Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer
Abstract
:1. Introduction
2. Mast Cells and Tumour Biology
3. Mast Cells in Tumour Angiogenesis and Lymphangiogenesis
4. Mast Cells in the Immune Contexture of Cancer
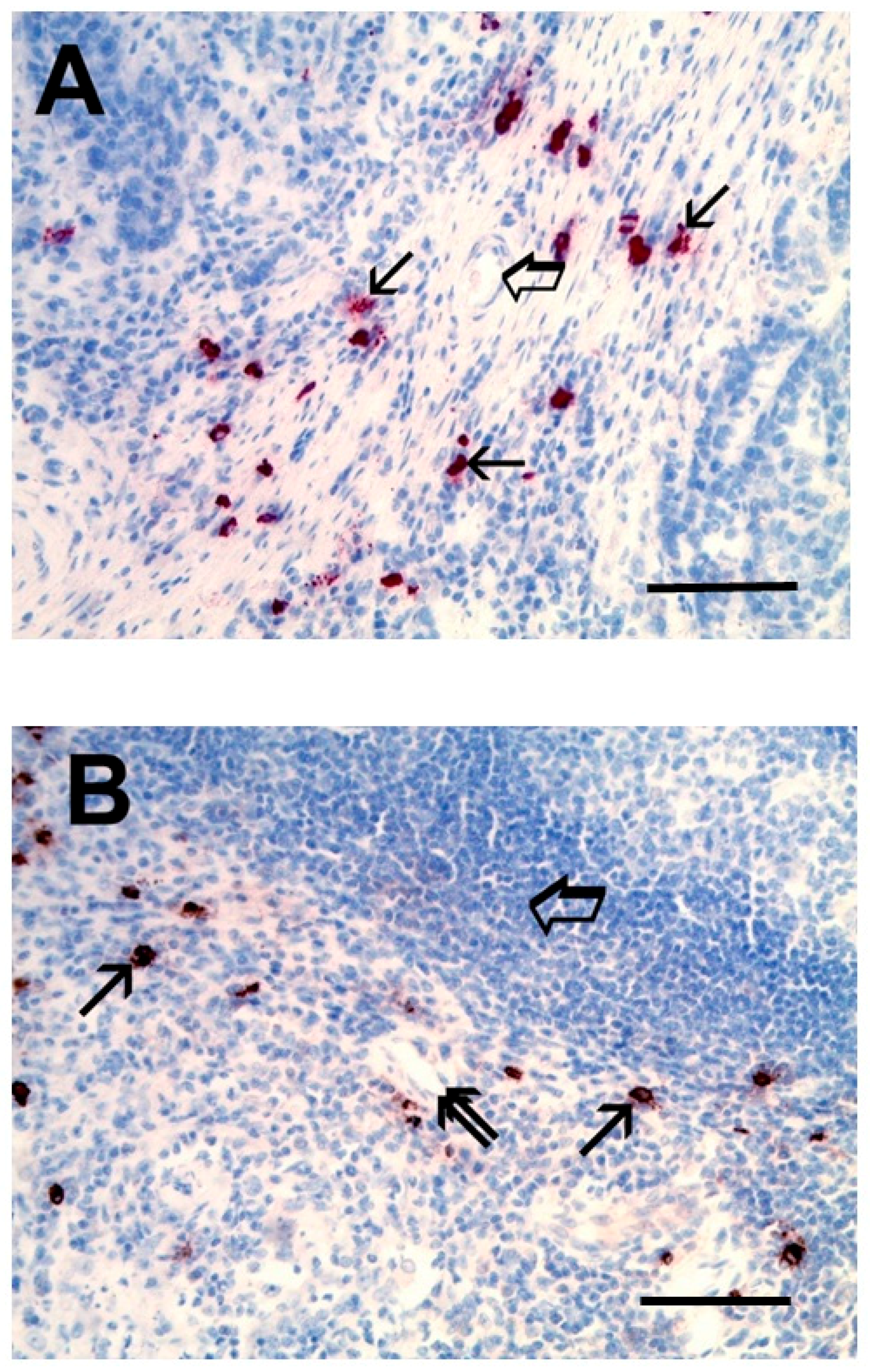
5. Mast Cells in the Immune Contexture of Human Gastric Cancer
6. Outstanding Questions and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANGPT | angiopoietin |
| BEC | blood endothelial cell |
| CD40 | cluster of differentiation 40 protein |
| CD40L | cluster of differentiation 40 ligand |
| COX | cyclooxygenase |
| DC | dendritic cell |
| ECM | extracellular matrix |
| FLC | free light chain |
| ICI | immune checkpoint inhibitor |
| ICOS | inducible costimulator |
| ICOS-L | inducible costimulator ligand |
| IFN | interferon |
| IL | interleukin |
| KIT | stem cell factor receptor |
| LEC | lymphatic endothelial cell |
| mAb | monoclonal antibody |
| MDCS | myeloid-derived suppressor cell |
| MMP | matrix metalloproteinase |
| NK | natural killer cell |
| NKT | natural killer T cell |
| NSCLC | non-small-cell lung cancer |
| PAR | protease-activated receptor |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death ligand 1 |
| PD-L2 | programmed death ligand 2 |
| PGE | prostaglandin E |
| PlGF | placental growth factor |
| PMN | polymorphonuclear leukocyte |
| SCF | stem cell factor |
| SCID | severe combined immunodeficiency |
| TAM | tumour-associated macrophage |
| TAMC | tumour-associated mast cell |
| TAN | tumour-associated neutrophils |
| TDLN | tumour draining lymph node |
| Tfh | T follicular helper cells |
| TIE | Tyrosine kinase with immunoglobulin-like and EGF-like domains |
| TIM-3 | T cell immunoglobulin and mucin domain-containing protein 3 |
| TME | tumour microenvironment |
| TNF | tumour necrosis factor |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Lott, P.C.; Carvajal-Carmona, L.G. Resolving gastric cancer aetiology: An update in genetic predisposition. Lancet Gastroenterol Hepatol. 2018, 3, 874–883. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet. 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Russo, A.E.; Strong, V.E. Gastric Cancer Etiology and Management in Asia and the West. Annu. Rev. Med. 2019, 70, 353–367. [Google Scholar] [CrossRef]
- Shim, J.H.; Song, K.Y.; Jeon, H.M.; Park, C.H.; Jacks, L.M.; Gonen, M.; Shah, M.A.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann. Surg. Oncol. 2014, 21, 2332–2339. [Google Scholar] [CrossRef]
- Gullo, I.; Carneiro, F.; Oliveira, C.; Almeida, G.M. Heterogeneity in Gastric Cancer: From Pure Morphology to Molecular Classifications. Pathobiology 2018, 85, 50–63. [Google Scholar] [CrossRef]
- Gao, J.P.; Xu, W.; Liu, W.T.; Yan, M.; Zhu, Z.G. Tumor heterogeneity of gastric cancer: From the perspective of tumor-initiating cell. World J. Gastroenterol. 2018, 24, 2567–2581. [Google Scholar] [CrossRef] [PubMed]
- Chia, N.Y.; Tan, P. Molecular classification of gastric cancer. Ann. Oncol. 2016, 27, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Cislo, M.; Filip, A.A.; Arnold Offerhaus, G.J.; Cisel, B.; Rawicz-Pruszynski, K.; Skierucha, M.; Polkowski, W.P. Distinct molecular subtypes of gastric cancer: From Lauren to molecular pathology. Oncotarget 2018, 9, 19427–19442. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, N.; Yang, H.H.; Wang, L.; Su, H.; Wang, C.; Clifford, R.; Dawsey, E.M.; Li, J.M.; Ding, T.; et al. Comparison of global gene expression of gastric cardia and noncardia cancers from a high-risk population in china. PLoS ONE 2013, 8, e63826. [Google Scholar] [CrossRef]
- Shah, M.A.; Khanin, R.; Tang, L.; Janjigian, Y.Y.; Klimstra, D.S.; Gerdes, H.; Kelsen, D.P. Molecular classification of gastric cancer: A new paradigm. Clin. Cancer Res. 2011, 17, 2693–2701. [Google Scholar] [CrossRef]
- Tay, S.T.; Leong, S.H.; Yu, K.; Aggarwal, A.; Tan, S.Y.; Lee, C.H.; Wong, K.; Visvanathan, J.; Lim, D.; Wong, W.K.; et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003, 63, 3309–3316. [Google Scholar]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Leung, S.Y.; Yuen, S.T.; Chu, K.M.; Ji, J.; Li, R.; Chan, A.S.; Law, S.; Troyanskaya, O.G.; Wong, J.; et al. Variation in gene expression patterns in human gastric cancers. Mol. Biol. Cell 2003, 14, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.B.; Ivanova, T.; Lim, K.H.; Ong, C.W.; Deng, N.; Lee, J.; Tan, S.H.; Wu, J.; Lee, M.H.; Ooi, C.H.; et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology 2011, 141, 476–485, 485.e1–485.e11. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lim, J.Y.; Cheong, J.H.; Park, Y.Y.; Yoon, S.L.; Kim, S.M.; Kim, S.B.; Kim, H.; Hong, S.W.; Park, Y.N.; et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin. Cancer Res. 2011, 17, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Goh, L.K.; Wang, H.; Das, K.; Tao, J.; Tan, I.B.; Zhang, S.; Lee, M.; Wu, J.; Lim, K.H.; et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012, 61, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, C.; Choi, I.S.; Yao, J.C.; Worah, S.; Xie, K.; Mansfield, P.F.; Ajani, J.A.; Rashid, A.; Hamilton, S.R.; Wu, T.T. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin. Cancer Res. 2005, 11, 656–663. [Google Scholar]
- Liu, Z.; Zhang, J.; Gao, Y.; Pei, L.; Zhou, J.; Gu, L.; Zhang, L.; Zhu, B.; Hattori, N.; Ji, J.; et al. Large-scale characterization of DNA methylation changes in human gastric carcinomas with and without metastasis. Clin. Cancer Res. 2014, 20, 4598–4612. [Google Scholar] [CrossRef] [Green Version]
- Zouridis, H.; Deng, N.; Ivanova, T.; Zhu, Y.; Wong, B.; Huang, D.; Wu, Y.H.; Wu, Y.; Tan, I.B.; Liem, N.; et al. Methylation subtypes and large-scale epigenetic alterations in gastric cancer. Sci. Transl. Med. 2012, 4, 156ra140. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef]
- Kakiuchi, M.; Nishizawa, T.; Ueda, H.; Gotoh, K.; Tanaka, A.; Hayashi, A.; Yamamoto, S.; Tatsuno, K.; Katoh, H.; Watanabe, Y.; et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat. Genet. 2014, 46, 583–587. [Google Scholar] [CrossRef]
- Liu, J.; McCleland, M.; Stawiski, E.W.; Gnad, F.; Mayba, O.; Haverty, P.M.; Durinck, S.; Chen, Y.J.; Klijn, C.; Jhunjhunwala, S.; et al. Integrated exome and transcriptome sequencing reveals ZAK isoform usage in gastric cancer. Nat. Commun. 2014, 5, 3830. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.S.; Kim, K.M.; Ting, J.C.; Yu, K.; Fu, J.; Liu, S.; Cristescu, R.; Nebozhyn, M.; Gong, L.; Yue, Y.G.; et al. Genomic landscape and genetic heterogeneity in gastric adenocarcinoma revealed by whole-genome sequencing. Nat. Commun. 2014, 5, 5477. [Google Scholar] [CrossRef] [Green Version]
- Ooi, C.H.; Ivanova, T.; Wu, J.; Lee, M.; Tan, I.B.; Tao, J.; Ward, L.; Koo, J.H.; Gopalakrishnan, V.; Zhu, Y.; et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009, 5, e1000676. [Google Scholar] [CrossRef]
- Wu, Y.; Grabsch, H.; Ivanova, T.; Tan, I.B.; Murray, J.; Ooi, C.H.; Wright, A.I.; West, N.P.; Hutchins, G.G.; Wu, J.; et al. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut 2013, 62, 1100–1111. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T.; Huntly, B.J. Targeting epigenetic readers in cancer. N. Engl. J. Med. 2012, 367, 647–657. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Andre, F.; Tesniere, A.; Kroemer, G. The anticancer immune response: Indispensable for therapeutic success? J. Clin. Investig. 2008, 118, 1991–2001. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F. Angiogenesis, lymphangiogenesis and clinical implications. Preface. Chem. Immunol. Allergy 2014, 99, XI–XII. [Google Scholar]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell. Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Granata, F.; Borriello, F. Controversial role of mast cells in skin cancers. Exp. Dermatol. 2017, 26, 11–17. [Google Scholar] [CrossRef]
- Mulero, I.; Sepulcre, M.P.; Meseguer, J.; Garcia-Ayala, A.; Mulero, V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 19434–19439. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, P. Beitrage zur Theorie und Praxis der Histologischen Farbung. Master’s Thesis, University of Leipzig, Leipzig, Germany, 1878. [Google Scholar]
- Marone, G.; Galli, S.J.; Kitamura, Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002, 23, 425–427. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human mast cells and basophils-How are they similar how are they different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Kirshenbaum, A.S.; Goff, J.P.; Semere, T.; Foster, B.; Scott, L.M.; Metcalfe, D.D. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+) and expresses aminopeptidase N (CD13). Blood 1999, 94, 2333–2342. [Google Scholar]
- Liu, J.; Fu, T.; Song, F.; Xue, Y.; Xia, C.; Liu, P.; Wang, H.; Zhong, J.; Li, Q.; Chen, J.; et al. Mast Cells Participate in Corneal Development in Mice. Sci Rep. 2015, 5, 17569. [Google Scholar] [CrossRef] [Green Version]
- Kurashima, Y.; Amiya, T.; Fujisawa, K.; Shibata, N.; Suzuki, Y.; Kogure, Y.; Hashimoto, E.; Otsuka, A.; Kabashima, K.; Sato, S.; et al. The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity 2014, 40, 530–541. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016, 778, 146–151. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149. [Google Scholar] [CrossRef]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv. Immunol. 2014, 122, 211–252. [Google Scholar]
- Reid, A.C.; Silver, R.B.; Levi, R. Renin: At the heart of the mast cell. Immunol. Rev. 2007, 217, 123–140. [Google Scholar] [CrossRef]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Le Gall, S.; Renault, N.; et al. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef] [Green Version]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2017, 34, 351–361. [Google Scholar] [CrossRef]
- Giannou, A.D.; Marazioti, A.; Spella, M.; Kanellakis, N.I.; Apostolopoulou, H.; Psallidas, I.; Prijovich, Z.M.; Vreka, M.; Zazara, D.E.; Lilis, I.; et al. Mast cells mediate malignant pleural effusion formation. J. Clin. Investig. 2015, 125, 2317–2334. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Marone, G. The immune network in thyroid cancer. Oncoimmunology 2016, 5, e1168556. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Oskeritzian, C.A. Mast cell plasticity and sphingosine-1-phosphate in immunity, inflammation and cancer. Mol. Immunol. 2015, 63, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Rigoni, A.; Colombo, M.P.; Pucillo, C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin. Immunol. 2018, 35, 29–34. [Google Scholar] [CrossRef]
- Jarido, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Stephenson, K.; Francis, H. The emerging role of mast cells in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G89–G101. [Google Scholar] [CrossRef]
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef]
- Jimenez-Andrade, G.Y.; Ibarra-Sanchez, A.; Gonzalez, D.; Lamas, M.; Gonzalez-Espinosa, C. Immunoglobulin E induces VEGF production in mast cells and potentiates their pro-tumorigenic actions through a Fyn kinase-dependent mechanism. J. Hematol. Oncol. 2013, 6, 56. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Genovese, A.; Borgia, G.; Bjorck, L.; Petraroli, A.; de Paulis, A.; Piazza, M.; Marone, G. Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J. Immunol. 2003, 170, 1854–1861. [Google Scholar] [CrossRef]
- Marone, G.; Rossi, F.W.; Detoraki, A.; Granata, F.; Genovese, A.; Spadaro, G. Role of superallergens in allergic disorders. Chem. Immunol. Allergy. 2007, 93, 195–213. [Google Scholar]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010, 17, 121–134. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Powe, D.G.; Kuijpers, S.A.; Abudukelimu, A.; Fens, M.H.; Pieters, E.H.; Kassing van der Ven, W.W.; Habashy, H.O.; Ellis, I.O.; Blokhuis, B.R.; et al. Immunoglobulin free light chains are biomarkers of poor prognosis in basal-like breast cancer and are potential targets in tumor-associated inflammation. Oncotarget 2014, 5, 3159–3167. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Lei, Z.; Zhang, G.M.; Li, D.; Song, C.; Li, B.; Liu, Y.; Yuan, Y.; Unkeless, J.; Xiong, H.; et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood 2008, 112, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Oldford, S.A.; Haidl, I.D.; Howatt, M.A.; Leiva, C.A.; Johnston, B.; Marshall, J.S. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 2010, 185, 7067–7076. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Varricchi, G.; Loffredo, S.; Galdiero, M.R.; Marone, G.; Cristinziano, L.; Granata, F. Innate effector cells in angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 152–160. [Google Scholar] [CrossRef]
- Borriello, F.; Granata, F.; Varricchi, G.; Genovese, A.; Triggiani, M.; Marone, G. Immunopharmacological modulation of mast cells. Curr. Opin. Pharmacol. 2014, 17, 45–57. [Google Scholar] [CrossRef]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological roles of mast cells. Int. Arch. Allergy Immunol. 2019, in press. [Google Scholar] [CrossRef]
- Westphal, E. Uber Mastzellen. In Farbenanalytische Untersuchungen; Ehrlich, P., Ed.; Hirschwald: Berlin, Germany, 1891; pp. 17–41. [Google Scholar]
- Dvorak, A.M.; Mihm, M.C., Jr.; Osage, J.E.; Dvorak, H.F. Melanoma. An ultrastructural study of the host inflammatory and vascular responses. J. Investig. Dermatol. 1980, 75, 388–393. [Google Scholar] [CrossRef]
- Takahashi, K.; Mulliken, J.B.; Kozakewich, H.P.; Rogers, R.A.; Folkman, J.; Ezekowitz, R.A. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J. Clin. Investig. 1994, 93, 2357–2364. [Google Scholar] [CrossRef]
- Toth-Jakatics, R.; Jimi, S.; Takebayashi, S.; Kawamoto, N. Cutaneous malignant melanoma: Correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol. 2000, 31, 955–960. [Google Scholar]
- Aoki, M.; Pawankar, R.; Niimi, Y.; Kawana, S. Mast cells in basal cell carcinoma express VEGF, IL-8 and RANTES. Int. Arch. Allergy Immunol. 2003, 130, 216–223. [Google Scholar] [CrossRef]
- Ribatti, D.; Ennas, M.G.; Vacca, A.; Ferreli, F.; Nico, B.; Orru, S.; Sirigu, P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur. J. Clin. Investig. 2003, 33, 420–425. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Ria, R.; Marzullo, A.; Nico, B.; Filotico, R.; Roncali, L.; Dammacco, F. Neovascularisation, expression of fibroblast growth factor-2 and mast cells with tryptase activity increase simultaneously with pathological progression in human malignant melanoma. Eur. J. Cancer 2003, 39, 666–674. [Google Scholar] [CrossRef]
- Beer, T.W.; Ng, L.B.; Murray, K. Mast cells have prognostic value in Merkel cell carcinoma. Am. J. Dermatopathol. 2008, 30, 27–30. [Google Scholar] [CrossRef]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikstrom, P.; et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef]
- Ng, L.; Beer, T.W.; Murray, K. Vascular density has prognostic value in Merkel cell carcinoma. Am. J. Dermatopathol. 2008, 30, 442–445. [Google Scholar] [CrossRef]
- Ma, Y.; Hwang, R.F.; Logsdon, C.D.; Ullrich, S.E. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013, 73, 3927–3937. [Google Scholar] [CrossRef] [Green Version]
- Melillo, R.M.; Guarino, V.; Avilla, E.; Galdiero, M.R.; Liotti, F.; Prevete, N.; Rossi, F.W.; Basolo, F.; Ugolini, C.; de Paulis, A.; et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene 2010, 29, 6203–6215. [Google Scholar] [CrossRef] [Green Version]
- Pittoni, P.; Tripodo, C.; Piconese, S.; Mauri, G.; Parenza, M.; Rigoni, A.; Sangaletti, S.; Colombo, M.P. Mast cell targeting hampers prostate adenocarcinoma development but promotes the occurrence of highly malignant neuroendocrine cancers. Cancer Res. 2011, 71, 5987–5997. [Google Scholar] [CrossRef]
- Johnson, C.; Huynh, V.; Hargrove, L.; Kennedy, L.; Graf-Eaton, A.; Owens, J.; Trzeciakowski, J.P.; Hodges, K.; DeMorrow, S.; Han, Y.; et al. Inhibition of Mast Cell-Derived Histamine Decreases Human Cholangiocarcinoma Growth and Differentiation via c-Kit/Stem Cell Factor-Dependent Signaling. Am. J. Pathol. 2016, 186, 123–133. [Google Scholar] [CrossRef]
- Siiskonen, H.; Poukka, M.; Bykachev, A.; Tyynela-Korhonen, K.; Sironen, R.; Pasonen-Seppanen, S.; Harvima, I.T. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. 2015, 25, 479–485. [Google Scholar] [CrossRef]
- Acikalin, M.F.; Oner, U.; Topcu, I.; Yasar, B.; Kiper, H.; Colak, E. Tumour angiogenesis and mast cell density in the prognostic assessment of colorectal carcinomas. Dig. Liver Dis. 2005, 37, 162–169. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schlomm, T.; Kollermann, J.; Sekulic, N.; Huland, H.; Mirlacher, M.; Sauter, G.; Simon, R.; Erbersdobler, A. Immunological microenvironment in prostate cancer: High mast cell densities are associated with favorable tumor characteristics and good prognosis. Prostate 2009, 69, 976–981. [Google Scholar] [CrossRef]
- Andersen, M.D.; Kamper, P.; Nielsen, P.S.; Bendix, K.; Riber-Hansen, R.; Steiniche, T.; Hamilton-Dutoit, S.; Clausen, M.; d’Amore, F. Tumour-associated mast cells in classical Hodgkin’s lymphoma: Correlation with histological subtype, other tumour-infiltrating inflammatory cell subsets and outcome. Eur. J. Haematol. 2016, 96, 252–259. [Google Scholar] [CrossRef]
- Englund, A.; Molin, D.; Enblad, G.; Karlen, J.; Glimelius, I.; Ljungman, G.; Amini, R.M. The role of tumour-infiltrating eosinophils, mast cells and macrophages in Classical and Nodular Lymphocyte Predominant Hodgkin Lymphoma in children. Eur. J. Haematol. 2016, 97, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Franco, G.; Guarnotta, C.; Frossi, B.; Piccaluga, P.P.; Boveri, E.; Gulino, A.; Fuligni, F.; Rigoni, A.; Porcasi, R.; Buffa, S.; et al. Bone marrow stroma CD40 expression correlates with inflammatory mast cell infiltration and disease progression in splenic marginal zone lymphoma. Blood 2014, 123, 1836–1849. [Google Scholar] [CrossRef] [Green Version]
- Molin, D.; Edstrom, A.; Glimelius, I.; Glimelius, B.; Nilsson, G.; Sundstrom, C.; Enblad, G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br. J. Haematol. 2002, 119, 122–124. [Google Scholar] [CrossRef]
- Rabenhorst, A.; Schlaak, M.; Heukamp, L.C.; Forster, A.; Theurich, S.; von Bergwelt-Baildon, M.; Buttner, R.; Kurschat, P.; Mauch, C.; Roers, A.; et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood 2012, 120, 2042–2054. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Vacca, A.; Nico, B.; Quondamatteo, F.; Ria, R.; Minischetti, M.; Marzullo, A.; Herken, R.; Roncali, L.; Dammacco, F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br. J. Cancer 1999, 79, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Taskinen, M.; Karjalainen-Lindsberg, M.L.; Leppa, S. Prognostic influence of tumor-infiltrating mast cells in patients with follicular lymphoma treated with rituximab and CHOP. Blood 2008, 111, 4664–4667. [Google Scholar] [CrossRef] [Green Version]
- Tripodo, C.; Gri, G.; Piccaluga, P.P.; Frossi, B.; Guarnotta, C.; Piconese, S.; Franco, G.; Vetri, V.; Pucillo, C.E.; Florena, A.M.; et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am. J. Pathol. 2010, 177, 792–802. [Google Scholar] [CrossRef]
- Vyzoukaki, R.; Tsirakis, G.; Pappa, C.A.; Devetzoglou, M.; Tzardi, M.; Alexandrakis, M.G. The Impact of Mast Cell Density on the Progression of Bone Disease in Multiple Myeloma Patients. Int. Arch. Allergy Immunol. 2015, 168, 263–268. [Google Scholar] [CrossRef]
- Marichal, T.; Tsai, M.; Galli, S.J. Mast cells: Potential positive and negative roles in tumor biology. Cancer Immunol. Res. 2013, 1, 269–279. [Google Scholar] [CrossRef]
- Antsiferova, M.; Martin, C.; Huber, M.; Feyerabend, T.B.; Forster, A.; Hartmann, K.; Rodewald, H.R.; Hohl, D.; Werner, S. Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. J. Immunol. 2013, 191, 6147–6155. [Google Scholar] [CrossRef]
- Xia, Q.; Wu, X.J.; Zhou, Q.; Jing, Z.; Hou, J.H.; Pan, Z.Z.; Zhang, X.S. No relationship between the distribution of mast cells and the survival of stage IIIB colon cancer patients. J. Transl. Med. 2011, 9, 88. [Google Scholar] [CrossRef]
- Dundar, E.; Oner, U.; Peker, B.C.; Metintas, M.; Isiksoy, S.; Ak, G. The significance and relationship between mast cells and tumour angiogenesis in non-small cell lung carcinoma. J. Int. Med. Res. 2008, 36, 88–95. [Google Scholar] [CrossRef]
- Tuna, B.; Yorukoglu, K.; Unlu, M.; Mungan, M.U.; Kirkali, Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur. Urol. 2006, 50, 530–534. [Google Scholar] [CrossRef]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajenoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Xu, J.; Zhang, X.; Han, D.; Liu, J.; Xia, M.; Yi, L.; Shen, Q.; Xu, S.; et al. Adult Connective Tissue-Resident Mast Cells Originate from Late Erythro-Myeloid Progenitors. Immunity 2018, 49, 640–653.e5. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Chevrier, S.; Levine, J.H.; Zanotelli, V.R.T.; Silina, K.; Schulz, D.; Bacac, M.; Ries, C.H.; Ailles, L.; Jewett, M.A.S.; Moch, H.; et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell 2017, 169, 736–749.e18. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef]
- Jaillon, S.; Galdiero, M.R.; Del Prete, D.; Cassatella, M.A.; Garlanda, C.; Mantovani, A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Varricchi, G.; Loffredo, S.; Mantovani, A.; Marone, G. Roles of neutrophils in cancer growth and progression. J. Leukoc. Biol. 2018, 103, 457–464. [Google Scholar] [CrossRef]
- Yamamoto, T.; Katayama, I.; Nishioka, K. Expression of stem cell factor in basal cell carcinoma. Br. J. Dermatol. 1997, 137, 709–713. [Google Scholar] [CrossRef]
- Prevete, N.; Staiano, R.I.; Granata, F.; Detoraki, A.; Necchi, V.; Ricci, V.; Triggiani, M.; De Paulis, A.; Marone, G.; Genovese, A. Expression and function of Angiopoietins and their tie receptors in human basophils and mast cells. J. Biol. Regul. Homeost. Agents 2013, 27, 827–839. [Google Scholar]
- Visciano, C.; Liotti, F.; Prevete, N.; Cali, G.; Franco, R.; Collina, F.; de Paulis, A.; Marone, G.; Santoro, M.; Melillo, R.M. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene 2015, 34, 5175–5186. [Google Scholar] [CrossRef]
- Fischer, M.; Juremalm, M.; Olsson, N.; Backlin, C.; Sundstrom, C.; Nilsson, K.; Enblad, G.; Nilsson, G. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int. J. Cancer 2003, 107, 197–201. [Google Scholar] [CrossRef] [Green Version]
- Kryczek, I.; Lange, A.; Mottram, P.; Alvarez, X.; Cheng, P.; Hogan, M.; Moons, L.; Wei, S.; Zou, L.; Machelon, V.; et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005, 65, 465–472. [Google Scholar]
- Romagnani, P.; De Paulis, A.; Beltrame, C.; Annunziato, F.; Dente, V.; Maggi, E.; Romagnani, S.; Marone, G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am. J. Pathol. 1999, 155, 1195–1204. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Lv, J.Q.; Lin, Y.; Xiang, M.; Gao, B.H.; Shi, Y.F. Expression of chemokines CCL5 and CCL11 by smooth muscle tumor cells of the uterus and its possible role in the recruitment of mast cells. Gynecol. Oncol. 2007, 105, 650–656. [Google Scholar] [CrossRef]
- Polajeva, J.; Sjosten, A.M.; Lager, N.; Kastemar, M.; Waern, I.; Alafuzoff, I.; Smits, A.; Westermark, B.; Pejler, G.; Uhrbom, L.; et al. Mast cell accumulation in glioblastoma with a potential role for stem cell factor and chemokine CXCL12. PLoS ONE 2011, 6, e25222. [Google Scholar] [CrossRef]
- Lin, T.J.; Issekutz, T.B.; Marshall, J.S. SDF-1 induces IL-8 production and transendothelial migration of human cord blood-derived mast cells. Int. Arch. Allergy Immunol. 2001, 124, 142–145. [Google Scholar] [CrossRef]
- Juremalm, M.; Hjertson, M.; Olsson, N.; Harvima, I.; Nilsson, K.; Nilsson, G. The chemokine receptor CXCR4 is expressed within the mast cell lineage and its ligand stromal cell-derived factor-1alpha acts as a mast cell chemotaxin. Eur. J. Immunol. 2000, 30, 3614–3622. [Google Scholar] [CrossRef]
- Godot, V.; Arock, M.; Garcia, G.; Capel, F.; Flys, C.; Dy, M.; Emilie, D.; Humbert, M. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J. Allergy Clin. Immunol. 2007, 120, 827–834. [Google Scholar] [CrossRef]
- Weller, C.L.; Collington, S.J.; Hartnell, A.; Conroy, D.M.; Kaise, T.; Barker, J.E.; Wilson, M.S.; Taylor, G.W.; Jose, P.J.; Williams, T.J. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Natl. Acad. Sci. USA 2007, 104, 11712–11717. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, A.; Matsue, H.; Matsushima, H.; Aoki, R.; Nakamura, Y.; Kambe, N.; Kon, S.; Uede, T.; Shimada, S. Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells. Eur. J. Immunol. 2008, 38, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.L.; Gruber, H.E.; Wasserman, S.I. Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. USA 1984, 81, 6192–6196. [Google Scholar] [CrossRef]
- Gottfried, E.; Kreutz, M.; Mackensen, A. Tumor metabolism as modulator of immune response and tumor progression. Semin. Cancer Biol. 2012, 22, 335–341. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef]
- Granata, F.; Frattini, A.; Loffredo, S.; Staiano, R.I.; Petraroli, A.; Ribatti, D.; Oslund, R.; Gelb, M.H.; Lambeau, G.; Marone, G.; et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J. Immunol. 2010, 184, 5232–5241. [Google Scholar] [CrossRef]
- Visciano, C.; Prevete, N.; Liotti, F.; Marone, G. Tumor-Associated Mast Cells in Thyroid Cancer. Int. J. Endocrinol. 2015, 2015, 705169. [Google Scholar] [CrossRef]
- Gulliksson, M.; Carvalho, R.F.; Ulleras, E.; Nilsson, G. Mast cell survival and mediator secretion in response to hypoxia. PLoS ONE 2010, 5, e12360. [Google Scholar] [CrossRef]
- Walczak-Drzewiecka, A.; Ratajewski, M.; Wagner, W.; Dastych, J. HIF-1alpha is up-regulated in activated mast cells by a process that involves calcineurin and NFAT. J. Immunol. 2008, 181, 1665–1672. [Google Scholar] [CrossRef]
- Redegeld, F.A.; van der Heijden, M.W.; Kool, M.; Heijdra, B.M.; Garssen, J.; Kraneveld, A.D.; Van Loveren, H.; Roholl, P.; Saito, T.; Verbeek, J.S.; et al. Immunoglobulin-free light chains elicit immediate hypersensitivity-like responses. Nat. Med. 2002, 8, 694–701. [Google Scholar] [CrossRef]
- Thio, M.; Groot Kormelink, T.; Fischer, M.J.; Blokhuis, B.R.; Nijkamp, F.P.; Redegeld, F.A. Antigen binding characteristics of immunoglobulin free light chains: Crosslinking by antigen is essential to induce allergic inflammation. PLoS ONE 2012, 7, e40986. [Google Scholar] [CrossRef]
- Lv, Y.P.; Peng, L.S.; Wang, Q.H.; Chen, N.; Teng, Y.S.; Wang, T.T.; Mao, F.Y.; Zhang, J.Y.; Cheng, P.; Liu, Y.G.; et al. Degranulation of mast cells induced by gastric cancer-derived adrenomedullin prompts gastric cancer progression. Cell Death Dis. 2018, 9, 1034. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, B.; Li, D.; Lv, M.; Huang, C.; Shen, G.X.; Huang, B. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE 2010, 5, e8922. [Google Scholar] [CrossRef]
- Cheon, E.C.; Khazaie, K.; Khan, M.W.; Strouch, M.J.; Krantz, S.B.; Phillips, J.; Blatner, N.R.; Hix, L.M.; Zhang, M.; Dennis, K.L.; et al. Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice. Cancer Res. 2011, 71, 1627–1636. [Google Scholar] [CrossRef]
- Danelli, L.; Frossi, B.; Gri, G.; Mion, F.; Guarnotta, C.; Bongiovanni, L.; Tripodo, C.; Mariuzzi, L.; Marzinotto, S.; Rigoni, A.; et al. Mast cells boost myeloid-derived suppressor cell activity and contribute to the development of tumor-favoring microenvironment. Cancer Immunol. Res. 2015, 3, 85–95. [Google Scholar] [CrossRef]
- Saleem, S.J.; Martin, R.K.; Morales, J.K.; Sturgill, J.L.; Gibb, D.R.; Graham, L.; Bear, H.D.; Manjili, M.H.; Ryan, J.J.; Conrad, D.H. Cutting edge: Mast cells critically augment myeloid-derived suppressor cell activity. J. Immunol. 2012, 189, 511–515. [Google Scholar] [CrossRef]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, C.; Lee, J.Y.; Kim, S.; Kwon, P.J.; Kim, W.; Jeon, Y.H.; Lee, E.; Yoon, Y.S. Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci Rep. 2015, 5, 11019. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kataru, R.P.; Koh, G.Y. Inflammation-associated lymphangiogenesis: A double-edged sword? J. Clin. Investig. 2014, 124, 936–942. [Google Scholar] [CrossRef]
- Rivera, L.B.; Bergers, G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015, 36, 240–249. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef]
- Zachary, I. Neuropilins: Role in signalling, angiogenesis and disease. Chem. Immunol. Allergy 2014, 99, 37–70. [Google Scholar]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Tammela, T.; Saaristo, A.; Lohela, M.; Morisada, T.; Tornberg, J.; Norrmen, C.; Oike, Y.; Pajusola, K.; Thurston, G.; Suda, T.; et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005, 105, 4642–4648. [Google Scholar] [CrossRef] [Green Version]
- Karaman, S.; Leppanen, V.M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef]
- Heinolainen, K.; Karaman, S.; D’Amico, G.; Tammela, T.; Sormunen, R.; Eklund, L.; Alitalo, K.; Zarkada, G. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ. Res. 2017, 120, 1414–1425. [Google Scholar] [CrossRef]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front. Immunol. 2017, 8, 443. [Google Scholar] [CrossRef]
- Barleon, B.; Sozzani, S.; Zhou, D.; Weich, H.A.; Mantovani, A.; Marme, D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996, 87, 3336–3343. [Google Scholar]
- de Paulis, A.; Prevete, N.; Fiorentino, I.; Rossi, F.W.; Staibano, S.; Montuori, N.; Ragno, P.; Longobardi, A.; Liccardo, B.; Genovese, A.; et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J. Immunol. 2006, 177, 7322–7331. [Google Scholar] [CrossRef]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef]
- Bry, M.; Kivela, R.; Leppanen, V.M.; Alitalo, K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 2014, 94, 779–794. [Google Scholar] [CrossRef]
- Clauss, M.; Weich, H.; Breier, G.; Knies, U.; Rockl, W.; Waltenberger, J.; Risau, W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996, 271, 17629–17634. [Google Scholar] [CrossRef]
- Eklund, L.; Kangas, J.; Saharinen, P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. 2017, 131, 87–103. [Google Scholar] [CrossRef]
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280. [Google Scholar] [CrossRef]
- Bosisio, D.; Ronca, R.; Salvi, V.; Presta, M.; Sozzani, S. Dendritic cells in inflammatory angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 180–186. [Google Scholar] [CrossRef]
- Bosisio, D.; Salvi, V.; Gagliostro, V.; Sozzani, S. Angiogenic and antiangiogenic chemokines. Chem. Immunol. Allergy 2014, 99, 89–104. [Google Scholar]
- Varricchi, G.; Granata, F.; Loffredo, S.; Genovese, A.; Marone, G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J. Am. Acad. Dermatol. 2015, 73, 144–153. [Google Scholar] [CrossRef]
- Boesiger, J.; Tsai, M.; Maurer, M.; Yamaguchi, M.; Brown, L.F.; Claffey, K.P.; Dvorak, H.F.; Galli, S.J. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J. Exp. Med. 1998, 188, 1135–1145. [Google Scholar] [CrossRef]
- Abdel-Majid, R.M.; Marshall, J.S. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J. Immunol. 2004, 172, 1227–1236. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef]
- Wroblewski, M.; Bauer, R.; Cubas Cordova, M.; Udonta, F.; Ben-Batalla, I.; Legler, K.; Hauser, C.; Egberts, J.; Janning, M.; Velthaus, J.; et al. Mast cells decrease efficacy of anti-angiogenic therapy by secreting matrix-degrading granzyme B. Nat. Commun. 2017, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hammerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Yu, X.; Dong, Q.; Xu, X.; Li, J.; Xu, Q.; Ma, J.; Zhou, C. Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer. Oncol. Lett. 2018, 16, 3917–3922. [Google Scholar] [CrossRef]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The Pleiotropic Immunomodulatory Functions of IL-33 and Its Implications in Tumor Immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Varricchi, G.; Seaf, M.; Marone, G.; Levi-Schaffer, F. Bidirectional Mast Cell-Eosinophil Interactions in Inflammatory Disorders and Cancer. Front. Med. 2017, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Donato, G.; Zuccala, V.; Russo, E.; Luposella, M.; Vescio, G.; Rizzuto, A.; Patruno, R.; De Sarro, G.; et al. Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology 2013, 85, 111–116. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccala, V.; Romano, R.; Luposella, M.; Patruno, R.; Vallicelli, C.; Verdecchia, G.M.; et al. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol. Res. Pract. 2013, 2013, 703163. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, Y.; Wang, X.; Chen, N.; Mao, F.; Teng, Y.; Wang, T.; Peng, L.; Zhang, J.; Cheng, P.; et al. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-alpha-PD-L1 pathway. J. Immunother. Cancer 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Li, H.; Zhang, H.; Chen, Y.F.; Cao, Y.F.; Li, R.C.; Lin, C.; Wei, Y.C.; Xiang, X.N.; Fang, H.J.; et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2019, 30, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Z.; Ma, Y.; Ji, B.; Wang, H.; Deng, D.; Liu, Y.; Abbruzzese, J.L.; Liu, Y.J.; Logsdon, C.D.; Hwu, P. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2011, 17, 7015–7023. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Menicagli, M.; Funel, N.; Bergmann, F.; Boggi, U.; Mosca, F.; Bevilacqua, G.; Campani, D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J. Clin. Pathol. 2004, 57, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Soucek, L.; Buggy, J.J.; Kortlever, R.; Adimoolam, S.; Monclus, H.A.; Allende, M.T.; Swigart, L.B.; Evan, G.I. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia 2011, 13, 1093–1100. [Google Scholar] [CrossRef]
- Soucek, L.; Lawlor, E.R.; Soto, D.; Shchors, K.; Swigart, L.B.; Evan, G.I. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007, 13, 1211–1218. [Google Scholar] [CrossRef]
- Strouch, M.J.; Cheon, E.C.; Salabat, M.R.; Krantz, S.B.; Gounaris, E.; Melstrom, L.G.; Dangi-Garimella, S.; Wang, E.; Munshi, H.G.; Khazaie, K.; et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin. Cancer Res. 2010, 16, 2257–2265. [Google Scholar] [CrossRef] [Green Version]
- Rao, Q.; Chen, Y.; Yeh, C.R.; Ding, J.; Li, L.; Chang, C.; Yeh, S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERbeta/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 2016, 7, 7842–7855. [Google Scholar] [CrossRef]
- Suzuki, S.; Ichikawa, Y.; Nakagawa, K.; Kumamoto, T.; Mori, R.; Matsuyama, R.; Takeda, K.; Ota, M.; Tanaka, K.; Tamura, T.; et al. High infiltration of mast cells positive to tryptase predicts worse outcome following resection of colorectal liver metastases. BMC Cancer 2015, 15, 840. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: Possible biological-clinical significance. PLoS ONE 2014, 9, e99512. [Google Scholar] [CrossRef]
- Malfettone, A.; Silvestris, N.; Saponaro, C.; Ranieri, G.; Russo, A.; Caruso, S.; Popescu, O.; Simone, G.; Paradiso, A.; Mangia, A. High density of tryptase-positive mast cells in human colorectal cancer: A poor prognostic factor related to protease-activated receptor 2 expression. J. Cell. Mol. Med. 2013, 17, 1025–1037. [Google Scholar] [CrossRef]
- Gulubova, M.; Vlaykova, T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J. Gastroenterol. Hepatol. 2009, 24, 1265–1275. [Google Scholar] [CrossRef]
- Tu, J.F.; Pan, H.Y.; Ying, X.H.; Lou, J.; Ji, J.S.; Zou, H. Mast Cells Comprise the Major of Interleukin 17-Producing Cells and Predict a Poor Prognosis in Hepatocellular Carcinoma. Medicine 2016, 95, e3220. [Google Scholar] [CrossRef] [Green Version]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Piardi, T.; Zuccala, V.; Patruno, R.; Zullo, A.; Zizzo, N.; Nardo, B.; Marech, I.; et al. Mast cells positive to tryptase, endothelial cells positive to protease-activated receptor-2 and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. Oncol. Targets Ther. 2016, 9, 4465–4471. [Google Scholar]
- Ju, M.J.; Qiu, S.J.; Gao, Q.; Fan, J.; Cai, M.Y.; Li, Y.W.; Tang, Z.Y. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci. 2009, 100, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, T.; Yao, L.; Tosato, G. Mast cell-derived angiopoietin-1 plays a critical role in the growth of plasma cell tumors. J. Clin. Investig. 2004, 114, 1317–1325. [Google Scholar] [CrossRef]
- Amini, R.M.; Aaltonen, K.; Nevanlinna, H.; Carvalho, R.; Salonen, L.; Heikkila, P.; Blomqvist, C. Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer 2007, 7, 165. [Google Scholar] [CrossRef]
- Dabiri, S.; Huntsman, D.; Makretsov, N.; Cheang, M.; Gilks, B.; Bajdik, C.; Gelmon, K.; Chia, S.; Hayes, M. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod. Pathol. 2004, 17, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Rajput, A.B.; Turbin, D.A.; Cheang, M.C.; Voduc, D.K.; Leung, S.; Gelmon, K.A.; Gilks, C.B.; Huntsman, D.G. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: A study of 4,444 cases. Breast Cancer Res. Treat. 2008, 107, 249–257. [Google Scholar] [CrossRef]
- Pittoni, P.; Colombo, M.P. The dark side of mast cell-targeted therapy in prostate cancer. Cancer Res. 2012, 72, 831–835. [Google Scholar] [CrossRef]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjostrom, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef]
- Carlini, M.J.; Dalurzo, M.C.; Lastiri, J.M.; Smith, D.E.; Vasallo, B.C.; Puricelli, L.I.; Lauria de Cidre, L.S. Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum. Pathol. 2010, 41, 697–705. [Google Scholar] [CrossRef]
- Shikotra, A.; Ohri, C.M.; Green, R.H.; Waller, D.A.; Bradding, P. Mast cell phenotype, TNFalpha expression and degranulation status in non-small cell lung cancer. Sci. Rep. 2016, 6, 38352. [Google Scholar] [CrossRef]
- Welsh, T.J.; Green, R.H.; Richardson, D.; Waller, D.A.; O’Byrne, K.J.; Bradding, P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 8959–8967. [Google Scholar] [CrossRef]
- Cai, S.W.; Yang, S.Z.; Gao, J.; Pan, K.; Chen, J.Y.; Wang, Y.L.; Wei, L.X.; Dong, J.H. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery 2011, 149, 576–584. [Google Scholar] [CrossRef]
- Holzel, M.; Landsberg, J.; Glodde, N.; Bald, T.; Rogava, M.; Riesenberg, S.; Becker, A.; Jonsson, G.; Tuting, T. A Preclinical Model of Malignant Peripheral Nerve Sheath Tumor-like Melanoma Is Characterized by Infiltrating Mast Cells. Cancer Res. 2016, 76, 251–263. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Rigoli, L.; Inferrera, C. Mast cell interaction with tumor cells in small early gastric cancer: Ultrastructural observations. Ultrastruct. Pathol. 1997, 21, 173–181. [Google Scholar] [CrossRef]
- Bruni, C.; Caschera, F. Quantità e distribuzione delle Mastzellen nel carcinoma dello stomaco-Ricerca sistematica. Lav. Anat. Pat. Perugia 1952, 12, 5–20. [Google Scholar]
- Yano, H.; Kinuta, M.; Tateishi, H.; Nakano, Y.; Matsui, S.; Monden, T.; Okamura, J.; Sakai, M.; Okamoto, S. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer 1999, 2, 26–32. [Google Scholar] [CrossRef]
- Kondo, K.; Muramatsu, M.; Okamoto, Y.; Jin, D.; Takai, S.; Tanigawa, N.; Miyazaki, M. Expression of chymase-positive cells in gastric cancer and its correlation with the angiogenesis. J. Surg. Oncol. 2006, 93, 36–43, discussion 42-3. [Google Scholar] [CrossRef]
- Nakajima, S.; Bamba, N.; Hattori, T. Histological aspects and role of mast cells in Helicobacter pylori-infected gastritis. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. 1), 165–170. [Google Scholar] [CrossRef] [Green Version]
- Moorchung, N.; Srivastava, A.N.; Gupta, N.K.; Malaviya, A.K.; Achyut, B.R.; Mittal, B. The role of mast cells and eosinophils in chronic gastritis. Clin. Exp. Med. 2006, 6, 107–114. [Google Scholar] [CrossRef]
- de Paulis, A.; Prevete, N.; Rossi, F.W.; Rivellese, F.; Salerno, F.; Delfino, G.; Liccardo, B.; Avilla, E.; Montuori, N.; Mascolo, M.; et al. Helicobacter pylori Hp(2-20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J. Immunol. 2009, 183, 3761–3769. [Google Scholar] [CrossRef]
- Piazuelo, M.B.; Camargo, M.C.; Mera, R.M.; Delgado, A.G.; Peek, R.M., Jr.; Correa, H.; Schneider, B.G.; Sicinschi, L.A.; Mora, Y.; Bravo, L.E.; et al. Eosinophils and mast cells in chronic gastritis: Possible implications in carcinogenesis. Hum. Pathol. 2008, 39, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Guidolin, D.; Marzullo, A.; Nico, B.; Annese, T.; Benagiano, V.; Crivellato, E. Mast cells and angiogenesis in gastric carcinoma. Int. J. Exp. Pathol. 2010, 91, 350–356. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, K.; Cai, K.; Zhai, R.; Tao, K.; Wang, G.; Wang, J. Increased numbers of gastric-infiltrating mast cells and regulatory T cells are associated with tumor stage in gastric adenocarcinoma patients. Oncol. Lett. 2012, 4, 755–758. [Google Scholar] [CrossRef] [Green Version]
- Blair, R.J.; Meng, H.; Marchese, M.J.; Ren, S.; Schwartz, L.B.; Tonnesen, M.G.; Gruber, B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Investig. 1997, 99, 2691–2700. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Vescio, G.; Zuccala, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Marech, I.; Ruggieri, R.; et al. Tryptase mast cell density, protease-activated receptor-2 microvascular density and classical microvascular density evaluation in gastric cancer patients undergoing surgery: Possible translational relevance. Ther. Adv. Gastroenterol. 2017, 10, 353–360. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Luposella, M.; Patruno, R.; Gadaleta, C.D.; Sarro, G.D.; Ranieri, G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus. Med. Hemother. 2016, 43, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccala, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef]
- Leporini, C.; Ammendola, M.; Marech, I.; Sammarco, G.; Sacco, R.; Gadaleta, C.D.; Oakley, C.; Russo, E.; De Sarro, G.; Ranieri, G. Targeting mast cells in gastric cancer with special reference to bone metastases. World J. Gastroenterol. 2015, 21, 10493–10501. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, H.; Ikeguchi, M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J. Surg. Res. 2012, 178, 685–691. [Google Scholar] [CrossRef]
- Zhuang, Y.; Peng, L.S.; Zhao, Y.L.; Shi, Y.; Mao, X.H.; Chen, W.; Pang, K.C.; Liu, X.F.; Liu, T.; Zhang, J.Y.; et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012, 143, 951–962.e8. [Google Scholar] [CrossRef]
- Hueber, A.J.; Asquith, D.L.; Miller, A.M.; Reilly, J.; Kerr, S.; Leipe, J.; Melendez, A.J.; McInnes, I.B. Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 2010, 184, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Taams, L.S.; Steel, K.J.A.; Srenathan, U.; Burns, L.A.; Kirkham, B.W. IL-17 in the immunopathogenesis of spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 453–466. [Google Scholar] [CrossRef]
- Suurmond, J.; Habets, K.L.; Dorjee, A.L.; Huizinga, T.W.; Toes, R.E. Expansion of Th17 Cells by Human Mast Cells Is Driven by Inflammasome-Independent IL-1beta. J. Immunol. 2016, 197, 4473–4481. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, H.; Zhang, G.; Lin, X.; Chen, C.; Sun, J.; Zhang, Y.; Zhang, Q.; Yu, J. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS ONE 2014, 9, e106834. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Zuccala, V.; Luposella, M.; Patruno, R.; Gadaleta, P.; Zizzo, N.; Gadaleta, C.D.; De Sarro, G.; Sammarco, G.; et al. Mast Cells Density Positive to Tryptase Correlate with Microvascular Density in both Primary Gastric Cancer Tissue and Loco-Regional Lymph Node Metastases from Patients That Have Undergone Radical Surgery. Int. J. Mol. Sci. 2016, 17, 1905. [Google Scholar] [CrossRef]
- Varricchi, G.; Loffredo, S.; Borriello, F.; Pecoraro, A.; Rivellese, F.; Genovese, A.; Marone, G.; Spadaro, G. Superantigenic Activation of Human Cardiac Mast Cells. Int. J. Mol. Sci. 2019, 20, 1828. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Loffredo, S.; Poto, R.; Rivellese, F.; Genovese, A.; Marone, G.; Spadaro, G. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front. Cell. Neurosci. 2019, in press. [Google Scholar]
- Guidolin, D.; Ruggieri, S.; Annese, T.; Tortorella, C.; Marzullo, A.; Ribatti, D. Spatial distribution of mast cells around vessels and glands in human gastric carcinoma. Clin. Exp. Med. 2017, 17, 531–539. [Google Scholar] [CrossRef]
- Sammarco, G.; Gadaleta, C.D.; Zuccala, V.; Albayrak, E.; Patruno, R.; Milella, P.; Sacco, R.; Ammendola, M.; Ranieri, G. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 1176. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef]
- Rabenhorst, A.; Leja, S.; Schwaab, J.; Gehring, M.; Forster, A.; Arock, M.; Reiter, A.; Raap, U.; Hartmann, K. Expression of programmed cell death ligand-1 in mastocytosis correlates with disease severity. J. Allergy Clin. Immunol. 2016, 137, 314–318. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Tocchetti, C.G. Cardiac Toxicity of Immune Checkpoint Inhibitors: Cardio-Oncology Meets Immunology. Circulation 2017, 136, 1989–1992. [Google Scholar] [CrossRef]
- Tocchetti, C.G.; Galdiero, M.R.; Varricchi, G. Cardiac Toxicity in Patients Treated With Immune Checkpoint Inhibitors: It Is Now Time for Cardio-Immuno-Oncology. J. Am. Coll. Cardiol. 2018, 71, 1765–1767. [Google Scholar] [CrossRef]
- Seo, A.N.; Kang, B.W.; Kwon, O.K.; Park, K.B.; Lee, S.S.; Chung, H.Y.; Yu, W.; Bae, H.I.; Jeon, S.W.; Kang, H.; et al. Intratumoural PD-L1 expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Br. J. Cancer 2017, 117, 1753–1760. [Google Scholar] [CrossRef]
- De Rosa, S.; Sahnane, N.; Tibiletti, M.G.; Magnoli, F.; Vanoli, A.; Sessa, F.; Chiaravalli, A.M. EBV(+) and MSI Gastric Cancers Harbor High PD-L1/PD-1 Expression and High CD8(+) Intratumoral Lymphocytes. Cancers 2018, 10, 102. [Google Scholar] [CrossRef]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; De Sarro, G.; Russo, E.; Ranieri, G. Targeting mast cells tryptase in tumor microenvironment: A potential antiangiogenetic strategy. Biomed. Res. Int. 2014, 2014, 154702. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Leung, L.H.; Liu, L.; Yang, F.; Yao, X. Efficacy and safety of angiogenesis inhibitors in advanced gastric cancer: A systematic review and meta-analysis. J. Hematol. Oncol. 2016, 9, 111. [Google Scholar] [CrossRef]
- Chan, D.L.; Sjoquist, K.M.; Goldstein, D.; Price, T.J.; Martin, A.J.; Bang, Y.J.; Kang, Y.K.; Pavlakis, N. The effect of anti-angiogenic agents on overall survival in metastatic oesophago-gastric cancer: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0172307. [Google Scholar] [CrossRef] [PubMed]
- Lotfi-Emran, S.; Ward, B.R.; Le, Q.T.; Pozez, A.L.; Manjili, M.H.; Woodfolk, J.A.; Schwartz, L.B. Human mast cells present antigen to autologous CD4(+) T cells. J. Allergy Clin. Immunol. 2018, 141, 311–321. [Google Scholar] [CrossRef]
- Kritikou, E.; van der Heijden, T.; Swart, M.; van Duijn, J.; Slutter, B.; Wezel, A.; Smeets, H.J.; Maffia, P.; Kuiper, J.; Bot, I. Hypercholesterolemia Induces a Mast Cell-CD4(+) T Cell Interaction in Atherosclerosis. J. Immunol. 2019, 202, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.; Jaffee, E.M. Immunotherapy transforms cancer treatment. J. Clin. Investig. 2019, 129, 46–47. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Y.; Jiang, J.; Zhao, J.; Zhang, X.G.; Xu, N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006, 108, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Derks, S.; Liao, X.; Chiaravalli, A.M.; Xu, X.; Camargo, M.C.; Solcia, E.; Sessa, F.; Fleitas, T.; Freeman, G.J.; Rodig, S.J.; et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016, 7, 32925–32932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, G.; Gabriele, L.; Mattei, F. The tumor microenvironment: A pitch for multiple players. Front. Oncol. 2013, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammarco, G.; Varricchi, G.; Ferraro, V.; Ammendola, M.; De Fazio, M.; Altomare, D.F.; Luposella, M.; Maltese, L.; Currò, G.; Marone, G.; et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 2106. https://doi.org/10.3390/ijms20092106
Sammarco G, Varricchi G, Ferraro V, Ammendola M, De Fazio M, Altomare DF, Luposella M, Maltese L, Currò G, Marone G, et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. International Journal of Molecular Sciences. 2019; 20(9):2106. https://doi.org/10.3390/ijms20092106
Chicago/Turabian StyleSammarco, Giuseppe, Gilda Varricchi, Valentina Ferraro, Michele Ammendola, Michele De Fazio, Donato Francesco Altomare, Maria Luposella, Lorenza Maltese, Giuseppe Currò, Gianni Marone, and et al. 2019. "Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer" International Journal of Molecular Sciences 20, no. 9: 2106. https://doi.org/10.3390/ijms20092106
APA StyleSammarco, G., Varricchi, G., Ferraro, V., Ammendola, M., De Fazio, M., Altomare, D. F., Luposella, M., Maltese, L., Currò, G., Marone, G., Ranieri, G., & Memeo, R. (2019). Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. International Journal of Molecular Sciences, 20(9), 2106. https://doi.org/10.3390/ijms20092106








