [Google Scholar] [CrossRef]
- Bernstein, K.A.; Gangloff, S.; Rothstein, R. The RecQ DNA Helicases in DNA Repair. Annu. Rev. Genet. 2010, 44, 393–417. [Google Scholar] [CrossRef]
- Mankouri, H.; Hickson, I. The RecQ helicase–topoisomerase III–Rmi1 complex: A DNA structure-specific ‘dissolvasome’? Trends Biochem. Sci. 2007, 32, 538–546. [Google Scholar] [CrossRef]
- Martin, C.-A.; Sarlós, K.; Logan, C.V.; Thakur, R.S.; Parry, D.A.; Bizard, A.H.; Leitch, A.; Cleal, L.; Ali, N.S.; Al-Owain, M.A.; et al. Mutations in TOP3A Cause a Bloom Syndrome-like Disorder. Am. J. Hum. Genet. 2018, 103, 221–231. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Saito, S.; Adachi, N.; Yano, K. Dynamic behavior of DNA topoisomerase IIβ in response to DNA double-strand breaks. Sci. Rep. 2018, 8, 10344. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, M. An integrated phylogenomic approach toward pinpointing the origin of mitochondria. Sci. Rep. 2015, 5, 7949. [Google Scholar] [CrossRef]
- Lavrov, D.V.; Pett, W. Animal Mitochondrial DNA as We Do Not Know It: Mt-Genome Organization and Evolution in Nonbilaterian Lineages. Genome Biol. Evol. 2016, 8, 2896–2913. [Google Scholar] [CrossRef]
- Ling, F. Recombination-dependent mtDNA partitioning: In vivo role of Mhr1p to promote pairing of homologous DNA. EMBO J. 2002, 21, 4730–4740. [Google Scholar] [CrossRef]
- Gerhold, J.M.; Aun, A.; Sedman, T.; Jõers, P.; Sedman, J. Strand Invasion Structures in the Inverted Repeat of Candida albicans Mitochondrial DNA Reveal a Role for Homologous Recombination in Replication. Mol. Cell 2010, 39, 851–861. [Google Scholar] [CrossRef]
- Freel, K.C.; Friedrich, A.; Schacherer, J. Mitochondrial genome evolution in yeasts: An all-encompassing view. FEMS Yeast Res. 2015, 15. [Google Scholar] [CrossRef]
- Williamson, D. The curious history of yeast mitochondrial DNA. Nat. Rev. Genet. 2002, 3, 475–481. [Google Scholar] [CrossRef]
- Yurina, N.P.; Odintsova, M.S. Mitochondrial genome structure of photosynthetic eukaryotes. Biochemistry 2016, 81, 101–113. [Google Scholar] [CrossRef]
- Voigt, O.; Erpenbeck, D.; Worheide, G. A fragmented metazoan organellar genome: The two mitochondrial chromosomes of Hydra magnipapillata. BMC Genomics 2008, 9, 350. [Google Scholar] [CrossRef]
- Roy, J.; Faktorová, D.; Lukeš, J.; Burger, G. Unusual Mitochondrial Genome Structures throughout the Euglenozoa. Protist 2007, 158, 385–396. [Google Scholar] [CrossRef]
- Bendich, A.J. Reaching for the ring: The study of mitochondrial genome structure. Curr. Genet. 1993, 24, 279–290. [Google Scholar] [CrossRef]
- Gray, M.W.; Lang, B.F.; Burger, G. Mitochondria of Protists. Annu. Rev. Genet. 2004, 38, 477–524. [Google Scholar] [CrossRef]
- Marcadé, I.; Cordaux, R.; Doublet, V.; Debenest, C.; Bouchon, D.; Raimond, R. Structure and Evolution of the Atypical Mitochondrial Genome of Armadillidium vulgare (Isopoda, Crustacea). J. Mol. Evol. 2007, 65, 651–659. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Goffart, S.; Tyynismaa, H.; Willcox, S.; Ide, T.; Kang, D.; Suomalainen, A.; Karhunen, P.J.; Griffith, J.D.; Holt, I.J.; et al. Human Heart Mitochondrial DNA Is Organized in Complex Catenated Networks Containing Abundant Four-way Junctions and Replication Forks. J. Biol. Chem. 2009, 284, 21446–21457. [Google Scholar] [CrossRef]
- Chen, X.J.; Clark-Walker, G.D. Unveiling the mystery of mitochondrial DNA replication in yeasts. Mitochondrion 2018, 38, 17–22. [Google Scholar] [CrossRef]
- Oldenburg, D.J.; Bendich, A.J. Mitochondrial DNA from the liverwort Marchantia polymorpha: Circularly permuted linear molecules, head-to-tail concatemers, and a 5′ protein 1 1Edited by N.-M. Chua. J. Mol. Biol. 2001, 310, 549–562. [Google Scholar] [CrossRef]
- Lewis, S.C.; Joers, P.; Willcox, S.; Griffith, J.D.; Jacobs, H.T.; Hyman, B.C. A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in Caenorhabditis elegans. PLoS Genet. 2015, 11, e1004985. [Google Scholar] [CrossRef]
- King, I.F.; Yandava, C.N.; Mabb, A.M.; Hsiao, J.S.; Huang, H.-S.; Pearson, B.L.; Calabrese, J.M.; Starmer, J.; Parker, J.S.; Magnuson, T.; et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature 2013, 501, 58–62. [Google Scholar] [CrossRef]
- Valach, M.; Farkas, Z.; Fricova, D.; Kovac, J.; Brejova, B.; Vinar, T.; Pfeiffer, I.; Kucsera, J.; Tomaska, L.; Lang, B.F.; et al. Evolution of linear chromosomes and multipartite genomes in yeast mitochondria. Nucleic Acids Res. 2011, 39, 4202–4219. [Google Scholar] [CrossRef]
- Mosig, G.; Gewin, J.; Luder, A.; Colowick, N.; Vo, D. Two recombination-dependent DNA replication pathways of bacteriophage T4, and their roles in mutagenesis and horizontal gene transfer. Proc. Natl. Acad. Sci. USA 2001, 98, 8306–8311. [Google Scholar] [CrossRef]
- Jensen, R.E.; Englund, P.T. Network News: The Replication of Kinetoplast DNA. Annu. Rev. Microbiol. 2012, 66, 473–491. [Google Scholar] [CrossRef]
- Hangas, A.; Aasumets, K.; Kekäläinen, N.J.; Paloheinä, M.; Pohjoismäki, J.L.; Gerhold, J.M.; Goffart, S. Ciprofloxacin impairs mitochondrial DNA replication initiation through inhibition of Topoisomerase 2. Nucleic Acids Res. 2018, 46, 9625–9636. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Wanrooij, S.; Hyvärinen, A.K.; Goffart, S.; Holt, I.J.; Spelbrink, J.N.; Jacobs, H.T. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006, 34, 5815–5828. [Google Scholar] [CrossRef]
- Raynard, S.; Bussen, W.; Sung, P. A Double Holliday Junction Dissolvasome Comprising BLM, Topoisomerase IIIα, and BLAP75. J. Biol. Chem. 2006, 281, 13861–13864. [Google Scholar] [CrossRef]
- Bizard, A.H.; Hickson, I.D. The Dissolution of Double Holliday Junctions. Cold Spring Harb. Perspect. Biol. 2014, 6, a016477. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Moens, P.B.; Wang, J.C. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase III. Proc. Natl. Acad. Sci. USA 2003, 100, 2526–2531. [Google Scholar] [CrossRef]
- Xu, D.; Shen, W.; Guo, R.; Xue, Y.; Peng, W.; Sima, J.; Yang, J.; Sharov, A.; Srikantan, S.; Yang, J.; et al. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 2013, 16, 1238–1247. [Google Scholar] [CrossRef]
- Turley, H.; Comley, M.; Houlbrook, S.; Nozaki, N.; Kikuchi, A.; Hickson, I.; Gatter, K.; Harris, A. The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br. J. Cancer 1997, 75, 1340–1346. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsutsui, K.; Tsutsui, K.; Inoue, Y. Differential expressions of the topoisomerase IIα and IIβ mRNAs in developing rat brain. Neurosci. Res. 1994, 19, 51–57. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, Y.L.; Wang, J.C. Dual localization of human DNA topoisomerase III to mitochondria and nucleus. Proc. Natl. Acad. Sci. USA 2002, 99, 12114–12119. [Google Scholar] [CrossRef]
- Moriyama, T.; Sato, N. Enzymes involved in organellar DNA replication in photosynthetic eukaryotes. Front. Plant Sci. 2014, 5, 480. [Google Scholar] [CrossRef]
- Wall, M.K.; Mitchenall, L.A.; Maxwell, A. Arabidopsis thaliana DNA gyrase is targeted to chloroplasts and mitochondria. Proc. Natl. Acad. Sci. USA 2004, 101, 7821–7826. [Google Scholar] [CrossRef]
- Carrie, C.; Kühn, K.; Murcha, M.W.; Duncan, O.; Small, I.D.; O’Toole, N.; Whelan, J. Approaches to defining dual-targeted proteins in Arabidopsis. Plant J. 2009, 57, 1128–1139. [Google Scholar] [CrossRef]
- Cupp, J.D.; Nielsen, B.L. Minireview: DNA replication in plant mitochondria. Mitochondrion 2014, 19, 231–237. [Google Scholar] [CrossRef]
- Aravind, L.; Iyer, L.M.; Wellems, T.E.; Miller, L.H. Plasmodium Biology. Cell 2003, 115, 771–785. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Godinho, J.L.P.; Vinayagam, J.; Zuma, A.A.; Silva, S.T.D.M.; Jaisankar, P.; Rodrigues, J.C.F.; De Souza, W.; Majumder, H.K. Isobenzofuranone derivative JVPH3, an inhibitor of L. donovani topoisomerase II, disrupts mitochondrial architecture in trypanosomatid parasites. Sci. Rep. 2018, 8, 11940. [Google Scholar] [CrossRef]
- Yang, G.; Choi, G.; No, J.H. Antileishmanial Mechanism of Diamidines Involves Targeting Kinetoplasts. Antimicrob. Agents Chemother. 2016, 60, 6828–6836. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Gluenz, E.; Gull, K.; Englund, P.T. A new function of Trypanosoma brucei mitochondrial topoisomerase II is to maintain kinetoplast DNA network topology. Mol. Microbiol. 2008, 70, 1465–1476. [Google Scholar] [CrossRef]
- Wu, J.; Feng, L.; Hsieh, T.-S. Drosophila topo III is required for the maintenance of mitochondrial genome and male germ-line stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6228–6233. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.-W.; Yasukawa, T.; Dalla Rosa, I.; Khiati, S.; Pommier, Y. Increased negative supercoiling of mtDNA in TOP1mt knockout mice and presence of topoisomerases II and II in vertebrate mitochondria. Nucleic Acids Res. 2014, 42, 7259–7267. [Google Scholar] [CrossRef]
- Sobek, S.; Dalla Rosa, I.; Pommier, Y.; Bornholz, B.; Kalfalah, F.; Zhang, H.; Wiesner, R.J.; von Kleist-Retzow, J.-C.; Hillebrand, F.; Schaal, H.; et al. Negative regulation of mitochondrial transcription by mitochondrial topoisomerase I. Nucleic Acids Res. 2013, 41, 9848–9857. [Google Scholar] [CrossRef]
- Khiati, S.; Baechler, S.A.; Factor, V.M.; Zhang, H.; Huang, S.N.; Dalla Rosa, I.; Sourbier, C.; Neckers, L.; Thorgeirsson, S.S.; Pommier, Y. Lack of mitochondrial topoisomerase I (TOP1mt) impairs liver regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 11282–11287. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, T.J.; Nadalutti, C.A.; Motori, E.; Sommerville, E.W.; Gorman, G.S.; Basu, S.; Hoberg, E.; Turnbull, D.M.; Chinnery, P.F.; Larsson, N.-G.; et al. Topoisomerase 3α Is Required for Decatenation and Segregation of Human mtDNA. Mol. Cell 2018, 69, 9–23.e6. [Google Scholar] [CrossRef]
- Zhang, H.; Barcelo, J.M.; Lee, B.; Kohlhagen, G.; Zimonjic, D.B.; Popescu, N.C.; Pommier, Y. Human mitochondrial topoisomerase I. Proc. Natl. Acad. Sci. USA 2001, 98, 10608–10613. [Google Scholar] [CrossRef]
- Rosa, I.D.; Goffart, S.; Wurm, M.; Wiek, C.; Essmann, F.; Sobek, S.; Schroeder, P.; Zhang, H.; Krutmann, J.; Hanenberg, H.; et al. Adaptation of topoisomerase I paralogs to nuclear and mitochondrial DNA. Nucleic Acids Res. 2009, 37, 6414–6428. [Google Scholar] [CrossRef]
- Dalla Rosa, I.; Huang, S.N.; Agama, K.; Khiati, S.; Zhang, H.; Pommier, Y. Mapping Topoisomerase Sites in Mitochondrial DNA with a Poisonous Mitochondrial Topoisomerase I (Top1mt). J. Biol. Chem. 2014, 289, 18595–18602. [Google Scholar] [CrossRef]
- Dalla Rosa, I.; Zhang, H.; Khiati, S.; Wu, X.; Pommier, Y. Transcription profiling suggests that mitochondrial topoisomerase IB acts as a topological barrier and regulator of mitochondrial DNA transcription. J. Biol. Chem. 2017, 292, 20162–20172. [Google Scholar] [CrossRef]
- Douarre, C.; Sourbier, C.; Dalla Rosa, I.; Brata Das, B.; Redon, C.E.; Zhang, H.; Neckers, L.; Pommier, Y. Mitochondrial Topoisomerase I is Critical for Mitochondrial Integrity and Cellular Energy Metabolism. PLoS ONE 2012, 7, e41094. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, R.; Sun, L.; Xia, J.; Yang, X.; Pan, C.; Huang, N.; Shi, M.; Bin, J.; Liao, Y.; et al. TOP1MT deficiency promotes GC invasion and migration via the enhancements of LDHA expression and aerobic glycolysis. Endocr. Relat. Cancer 2017, 24, 565–578. [Google Scholar] [CrossRef]
- Zhang, H.; Seol, Y.; Agama, K.; Neuman, K.C.; Pommier, Y. Distribution bias and biochemical characterization of TOP1MT single nucleotide variants. Sci. Rep. 2017, 7, 8614. [Google Scholar] [CrossRef]
- Baechler, S.A.; Factor, V.M.; Dalla Rosa, I.; Ravji, A.; Becker, D.; Khiati, S.; Miller Jenkins, L.M.; Lang, M.; Sourbier, C.; Michaels, S.A.; et al. The mitochondrial type IB topoisomerase drives mitochondrial translation and carcinogenesis. Nat. Commun. 2019, 10, 83. [Google Scholar] [CrossRef]
- Khiati, S.; Dalla Rosa, I.; Sourbier, C.; Ma, X.; Rao, V.A.; Neckers, L.M.; Zhang, H.; Pommier, Y. Mitochondrial Topoisomerase I (Top1mt) Is a Novel Limiting Factor of Doxorubicin Cardiotoxicity. Clin. Cancer Res. 2014, 20, 4873–4881. [Google Scholar] [CrossRef]
- Castora, F.J.; Simpson, M.V. Search for a DNA gyrase in mammalian mitochondria. J. Biol. Chem. 1979, 254, 11193–11195. [Google Scholar]
- Castora, F.J.; Vissering, F.F.; Simpson, M.V. The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria. Biochim. Biophys. Acta Gene Struct. Expr. 1983, 740, 417–427. [Google Scholar] [CrossRef]
- Lin, J.-H.; Castora, F.J. DNA topoisomerase II from mammalian mitochondria is inhibited by the antitumor drugs, m-AMSA and VM-26. Biochem. Biophys. Res. Commun. 1991, 176, 690–697. [Google Scholar] [CrossRef]
- Castora, F.J.; Lazarus, G.M. Isolation of a mitochondrial DNA topoisomerase from human leukemia cells. Biochem. Biophys. Res. Commun. 1984, 121, 77–86. [Google Scholar] [CrossRef]
- Castora, F.J.; Lazarus, G.M.; Kunes, D. The presence of two mitochondrial DNA topoisomerases in human acute leukemia cells. Biochem. Biophys. Res. Commun. 1985, 130, 854–866. [Google Scholar] [CrossRef]
- Low, R.L.; Orton, S.; Friedman, D.B. A truncated form of DNA topoisomerase IIβ associates with the mtDNA genome in mammalian mitochondria. Eur. J. Biochem. 2003, 270, 4173–4186. [Google Scholar] [CrossRef]
- Austin, C.A.; Sng, J.-H.; Patel, S.; Fisher, L.M. Novel HeLa topoisomerase II is the IIβ isoform: Complete coding sequence and homology with other type II topoisomerases. Biochim. Biophys. Acta Gene Struct. Expr. 1993, 1172, 283–291. [Google Scholar] [CrossRef]
- Yang, X. DNA Topoisomerase II and Neural Development. Science 2000, 287, 131–134. [Google Scholar] [CrossRef]
- Tsai, H.-Z.; Lin, R.-K.; Hsieh, T.-S. Drosophila mitochondrial topoisomerase III alpha affects the aging process via maintenance of mitochondrial function and genome integrity. J. Biomed. Sci. 2016, 23, 38. [Google Scholar] [CrossRef]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef]
- Cutts, S.; Rephaeli, A.; Nudelman, A.; Ugarenko, M.; Phillips, D. Potential Therapeutic Advantages of Doxorubicin when Activated by Formaldehyde to Function as a DNA Adduct-Forming Agent. Curr. Top. Med. Chem. 2015, 15, 1409–1422. [Google Scholar] [CrossRef]
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Ashley, N.; Poulton, J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem. Biophys. Res. Commun. 2009, 378, 450–455. [Google Scholar] [CrossRef]
- Williams, G.M.; Brunnemann, K.D.; Smart, D.J.; Molina, D.; Jeffrey, A.M.; Duan, J.-D.; Krebsfaenger, N.; Kampkoetter, A.; Schmuck, G. Relationship of cellular topoisomerase IIα inhibition to cytotoxicity and published genotoxicity of fluoroquinolone antibiotics in V79 cells. Chem. Biol. Interact. 2013, 203, 386–390. [Google Scholar] [CrossRef]
- Kloskowski, T.; Gurtowska, N.; Olkowska, J.; Nowak, J.M.; Adamowicz, J.; Tworkiewicz, J.; DĘbski, R.; Grzanka, A.; Drewa, T. Ciprofloxacin is a potential topoisomerase II inhibitor for the treatment of NSCLC. Int. J. Oncol. 2012, 41, 1943–1949. [Google Scholar] [CrossRef]
- Smart, D.J.; Lynch, A.M. Evaluating the genotoxicity of topoisomerase-targeted antibiotics. Mutagenesis 2012, 27, 359–365. [Google Scholar] [CrossRef]
- Ilgin, S.; Can, O.D.; Atli, O.; Ucel, U.I.; Sener, E.; Guven, I. Ciprofloxacin-induced neurotoxicity: Evaluation of possible underlying mechanisms. Toxicol. Mech. Methods 2015, 25, 374–381. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Safety Considerations of Fluoroquinolones in the Elderly. Drugs Aging 2010, 27, 193–209. [Google Scholar] [CrossRef]
- Hollenstein, U.; Brunner, M.; Schmid, R.; Müller, M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. 2001, 25, 354–358. [Google Scholar] [CrossRef]
- Ghaly, H.; Jörns, A.; Rustenbeck, I. Effect of fluoroquinolones on mitochondrial function in pancreatic beta cells. Eur. J. Pharm. Sci. 2014, 52, 206–214. [Google Scholar] [CrossRef]
- Holtom, P.D.; Pavkovic, S.A.; Bravos, P.D.; Patzakis, M.J.; Shepherd, L.E.; Frenkel, B. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cellsin vitro. J. Orthop. Res. 2000, 18, 721–727. [Google Scholar] [CrossRef]
- Hsiao, C.-J.J.; Younis, H.; Boelsterli, U.A. Trovafloxacin, a fluoroquinolone antibiotic with hepatotoxic potential, causes mitochondrial peroxynitrite stress in a mouse model of underlying mitochondrial dysfunction. Chem. Biol. Interact. 2010, 188, 204–213. [Google Scholar] [CrossRef]
- Rowe, T.C.; Weissig, V.; Lawrence, J.W. Mitochondrial DNA metabolism targeting drugs. Adv. Drug Deliv. Rev. 2001, 49, 175–187. [Google Scholar] [CrossRef]
- Nadanaciva, S.; Dillman, K.; Gebhard, D.F.; Shrikhande, A.; Will, Y. High-Content Screening for Compounds That Affect mtDNA-Encoded Protein Levels in Eukaryotic Cells. J. Biomol. Screen. 2010, 15, 937–948. [Google Scholar] [CrossRef]
- Németh, A.; Orgovan, N.; Sódar, B.W.; Osteikoetxea, X.; Pálóczi, K.; Szabó-Taylor, K.É.; Vukman, K.V.; Kittel, Á.; Turiák, L.; Wiener, Z.; et al. Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci. Rep. 2017, 7, 8202. [Google Scholar] [CrossRef]
- Idowu, T.; Schweizer, F. Ubiquitous Nature of Fluoroquinolones: The Oscillation between Antibacterial and Anticancer Activities. Antibiotics 2017, 6, 26. [Google Scholar] [CrossRef]
- Song, M.; Wu, H.; Wu, S.; Ge, T.; Wang, G.; Zhou, Y.; Sheng, S.; Jiang, J. Antibiotic drug levofloxacin inhibits proliferation and induces apoptosis of lung cancer cells through inducing mitochondrial dysfunction and oxidative damage. Biomed. Pharmacother. 2016, 84, 1137–1143. [Google Scholar] [CrossRef]
- Khiati, S.; Seol, Y.; Agama, K.; Rosa, I.D.; Agrawal, S.; Fesen, K.; Zhang, H.; Neuman, K.C.; Pommier, Y. Poisoning of Mitochondrial Topoisomerase I by Lamellarin D. Mol. Pharmacol. 2014, 86, 193–199. [Google Scholar] [CrossRef]
- Daroui, P.; Desai, S.D.; Li, T.-K.; Liu, A.A.; Liu, L.F. Hydrogen Peroxide Induces Topoisomerase I-mediated DNA Damage and Cell Death. J. Biol. Chem. 2004, 279, 14587–14594. [Google Scholar] [CrossRef]
- Pourquier, P.; Ueng, L.-M.; Fertala, J.; Wang, D.; Park, H.-J.; Essigmann, J.M.; Bjornsti, M.-A.; Pommier, Y. Induction of Reversible Complexes between Eukaryotic DNA Topoisomerase I and DNA-containing Oxidative Base Damages. J. Biol. Chem. 1999, 274, 8516–8523. [Google Scholar] [CrossRef]
- Pommier, Y.; Huang, S.N.; Gao, R.; Das, B.B.; Murai, J.; Marchand, C. Tyrosyl-DNA-phosphodiesterases (TDP1 and TDP2). DNA Repair 2014, 19, 114–129. [Google Scholar] [CrossRef]
- Chiang, S.-C.; Meagher, M.; Kassouf, N.; Hafezparast, M.; McKinnon, P.J.; Haywood, R.; El-Khamisy, S.F. Mitochondrial protein-linked DNA breaks perturb mitochondrial gene transcription and trigger free radical–induced DNA damage. Sci. Adv. 2017, 3, e1602506. [Google Scholar] [CrossRef]
- Banerjee, B.; Roy, A.; Sen, N.; Majumder, H.K. A tyrosyl DNA phosphodiesterase 1 from kinetoplastid parasite Leishmania donovani (LdTdp1) capable of removing topo I-DNA covalent complexes. Mol. Microbiol. 2010, 78, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Dexheimer, T.S.; Maddali, K.; Pommier, Y. Role of tyrosyl-DNA phosphodiesterase (TDP1) in mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 19790–19795. [Google Scholar] [CrossRef] [PubMed]
- Fam, H.K.; Chowdhury, M.K.; Walton, C.; Choi, K.; Boerkoel, C.F.; Hendson, G. Expression profile and mitochondrial colocalization of Tdp1 in peripheral human tissues. J. Mol. Histol. 2013, 44, 481–494. [Google Scholar] [CrossRef]
- Huang, S.N.; Dalla Rosa, I.; Michaels, S.A.; Tulumello, D.V.; Agama, K.; Khiati, S.; Jean, S.R.; Baechler, S.A.; Factor, V.M.; Varma, S.; et al. Mitochondrial tyrosyl-DNA phosphodiesterase 2 and its TDP2 S short isoform. EMBO Rep. 2018, 19, e42139. [Google Scholar] [CrossRef]
- Dykhuizen, E.C.; Hargreaves, D.C.; Miller, E.L.; Cui, K.; Korshunov, A.; Kool, M.; Pfister, S.; Cho, Y.-J.; Zhao, K.; Crabtree, G.R. BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 2013, 497, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Begum, N.A.; Taniguchi, T.; Taniguchi, H.; Kobayashi, M.; Honjo, T. Chromatin remodeller SMARCA4 recruits topoisomerase 1 and suppresses transcription-associated genomic instability. Nat. Commun. 2016, 7, 10549. [Google Scholar] [CrossRef]
- Cowell, I.G.; Okorokov, A.L.; Cutts, S.A.; Padget, K.; Bell, M.; Milner, J.; Austin, C.A. Human Topoisomerase IIα and IIβ Interact with the C-Terminal Region of p53. Exp. Cell Res. 2000, 255, 86–94. [Google Scholar] [CrossRef]
- Park, J.-H.; Zhuang, J.; Li, J.; Hwang, P.M. p53 as guardian of the mitochondrial genome. FEBS Lett. 2016, 590, 924–934. [Google Scholar] [CrossRef]
- Zhuang, J.; Ma, W.; Lago, C.U.; Hwang, P.M. Metabolic regulation of oxygen and redox homeostasis by p53: Lessons from evolutionary biology? Free Radic. Biol. Med. 2012, 53, 1279–1285. [Google Scholar] [CrossRef][Green Version]
- Sabourin, M.; Osheroff, N. Sensitivity of human type II topoisomerases to DNA damage: Stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000, 28, 1947–1954. [Google Scholar] [CrossRef]
- Leducq, J.-B.; Henault, M.; Charron, G.; Nielly-Thibault, L.; Terrat, Y.; Fiumera, H.L.; Shapiro, B.J.; Landry, C.R. Mitochondrial Recombination and Introgression during Speciation by Hybridization. Mol. Biol. Evol. 2017, 34, 1947–1959. [Google Scholar] [CrossRef]
- Gualberto, J.M.; Newton, K.J. Plant Mitochondrial Genomes: Dynamics and Mechanisms of Mutation. Annu. Rev. Plant Biol. 2017, 68, 225–252. [Google Scholar] [CrossRef]
- Dahal, S.; Dubey, S.; Raghavan, S.C. Homologous recombination-mediated repair of DNA double-strand breaks operates in mammalian mitochondria. Cell. Mol. Life Sci. 2018, 75, 1641–1655. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.; Goffart, S. The role of mitochondria in cardiac development and protection. Free Radic. Biol. Med. 2017, 106, 345–354. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Goffart, S. Of circles, forks and humanity: Topological organisation and replication of mammalian mitochondrial DNA. BioEssays 2011, 33, 290–299. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y. Borrowing Nuclear DNA Helicases to Protect Mitochondrial DNA. Int. J. Mol. Sci. 2015, 16, 10870–10887. [Google Scholar] [CrossRef]
- Sen, D.; Patel, G.; Patel, S.S. Homologous DNA strand exchange activity of the human mitochondrial DNA helicase TWINKLE. Nucleic Acids Res. 2016, 44, 4200–4210. [Google Scholar] [CrossRef]
- Pohjoismäki, J.L.O.; Forslund, J.M.E.; Goffart, S.; Torregrosa-Muñumer, R.; Wanrooij, S. Known Unknowns of Mammalian Mitochondrial DNA Maintenance. BioEssays 2018, 40, 1800102. [Google Scholar] [CrossRef]
- Herbers, E.; Kekäläinen, N.J.; Hangas, A.; Pohjoismäki, J.L.; Goffart, S. Tissue specific differences in mitochondrial DNA maintenance and expression. Mitochondrion 2019, 44, 85–92. [Google Scholar] [CrossRef]
- Mi, J.; Tian, G.; Liu, S.; Li, X.; Ni, T.; Zhang, L.; Wang, B. The Relationship Between Altered Mitochondrial DNA Copy Number And Cancer Risk: A Meta-Analysis. Sci. Rep. 2015, 5, 10039. [Google Scholar] [CrossRef]

| Features | Top1mt | Top2α | Top2β | Top3α |
|---|---|---|---|---|
| Classification | Type IB | Type IIA | Type IIA | Type IA |
| Shown mitochondrial function | Regulation of transcription [56,57] and replication [58] | Regulation of replication [38] | Decatenation of hemicatenates [59] | |
| Potential mitochondrial functions | Regulation of translation [59] | Scaffolding of the non-coding region, 7S protection [56] | Decatenation, recombination | Recombination, replication |
| Encoding details | Mitochondria-specific gene [60] | Probably identical to nuclear protein | Probably identical to nuclear protein | Alternative start codon [46] |
| Mitochondrial targeting sequence | Yes [60] | Unknown | Unknown | Yes [46] |
| Enzyme structure | Monomer | Homodimer | Homodimer | Monomer |
| Cofactors | Stimulated by Mg2+ or Ca2+ [60] | Mg2+, ATP | Mg2+, ATP | Mg2+ |
| Protein size | 70 kDa | 174 kDa | 180 kDa | 110 kDa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goffart, S.; Hangas, A.; Pohjoismäki, J.L.O. Twist and Turn—Topoisomerase Functions in Mitochondrial DNA Maintenance. Int. J. Mol. Sci. 2019, 20, 2041. https://doi.org/10.3390/ijms20082041
Goffart S, Hangas A, Pohjoismäki JLO. Twist and Turn—Topoisomerase Functions in Mitochondrial DNA Maintenance. International Journal of Molecular Sciences. 2019; 20(8):2041. https://doi.org/10.3390/ijms20082041
Chicago/Turabian StyleGoffart, Steffi, Anu Hangas, and Jaakko L. O. Pohjoismäki. 2019. "Twist and Turn—Topoisomerase Functions in Mitochondrial DNA Maintenance" International Journal of Molecular Sciences 20, no. 8: 2041. https://doi.org/10.3390/ijms20082041
APA StyleGoffart, S., Hangas, A., & Pohjoismäki, J. L. O. (2019). Twist and Turn—Topoisomerase Functions in Mitochondrial DNA Maintenance. International Journal of Molecular Sciences, 20(8), 2041. https://doi.org/10.3390/ijms20082041




