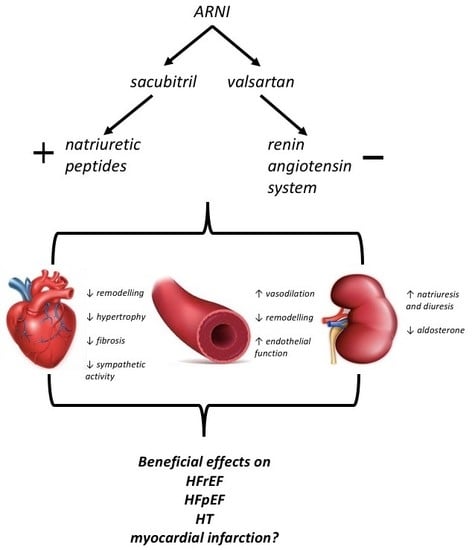
ARNi: A Novel Approach to Counteract Cardiovascular Diseases
Abstract
:1. Inhibition of Neurohormonal Systems in Cardiovascular Diseases
2. Natriuretic Peptides—Biological Properties
3. Mechanisms of Degradation of NPs
4. Therapeutic Strategies Involving NP Metabolism in Cardiovascular Diseases
5. Clinical Applications of ARNi
5.1. Heart Failure with Reduced Ejection Fraction
5.2. Heart Failure with Preserved Ejection Fraction
5.3. Hypertension
6. Future Perspective of NP-Based Therapies
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.; et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Yandrapalli, S.; Aronow, W.S.; Mondal, P.; Chabbott, D.R. The evolution of natriuretic peptide augmentation in management of heart failure and the role of sacubitril/valsartan. Arch. Med. Sci. 2017, 13, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Noe, A.; Chandra, P.; Al-Fayoumi, S.; Ligueros-Saylan, M.; Sarangapani, R.; Maahs, S.; Ksander, G.; Rigel, D.F.; Jeng, A.Y.; et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J. Clin. Pharmacol. 2010, 50, 401–414. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [PubMed]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M.; et al. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar]
- Melo, L.G.; Steinhelper, M.E.; Pang, S.C.; Tse, Y.; Ackermann, U. ANP in regulation of arterial pressure and fluid-electrolyte balance: Lessons from genetic mouse models. Physiol. Genomics 2000, 3, 4–58. [Google Scholar] [CrossRef]
- Volpe, M.; Battistoni, A.; Rubattu, S. Natriuretic peptides in heart failure: Current achievements and future perspectives. Int. J. Cardiol. 2019, 281, 186–189. [Google Scholar] [CrossRef]
- Elesgaray, R.; Caniffi, C.; Ierace, D.R.; Jaime, M.F.; Fellet, A.; Arranz, C.; Costa, M.A. Signaling cascade that mediates endothelial nitric oxide synthase activation induced by atrial natriuretic peptide. Regul. Pept. 2008, 151, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Elesgaray, R.; Balaszczuk, A.M.; Arranz, C. Role of NPR-C natriuretic receptor in nitric oxide system activation induced by atrial natriuretic peptide. Regul. Pept. 2006, 135, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Theilig, F.; Wu, Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal Physiol. 2015, 308, F1047–F1055. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, A.; Della Bruna, R.; Pfeilschifter, J.; Taugner, R.; Bauer, C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc. Natl. Acad. Sci. USA 1986, 83, 4769–4773. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Ballermann, B.J.; Gunning, M.E.; Zeidel, M.L. Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 1990, 70, 665–699. [Google Scholar] [CrossRef]
- Woods, R.L. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: A brief review. Clin. Exp. Pharmacol. Physiol. 2004, 31, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Schreiber, C.A.; Nini, R.; Tonne, J.M.; Peng, K.W.; Geurts, A.; Jacob, H.J.; Burnett, J.C.; Cataliotti, A.; Ikeda, Y. B-type natriuretic peptide deletion leads to progressive hypertension, associated organ damage, and reduced survival: Novel model for human hypertension. Hypertension. 2015, 66, 199–210. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Cuocolo, A.; Vecchione, F.; Mele, A.F.; Condorelli, M.; Trimarco, B. Vagal mediation of the effects of atrial natriuretic factor on blood pressure and arterial baroreflexes in the rabbit. Circ. Res. 1987, 60, 747–755. [Google Scholar] [CrossRef]
- Van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Ahluwalia, A.; MacAllister, R.J.; Hobbs, A.J. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res. Cardiol. 2004, 99, 83–89. [Google Scholar] [CrossRef]
- Rubattu, S.; Sciarretta, S.; Morriello, A.; Calvieri, C.; Battistoni, A.; Volpe, M. NPR-C: A component of the natriuretic peptide family with implications in human diseases. J. Mol. Med. (Berl.) 2010, 88, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Nussenzveig, D.; Lewicki, J.; Maack, T. Cellular mechanisms of the clearance function of Type C receptors of atrial natriuretic factor. J. Biol. Chem. 1990, 265, 20952–20958. [Google Scholar] [PubMed]
- Mangiafico, S.; Costello-Boerrigter, L.C.; Andersen, I.A.; Cataliotti, A.; Burnett, J.C., Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: An evolving strategy in cardiovascular therapeutics. Eur. Heart J. 2013, 34, 886c–893c. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Pankow, K.; Schwiebs, A.; Becker, M.; Siems, W.E.; Krause, G.; Walther, T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J. Mol. Biol. 2009, 393, 496–503. [Google Scholar] [CrossRef]
- Palmer, S.C.; Yandle, T.G.; Nicholls, M.G.; Frampton, C.M.; Richards, A.M. Regional clearance of amino-terminal pro-brain natriuretic peptide from human plasma. Eur. J. Heart Fail. 2009, 11, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.C.; Yandle, T.G.; Nicholls, M.G.; Frampton, C.M.; Richards, A.M. Neprilysin inhibitors potentiate effects of bradykinin on B2 receptor. Hypertension 2002, 39, 619–623. [Google Scholar]
- Kozlovski, V.I.; Lomnicka, M.; Jakubowski, A.; Chlopicki, S. Inhibition of neutral endopeptidase by thiorphan does not modify coronary vascular responses to angiotensin I, angiotensin II and bradykinin in the isolated guinea pig heart. Pharmacol. Rep. 2007, 59, 421–427. [Google Scholar] [PubMed]
- Dalzell, J.R.; Seed, A.; Berry, C.; Whelan, C.J.; Petrie, M.C.; Padmanabhan, N.; Clarke, A.; Biggerstaff, F.; Hillier, C.; McMurray, J.J. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: Studies with thiorphan and omapatrilat. Cardiovasc. Ther. 2014, 32, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.B.; Touzin, K.; Sile, S.; Gainer, J.V.; Yu, C.; Nadeau, J.; Adam, A.; Brown, N.J. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor-associated angioedema. Hypertension 2008, 51, 141–147. [Google Scholar] [CrossRef]
- Nakamura, M.; Yoshida, H.; Hiramori, K. Comparison of vasodilator potency of adrenomedulling and proadrenomedullin N-terminal 20 peptide in human. Life Sci. 1999, 65, 2151–2156. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; McEniery, C.M.; Bongaerts, K.H.; MacCallum, H.; Webb, D.J.; Cockcroft, J.R. Adrenomedullin (ADM) in the human forearm vascular bed: Effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP). Br. J. Clin. Pharmacol. 2001, 52, 159–164. [Google Scholar] [CrossRef]
- Pinheiro, S.V.; Simones, E.; Silva, A.C. Angiotensin converting enzyme 2, angiotensin-(1–7), and receptor MAS axis in the kidney. Int. J. Hypertens. 2012, 22, 224–233. [Google Scholar] [CrossRef]
- Newby, D.E.; McDonagh, T.; Currie, P.F.; Northridge, D.B.; Boon, N.A.; Dargie, H.J. Candoxatril improves exercise capacity in patients with chronic heart failure receiving angiotensin converting enzyme inhibition. Eur. Heart J. 1998, 19, 1808–1813. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, S.L.; Kenny, A.J. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem. J. 1987, 241, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Ferro, C.J.; Spratt, J.C.; Haynes, W.G.; Webb, D.J. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation 1998, 97, 2323–2330. [Google Scholar] [CrossRef]
- Kawanabe, Y.; Nauli, S.M. Endothelin. Cell. Mol. Life Sci. 2011, 68, 195–203. [Google Scholar] [CrossRef]
- Shubeita, H.E.; McDonough, P.M.; Harris, A.N.; Knowlton, K.U.; Glembotski, C.C.; Brown, J.H.; Chien, K.R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricularmyocytes: A paracrine mechanism for myocardial cell hypertrophy. J. Biol. Chem. 1990, 265, 20555–20562. [Google Scholar] [PubMed]
- Rubattu, S.; Calvieri, C.; Pagliaro, B.; Volpe, M. Atrial natriuretic peptide and regulation of vascular function in hypertension and heart failure: Implications for novel therapeutic strategies. J. Hypertens. 2013, 31, 1061–1072. [Google Scholar] [CrossRef]
- McDowell, G.; Coutie, W.; Shaw, C.; Buchanan, K.D.; Struthers, A.D.; Nicholls, D.P. The Effect of the Neutral Endopeptidase Inhibitor Drug, Candoxatril, on Circulating Levels of Two of the Most Potent Vasoactive Peptides. Br. J. Clin. Pharmacol. 1997, 43, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Califf, R.M.; Konstam, M.A.; Krum, H.; McMurray, J.J.; Rouleau, J.L.; Swedberg, K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002, 106, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Tognoni, G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Nougué, H.; Pezel, T.; Picard, F.; Sadoune, M.; Arrigo, M.; Beauvais, F.; Launay, J.M.; Cohen-Solal, A.; Vodovar, N.; Logeart, D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: A mechanistic clinical study. Eur. J. Heart Fail. 2018. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. For the PARADIGM-HF investigators and coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Vicent, L.; Cinca, J.; Vazquez-García, R.; Gonzalez-Juanatey, J.R.; Rivera, M.; Segovia, J.; Pascual-Figal, D.; Bover, R.; Worner, F.; Delgado-Jiménez, J.; et al. Discharge treatment with ACE inhibitor/ARB after a heart failure hospitalization is associated with a better prognosis irrespectively of left ventricular ejection fraction. Intern. Med. J. 2019. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Morrow, D.A.; DeVore, A.D.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Senni, M.; McMurray, J.J.V.; Wachter, R.; McIntyre, H.F.; Anand, I.S.; Duino, V.; Sarkar, A.; Shi, V.; Charney, A. Impact of systolic blood pressure on the safety and tolerability of initiating and up-titrating sacubitril/valsartan in patients with heart failure and reduced ejection fraction: Insights from the TITRATION study. Eur. J. Heart Fail. 2018, 20, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Jhund, P.S.; Baicu, C.F.; Claggett, B.L.; Pieske, B.; Voors, A.A.; Prescott, M.F.; Shi, V.; Lefkowitz, M.; McMurray, J.J.; et al. Prospective comparison of ARNi with ARB on management of heart failure with preserved ejection fraction (PARAMOUNT) investigators. Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: Data from the prospective comparison of ARNi with ARB on management of heart failure with preserved ejection fraction study. Circ. Heart Fail. 2016, 9, e002551. [Google Scholar] [PubMed]
- Williams, B.; Cockcroft, J.R.; Kario, K.; Zappe, D.H.; Brunel, P.C.; Wang, Q.; Guo, W. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: The PARAMETER study. Hypertension 2017, 69, 411–420. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Preiss, D.; Jhund, P.S.; Squire, I.; Cardoso, J.S.; Merkely, B.; Martinez, F.; Starling, R.C.; Desai, A.S.; Lefkowitz, M. Risk Related to Pre-Diabetes Mellitus and Diabetes Mellitus in Heart Failure With Reduced Ejection Fraction: Insights From Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ. Heart Fail. 2016, 9, e002560. [Google Scholar] [CrossRef]
- Suematsu, Y.; Miura, S.; Goto, M.; Matsuo, Y.; Arimura, T.; Kuwano, T.; Imaizumi, S.; Iwata, A.; Yahiro, E.; Saku, K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur. J. Heart Fail. 2016, 18, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roksnoer, L.C.; van Veghel, R.; de Vries, R.; Garrelds, I.M.; Bhaggoe, U.M.; Friesema, E.C.; Leijten, F.P.; Poglitsch, M.; Domenig, O.; Clahsen-van Groningen, M.C.; et al. Optimum AT1 receptor-neprilysin inhibition has superior cardioprotective effects compared with AT1 receptor blockade alone in hypertensive rats. Kidney Int. 2015, 88, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Roksnoer, L.C.; van Veghel, R.; van Groningen, M.C.; de Vries, R.; Garrelds, I.M.; Bhaggoe, U.M.; van Gool, J.M.; Friesema, E.C.; Leijten, F.P.; Hoorn, E.; et al. Blood pressure-independent renoprotection in diabetic rats treated with AT1 receptor-neprilysin inhibition versus AT1 receptor blockade alone. Clin. Sci. 2016, 30, 1209–1220. [Google Scholar] [CrossRef]
- Habibi, J.; Aroor, A.R.; Das, N.A.; Manrique-Acevedo, C.M.; Johnson, M.S.; Hayden, M.R.; Nistala, R.; Wiedmeyer, C.; Chandrasekar, B.; DeMarco, V.G.; et al. The combination of a neprilysin inhibitor (sacubitril) and angiotensin‑II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc. Diabetol. 2019, 18, 40. [Google Scholar] [CrossRef]
- Mogensen, U.M.; Køber, L.; Kristensen, S.L.; Jhund, P.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Swedberg, K.; et al. The effects of sacubitril/valsartan on coronary outcomes in PARADIGM-HF. Am. Heart J. 2017, 188, 35–41. [Google Scholar] [CrossRef]
- Maslov, M.Y.; Foianini, S.; Mayer, D.; Orlov, M.V.; Lovich, M.A. Synergy Between Sacubitril and Valsartan 1 Leads to Hemodynamic, Antifibrotic, and Exercise Tolerance Benefits in Rats with Preexisting Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H289–H297. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.R.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef]
- Jordan, J.; Stinkens, R.; Jax, T.; Engeli, S.; Blaak, E.E.; May, M.; Havekes, B.; Schindler, C.; Albrecht, D.; Pal, P.; et al. Improved Insulin Sensitivity with Angiotensin Receptor Neprilysin Inhibition in Individuals with Obesity and Hypertension. Clin. Pharmacol. Ther. 2017, 101, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Leung, N.; Lapointe, N.; Szeto, L.; Uffelman, K.D.; Giacca, A.; Rouleau, J.L.; Lewis, G.F. Vasopeptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats: Studies comparing a vasopeptidase inhibitor, angiotensin-converting enzyme inhibitor, and angiotensin II type I receptor blocker. Circulation 2003, 107, 1923–1929. [Google Scholar] [PubMed]
- Plamboeck, A.; Holst, J.J.; Carr, R.D.; Deacon, C.F. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 2005, 48, 1882–1890. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.P.; Coppey, L.J.; Shevalye, H.; Obrosov, A.; Yorek, M.A. Vascular and Neural Complications in Type 2 Diabetic Rats: Improvement by Sacubitril/Valsartan Greater Than Valsartan Alone. Diabetes 2018, 67, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; D’Elia, E.; Senni, M. Sacubitril/valsartan therapeutic strategy in HFpEF: Clinical insights and perspectives. Int. J. Cardiol. 2019, 281, 158–165. [Google Scholar] [CrossRef]
- Solomon, S.D.; Zile, M.; Pieske, B.; Voors, A.; Shah, A.; Kraigher-Krainer, E.; Shi, V.; Bransford, T.; Takeuchi, M.; Gong, J.; et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet 2012, 380, 1387–1395. [Google Scholar] [CrossRef]
- Jhund, P.S.; Claggett, B.; Packer, M.; Zile, M.R.; Voors, A.A.; Pieske, B.; Lefkowitz, M.; Shi, V.; Bransford, T.; McMurray, J.J.; et al. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: An analysis of the PARAMOUNT trial. Eur. J. Heart Fail. 2014, 16, 671–677. [Google Scholar] [CrossRef]
- Voors, A.A.; Gori, M.; Liu, L.C.; Claggett, B.; Zile, M.R.; Pieske, B.; McMurray, J.J.; Packer, M.; Shi, V.; Lefkowitz, M. For the PARAMOUNT investigators. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2015, 17, 510–517. [Google Scholar] [CrossRef]
- Solomon, S.D.; Rizkala, A.R.; Lefkowitz, M.P.; Shi, V.C.; Gong, J.; Anavekar, N.; Anker, S.D.; Arango, J.L.; Arenas, J.L.; Atar, D.; et al. Baseline Characteristics of Patients with Heart Failure and Preserved Ejection Fraction in the PARAGON-HF Trial. Circ. Heart Fail. 2018, 11, e004962. [Google Scholar] [CrossRef] [PubMed]
- A Randomized, Double-Blind Controlled Study Comparing LCZ696 to Medical Therapy for Comorbidities in HFpEF Patients (PARALLAX). Available online: http://clinicaltrials.gov/ct2/show/NCT03066804 (accessed on 28 December 2018).
- Rubattu, S.; Cotugno, M.; Forte, M. Effects of dual angiotensin type 1 receptor/neprilysin inhibition vs. angiotensin type 1 receptor inhibition on target organ injury in the stroke-prone spontaneously hypertensive rat. J. Hypertens. 2018, 36, 1902–1914. [Google Scholar] [CrossRef]
- Seki, T.; Goto, K.; Kansui, Y.; Ohtsubo, T.; Matsumura, K.; Kitazono, T. Angiotensin II Receptor–Neprilysin Inhibitor Sacubitril/Valsartan Improves Endothelial Dysfunction in Spontaneously Hypertensive Rats. J. Am. Heart Assoc. 2017, 6, e006617. [Google Scholar] [CrossRef]
- Kusaka, H.; Sueta, D. LCZ696, Angiotensin II Receptor-Neprilysin Inhibitor, Ameliorates High-Salt-Induced Hypertension and Cardiovascular Injury More Than Valsartan Alone. Am. J. Hypertens. 2015, 28, 1409–1417. [Google Scholar] [CrossRef] [Green Version]
- Ruilope, L.M.; Dukat, A.; Böhm, M.; Lacourcière, Y.; Gong, J.; Lefkowitz, M.P. Blood pressure reduction with LCZ696, a novel dualacting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebocontrolled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Supasyndh, O.; Wang, J.; Hafeez, K.; Zhang, Y.; Zhang, J.; Rakugi, H. Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared with Olmesartan in Elderly Asian Patients (≥65 Years) With Systolic Hypertension. Am. J. Hypertens. 2017, 30, 1163–1169. [Google Scholar] [CrossRef]
- Novartis. Effects of Sacubitril/Valsartan Therapy on Biomarkers, Myocardial Remodeling and Outcomes. (PROVE-HF). Available online: https://clinicaltrials.gov/ct2/show/NCT02887183 (accessed on 28 December 2018).
- Novartis. Study of Effects of Sacubitril/Valsartan vs. Enalapril on Aortic Stiffness in Patients with Mild to Moderate HF with Reduced Ejection Fraction (EVALUATE-HF). Available online: https://clinicaltrials.gov/ct2/show/NCT02874794 (accessed on 28 December 2018).
- Novartis. Pharmacological Reduction of Functional, Ischemic Mitral Regurgitation (PRIME). Available online: https:// clinicaltrials.gov/ct2/show/NCT02687932 (accessed on 28 December 2018).
- Novartis. Pulmonary artery pressure reduction with Entresto (sacubitril/valsartan) (PARENT). Available online: https:// clinicaltrials.gov/ct2/show/NCT02788656 (accessed on 28 December 2018).
- Novartis. Entresto TM (LCZ696) In Advanced Heart Failure (LIFE Study) (HFN-LIFE). Available online: https://clinicaltrials.gov/ct2/show/NCT02816736 (accessed on 28 December 2018).
- Novartis. Comparing ARNi with ACE inhibitor on endothelial function (PARADOR). Available online: https://clinicaltrials.gov/ct2/show/NCT03119623 (accessed on 28 December 2018).
- Ishii, M.; Kaikita, K.; Sato, K.; Sueta, D.; Fujisue, K.; Arima, Y.; Oimatsu, Y.; Mitsuse, T.; Onoue, Y.; Araki, S.; et al. Cardioprotective effects of LCZ696 (sacubitril/valsartan) after experimental acute myocardial infarction. JACC Basic Transl. Sci. 2017, 2, 655–668. [Google Scholar] [CrossRef]
- Imran, M.; Hassan, M.Q.; Akhtar, M.S.; Rahman, O.; Akhtar, M.; Najmi, A.K. Sacubitril and valsartan protect from experimental myocardial infarction by ameliorating oxidative damage in Wistar rats. Clin. Exp. Hypertens. 2019, 41, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Cain, C.; Mauro, A.G.; Romeo, F.; Ockaili, R.; Chau, V.Q.; Nestler, J.A.; Devarakonda, T.; Ghosh, S.; Das, A.; et al. Sacubitril/Valsartan Averts Adverse Post-Infarction Ventricular Remodeling and Preserves Systolic Function in Rabbits. J. Am. Coll. Cardiol. 2018, 72, 2342–2356. [Google Scholar] [CrossRef]
- Pfau, D.; Thorn, S.L.; Zhang, J.; Mikush, N.; Renaud, J.M.; Klein, R.; deKemp, R.A.; Wu, X.; Hu, X.; Sinusas, A.J.; et al. Angiotensin Receptor Neprilysin Inhibitor Attenuates Myocardial Remodeling and Improves Infarct Perfusion in Experimental Heart Failure. Sci. Rep. 2019, 9, 5791. [Google Scholar] [CrossRef]
- Novartis. Prospective ARNi vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI (PARADISE-MI). Available online: http://clinicaltrials.gov/ct2/show/NCT02924727 (accessed on 28 December 2018).
- Suematsu, Y.; Jing, W.; Nunes, A.; Kashyap, M.L.; Khazaeli, M.; Vaziri, N.D.; Moradi, H. LCZ696 (Sacubitril/valsartan), an Angiotensin-Receptor Neprilysin Inhibitor, Attenuates Cardiac Hypertrophy, Fibrosis and Vasculopathy in a Rat Model of Chronic Kidney Disease. J. Card. Fail. 2018, 24, 266–275. [Google Scholar] [CrossRef] [PubMed]
| Study; Aim | Study Population | Design | Outcomes |
|---|---|---|---|
| PARADIGM-HF (and post hoc analysis) Comparison of efficacy of LCZ696 versus enalapril in patients with HFrEF (LVEF ≤35%) [5,47] | Patients with symptomatic HFrEF (NYHA functional classes II to IV), and elevated B-type natriuretic peptide levels or hospitalization for HF within the previous 12 months; n = 8442 | Multicenter, randomized, double-blind study | LCZ696 reduced the composite primary of CV death or HF hospitalization more than enalapril; LCZ696 reduced secondary endpoint more than enalapril: • any CV death; • first worsening HF hospitalization; • all-cause mortality Moreover, LCZ696 group had fewer hospitalizations for worsening HF, less necessity to receive intensive care, intravenous positive inotropic agents, and to have implantation of a HF device or cardiac transplantation. |
| TRANSITION To assess the safety and tolerability of starting a therapy with LCZ696 while still in the hospital or after discharge [48] | HFrEF patients hospitalized for ADHF, after stabilization n = 1002 | Multicenter, randomized, open-label, parallel-group study | The percentage of patients taking target dose of sacubitril/valsartan 200 mg BID at 10 weeks post randomization was the same among patients who started taking LCZ696 during hospitalization or after discharge |
| PIONEER-HF To assess the percentage change from baseline in NTproBNP levels with LCZ696 [49] | HFrEF patients hospitalized for ADHF after stabilization n = 736 | Multicenter, randomized, double-blind study | LCZ696 led to a reduction in the NTproBNP concentration than a therapy with enalapril at 4 and 8 weeks; LCZ696 led to a reduction in the level of high-sensitivity cardiac troponin T; LCZ696 led to lower rate of rehospitalization for HF |
| TITRATION To assess the tolerability of initiating/uptitrating LCZ696 from 50 to 200 mg BID over 3 and 6 weeks [50] | Patients with symptomatic HFrEF (NYHA functional classes II to IV) + one or more of the following additional eligibility requirements: for outpatients currently treated with ACEi/ARB, the dose must have been stable for at least 2 weeks; to be classified as ACEi/ARB-naïve, the patient must not have taken ACEi/ARB for at least 4weeks; hospitalized patients had to be either ACEi/ARB-naïve, or on a tolerated dose of an ACEi/ARB at screening n = 429 | Multicenter, randomized, double bind, parallel study | Initiation/uptitration of LCZ696 from 50 to 200 mg BID had a tolerability profile in line with other HF treatments. |
| PARAMOUNT To assess the efficacy of LCZ96 versus valsartan to change NTproBNP levels from baseline [51] | Patients with signs and symptoms of HF, ≥40 years, with NTproBNP ≥400 pg/mL and a LVEF ≥45%, while on active diuretic therapy n = 301 | Multicenter, randomized, double-blind study | The decline in NTproBNP at 12 weeks after initiation of the treatment was greater in the LCZ696 group. LCZ969 was also able to ameliorate LA size and NHYA class (secondary endpoints) |
| PARAMETER To assess the efficacy of LCZ696 versus olmesartan in reducing arterial stiffness [52] | Elderly patients (aged ≥60 years) with systolic hypertension and pulse pressure >60 mmHg n = 454 | Multicenter, randomized, double-blind study | LCZ696 reduced central aortic SBP more than olmesartan and reduced mean 24-hour ambulatory brachial and central aortic SBP |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpe, M.; Rubattu, S.; Battistoni, A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 2092. https://doi.org/10.3390/ijms20092092
Volpe M, Rubattu S, Battistoni A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. International Journal of Molecular Sciences. 2019; 20(9):2092. https://doi.org/10.3390/ijms20092092
Chicago/Turabian StyleVolpe, Massimo, Speranza Rubattu, and Allegra Battistoni. 2019. "ARNi: A Novel Approach to Counteract Cardiovascular Diseases" International Journal of Molecular Sciences 20, no. 9: 2092. https://doi.org/10.3390/ijms20092092
APA StyleVolpe, M., Rubattu, S., & Battistoni, A. (2019). ARNi: A Novel Approach to Counteract Cardiovascular Diseases. International Journal of Molecular Sciences, 20(9), 2092. https://doi.org/10.3390/ijms20092092