Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray
Abstract
:1. Introduction
2. Results
2.1. Micropropagation
2.2. Content of Bioactive Compounds
3. Discussion
4. Methods
4.1. Micropropagation
4.2. Extraction and Quantification of Carotenoids and Tocopherols
4.3. Lipid Extraction and Fatty Acid Methyl Ester (FAME) Preparation
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, H.Y.; Kim, D.H.; Sivanesan, I. Micropropagation of Ajuga species: A mini review. Biotechnol. Lett. 2017, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.R.; Sivanesan, I. Direct adventitious shoot regeneration, in vitro flowering, fruiting, secondary metabolite content and antioxidant activity of Scrophularia takesimensis Nakai. Plant. Cell Tissue Organ. Cult. 2015, 123, 607–618. [Google Scholar] [CrossRef]
- Sivanesan, I.; Saini, R.K.; Noorzai, R.; Zamany, A.J.; Kim, D.H. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3 Biotech 2016, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Lucioli, S.; Di Bari, C.; Nota, P.; Frattarelli, A.; Forni, C.; Caboni, E. Methyl jasmonate promotes anthocyanins’ production in Prunus salicina × Prunus persica in vitro shoot cultures. Plant. Biosyst. 2017, 151, 788–791. [Google Scholar] [CrossRef]
- Jeong, B.R.; Sivanesan, I. Impact of light quality and sucrose on adventitious shoot regeneration and bioactive compound accumulation in Ajuga multiflora Bunge. Sci. Hortic. 2018, 236, 222–228. [Google Scholar] [CrossRef]
- Panigrahi, J.; Dholu, P.; Shah, T.J.; Gantait, S. Silver nitrate-induced in vitro shoot multiplication and precocious flowering in Catharanthus roseus (L.) G. Don, a rich source of terpenoid indole alkaloids. Plant. Cell Tissue Organ. Cult. 2018, 132, 579–584. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [PubMed]
- Scott, G.A.M. Mertensia maritima (L.) S. F. Gray. J. Ecol. 1963, 51, 733–742. [Google Scholar] [CrossRef]
- Alton, S.; FitzGerald, R. Mertensia maritima Boraginaceae. Curtis’s Bot. Mag. 2009, 26, 96–110. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Chapuis, C.; Rubin, M.; Starken-mann, C.J. Volatile composition of oyster leaf (Mertensia maritima (L.) Gray). J. Agric. Food Chem. 2012, 60, 11681–11690. [Google Scholar] [CrossRef] [PubMed]
- Skarpaas, O.; Stabbetorp, E. Diaspore ecology of Mertensia maritima: Effects of physical treatments and their relative timing on dispersal and germination. Oikos 2001, 95, 374–382. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Inyushkina, Y.V.; Bulgakov, V.P.; Veselova, M.V.; Tchernoded, G.K.; Gerasimenko, A.V.; Zhuravlev, Y.N. Production of allantoin, rabdosiin and rosmarinic acid in callus cultures of the seacostal plant Mertensia maritima (Boraginaceae). Plant. Cell Tissue Organ. Cult. 2012, 110, 183–188. [Google Scholar] [CrossRef]
- Pal, M.; Chaudhury, A. High frequency direct plant regeneration, micropropagation and shikonin induction in Arnebia hispidissima. J. Crop. Sci. Biotech. 2010, 13, 13–19. [Google Scholar] [CrossRef]
- Phulwaria, M.; Shekhawat, N.S. An efficient in vitro shoot regeneration from immature inflorescence and ex vitro rooting of Arnebia hispidissima (Lehm). DC.—A red dye (Alkannin) yielding plant. Physiol. Mol. Biol. Plants 2013, 19, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Lameira, O.A.; Pinto, J.E.B.P. In vitro propagation of Cordia verbenacea (Boraginaceae). Rev. Bras. Plant Med. Botucatu. 2006, 8, 102–104. [Google Scholar]
- Edson, J.L.; Leege-Brusven, A.D.; Everett, R.L.; Wenny, D.L. Minimizing growth regulators in shoot cultures of an endangered plant, Hackelia venusta (Boraginaceae). In vitro Cell. Dev. Biol. Plant 1996, 32, 267–271. [Google Scholar] [CrossRef]
- Kumar, M.S.; Rao, M.V. In Vitro micropropagation of Heliotropium indicum L.-an ayurvedic herb. Indian J. Biotechnol. 2007, 6, 245–249. [Google Scholar]
- Jain, S.C.; Pancholi, B.; Jain, R. Rapid in vitro multiplication and biological potentialities of Sericostoma pauciflorum stocks ex Wight. J. Med. Plants Res. 2014, 8, 45–51. [Google Scholar]
- Verma, N.; Koche, V.; Tiwari, K.L.; Mishra, S.K. Plant regeneration through organogenesis and shoot proliferation in Trichodesma indicum (Linn) R.Br.— A medicinal herb. Afr. J. Biotechnol. 2008, 7, 3632–3637. [Google Scholar]
- Mahesh, A.; Jeyachandran, R. Influence of plant growth regulators on micropropagation and in vitro flowering of Trichodesma indicum (Linn) R. Br. Plant. Biosyst. 2013, 147, 493–499. [Google Scholar] [CrossRef]
- Sivanesan, I.; Saini, R.K.; Kim, D.H. Bioactive compounds in hyperhydric and normal micropropagated shoots of Aronia melanocarpa (michx.) Elliott. Ind. Crops Prod. 2016, 83, 31–38. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; El-Sheikh, M.A.; Abdel-Salam, E.M.; Qahtan, A.A. Thidiazuron induced in vitro morphogenesis for sustainable supply of genetically true quality plantlets of Brahmi. Ind. Crops Prod. 2018, 118, 173–179. [Google Scholar] [CrossRef]
- Jeong, B.R.; Sivanesan, I. Micropropagation, berberine content and antitumor activity of Jeffersonia dubia (Maxim.) Benth et Hook. Plant. Cell Tissue Organ. Cult. 2015, 124, 453–458. [Google Scholar] [CrossRef]
- Huetteman, C.A.; Preece, J.E. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant. Cell Tissue Organ. Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Lu, C.Y. The use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Sivanesan, I.; Song, J.Y.; Hwang, S.J.; Jeong, B.R. Micropropagation of Cotoneaster wilsonii Nakai—A rare endemic ornamental plant. Plant. Cell Tissue Organ. Cult. 2011, 105, 55–63. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, K.W.; Sivanesan, I. Micropropagation of Haworthia retusa Duval. Propag. Ornam. Plants 2017, 17, 77–82. [Google Scholar]
- Shekhawat, M.S.; Shekhawat, N.S. Micropropagation of Arnebia hispidissima (Lehm). DC. and production of alkannin from callus and cell suspension culture. Acta Physiol. Plant. 2011, 33, 1445–1450. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant. 2015, 8, 68–82. [Google Scholar] [CrossRef]
- Park, H.Y.; Saini, R.K.; Gopal, J.; Keum, Y.-S.; Kim, D.H.; Lee, O.; Sivanesan, I. Micropropagation and subsequent enrichment of carotenoids, fatty acids, and tocopherol contents in Sedum dasyphyllum L. Front. Chem. 2017, 5, 77. [Google Scholar] [CrossRef]
- Afzal, M.; Obuekwe, C.; Shuaib, N.; Barakat, H. Photosynthetic pigment profile of Cordia myxa L. and its potential in folklore medicinal application. J. Food Agric. Environ. 2004, 2, 114–120. [Google Scholar]
- Kimura, M.; Rodriguez-Amaya, D.B. Harvestplus Handbook for Carotenoid Analysis; HarvestPlus: Washington, DC, USA, 2004; pp. 2–11. [Google Scholar]
- Minguez-Mosqueira, M.I.; Hornero-Méndez, D.; Pérez-Gálvez, A. Carotenoids and provitamin A in functional foods. In Methods of analysis for functional foods and nutraceuticals; CRC Press: Boca Raton, FL, USA, 2008; pp. 277–336. [Google Scholar]
- Mohajer, S.; Mohajer, M.; Ramli, R.B.; Taha, R.M. Phytochemical constituents and radical scavenging properties of Borago officinalis and Malva sylvestris. Ind. Crops Prod. 2016, 94, 673–681. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary carotenoid roles in redox homeostasis and human health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods-a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Garcia Maroto, F.; Vilches-Ferron, M.A.; Lopez-Alonso, D. Γ-linolenic acid from fourteen Boraginaceae species. Ind. Crops Prod. 2003, 18, 85–89. [Google Scholar] [CrossRef]
- Jaffel-Hamza, K.; Sai-Kachout, S.; Harrathi, J. Growth and fatty acid composition of borage (Borago officinalis L.) leaves and seeds cultivated in saline medium. J. Plant. Growth Regul. 2013, 32, 200–207. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Rincón-Cervera, M. Sardinian Boraginaceae are new potential sources of γ-linolenic acid. Food Chem. 2017, 218, 435–439. [Google Scholar] [CrossRef]
- Sewon, P.; Tyystjarvi, E. Stearidonic and γ-linolenic acid contents of common borage leaves. Phytochemistry 1993, 33, 1029–1032. [Google Scholar]
- Sayanova, O.; Napier, J.; Shewry, P.R. Δ6-Unsaturated fatty acids in species and tissues of the Primulaceae. Phytochemistry 1999, 52, 419–422. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Maroto, F.G.; Gimenez, A.G. Fatty acid profiles from forty-nine plant species that are potential new sources of γ-linolenic acid. J. Am. Oil. Chem. Soc. 2001, 78, 677–684. [Google Scholar] [CrossRef]
- Del-Rıo-Celestino, M.; Font, R.; de-Haro-Bailon, A. Distribution of fatty acids in edible organs and seed fractions of borage (Borago officinalis L.). J. Sci. Food Agric. 2008, 88, 248–255. [Google Scholar] [CrossRef]
- Stähler, K.; Quek, S.; Miller, M.R. Investigation of -linolenic acid and stearidonic acid biosynthesis during a life cycle of Borago officinalis L. J. Am. Oil Chem. Soc. 2011, 88, 1715–1725. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rincón-Cervera, M.Á.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; Venegas-Venegas, E. New seed oils of Boraginaceae rich in stearidonic and γ-linolenic acids from the Maghreb region. J. Food Comps. Anal. 2013, 31, 20–23. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Rincón-Cervera, M.; Jiménez-Beckerc, S.; de Bélaird, G. Fatty acid profiles and sn-2 fatty acid distribution of γ-linolenic acid-rich Borago species. J. Food Comps. Anal. 2018, 66, 74–80. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Saini, R.K.; Shetty, N.P.; Giridhar, P. GC-FID/MS analysis of fatty acids in Indian cultivars of Moringa oleifera: Potential sources of PUFA. J. Am. Oil Chem. Soc. 2014, 91, 1029–1034. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]



| Cytokinins (µM) | Shoot Induction (%) | Number of Shoots/Explant | ||
|---|---|---|---|---|
| Node | Shoot Tip | Node | Shoot Tip | |
| 0 | 0.0k | 0.0m | 0.0l | 0.0h |
| BA 1 | 14.2j | 24.7l | 1.8k | 1.2fg |
| 2 | 24.5i | 35.0k | 2.8ij | 1.7efg |
| 4 | 44.4f | 47.3h | 3.3ghi | 2.0def |
| 8 | 49.1e | 57.1fg | 4.4cdef | 2.6cd |
| 12 | 57.1cd | 59.4ef | 3.0hij | 2.3cde |
| 16 | 37.6g | 43.8i | 2.3jk | 1.4fg |
| KN 1 | 24.9i | 34.8k | 2.4ijk | 1.3fg |
| 2 | 32.7h | 36.6jk | 3.7fgh | 1.9defg |
| 4 | 38.4g | 45.0hi | 4.3def | 2.6cd |
| 8 | 58.6c | 65.4d | 5.2c | 3.4b |
| 12 | 54.7d | 60.7e | 4.0efg | 3.0bc |
| 16 | 47.7e | 55.9g | 3.2ghij | 1.1g |
| TDZ 1 | 36.9g | 38.5j | 4.7cde | 2.0def |
| 2 | 65.7b | 69.1c | 6.1b | 3.1bc |
| 4 | 84.7a | 87.0a | 8.9a | 4.8a |
| 8 | 68.2b | 72.7b | 5.1cd | 2.4cde |
| 12 | 55.6cd | 64.0d | 2.6ijk | 2.3cde |
| 16 | 23.9i | 25.9l | 1.8k | 1.7efg |
| TDZ (µM) | NAA (µM) | Shoot Induction (%) | Number of Shoots/Explant | ||
|---|---|---|---|---|---|
| Node | Shoot Tip | Node | Shoot Tip | ||
| 1 | 1 | 49.2g | 46.8f | 6.8de | 4.4d |
| 2 | 1 | 80.3c | 78.2d | 10.8b | 7.6b |
| 4 | 1 | 93.8a | 95.9a | 17.7a | 8.6a |
| 8 | 1 | 77.8cd | 85.0c | 8.1c | 4.6cd |
| 12 | 1 | 66.3e | 74.0e | 4.8gh | 2.9ef |
| 16 | 1 | 29.8i | 32.8g | 3.8h | 2.3f |
| 1 | 2 | 42.6h | 43.1f | 5.1fg | 2.0fg |
| 2 | 2 | 70.2e | 73.9e | 7.8cd | 3.8de |
| 4 | 2 | 89.4b | 91.1b | 11.7b | 5.4c |
| 8 | 2 | 74.4d | 78.1d | 6.2ef | 2.7f |
| 12 | 2 | 61.3f | 70.9e | 2.1i | 2.2f |
| 16 | 2 | 28.4i | 30.8g | 1.3i | 1.1g |
| Auxins (µM) | Root Induction (%) | No. of Roots/Shoot | Mean Root Length (cm) |
|---|---|---|---|
| 0 | 26.0j | 3.4j | 0.9i |
| IAA 2 | 47.9h | 5.2hi | 1.4h |
| 4 | 60.8f | 9.7fg | 1.9g |
| 8 | 68.8e | 11.0e | 2.4e |
| 12 | 55.2g | 6.7h | 2.1fg |
| IBA 2 | 72.2d | 15.0c | 3.1c |
| 4 | 97.4a | 25.4a | 4.2a |
| 8 | 90.9b | 20.7b | 3.8b |
| 12 | 84.3c | 11.4e | 2.8cd |
| NAA 2 | 58.3f | 5.9hi | 1.8g |
| 4 | 66.2e | 13.1d | 2.8d |
| 8 | 46.4h | 8.4g | 2.3ef |
| 12 | 35.7i | 4.9ij | 1.4h |
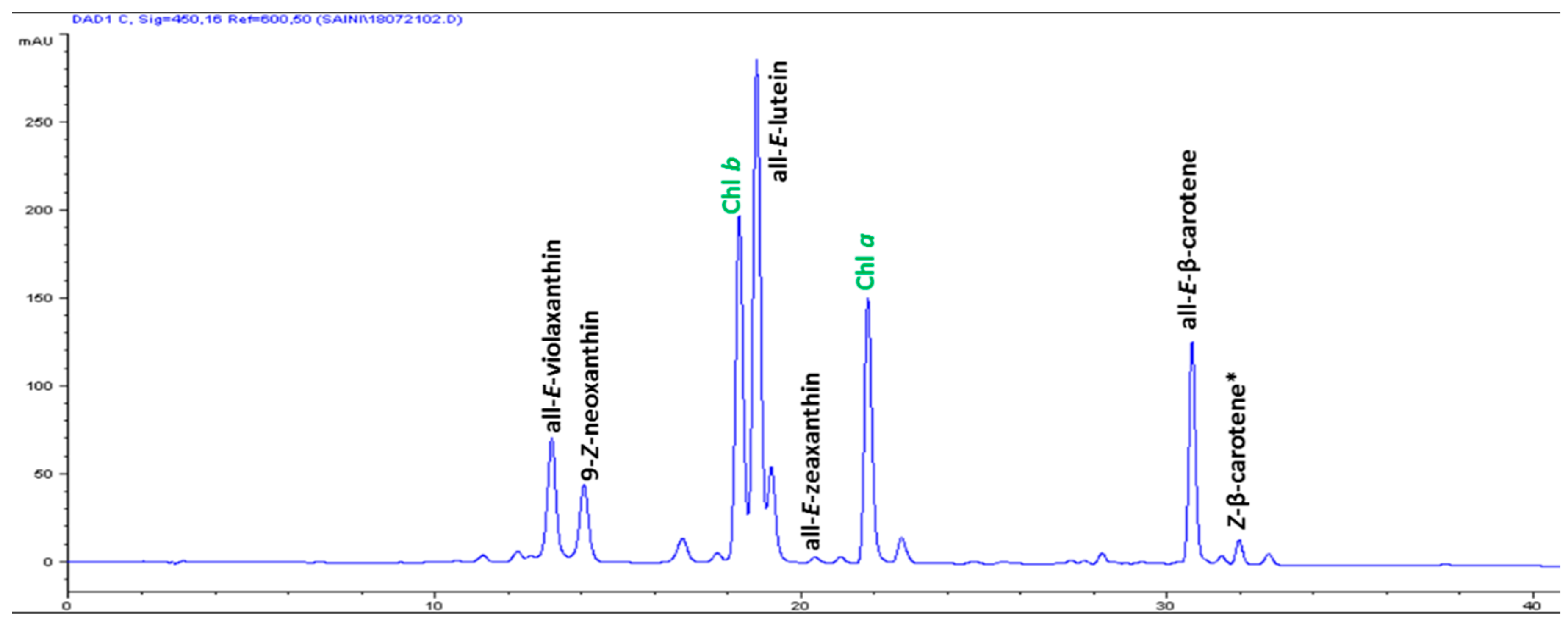
| S/No | Carotenoids | Retention Time (min) | Contents (μg g-1 FW) |
|---|---|---|---|
| 1 | All-E-violaxanthin | 13.176 | 5.59 ± 0.31 |
| 2 | 9-Z-neoxanthin | 14.058 | 3.44 ± 0.17 |
| 3 | All-E-lutein | 18.773 | 18.49 ± 0.74 |
| 4 | All-E-zeaxanthin | 20.364 | 0.29 ± 0.04 |
| 5 | All-E-β-carotene | 30.665 | 6.42 ± 0.86 |
| 6 | (Z)-β-carotene | 31.951 | 0.74 ± 0.08 |
| 7 | Total carotenoids | - | 34.97 ± 2.12 |
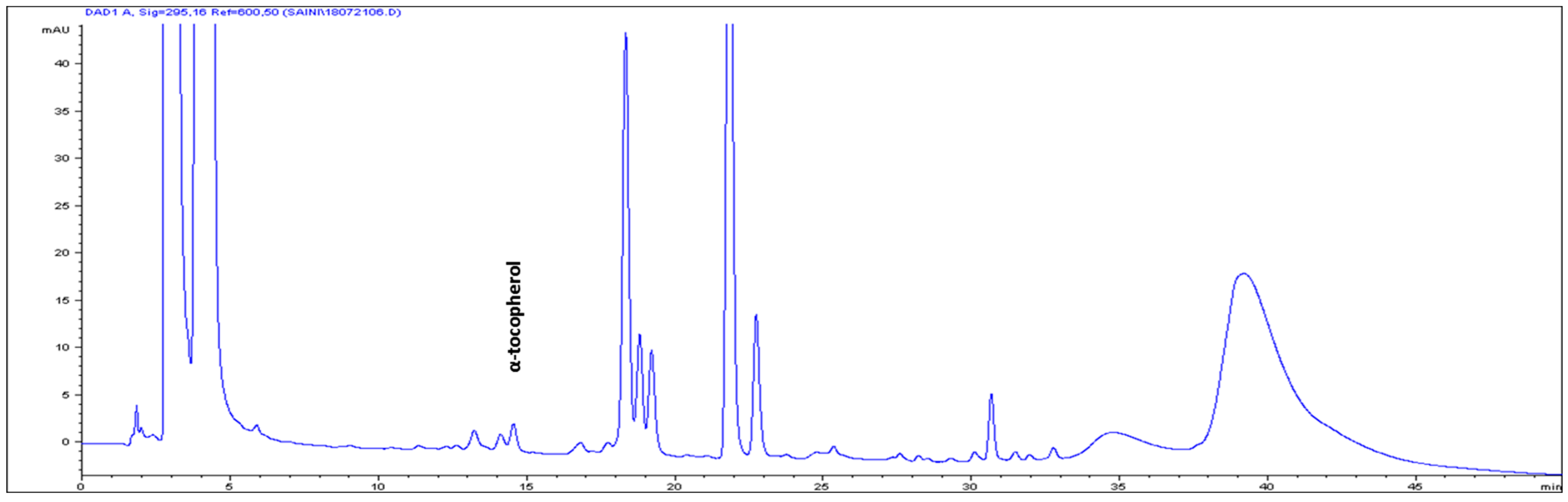
| 8 | α-tocopherol | 14.504 | 3.82 ± 0.13 |
| Retention Time (min) | FAME | % Composition |
|---|---|---|
| 11.42 | C12:0 (Lauric, SFA) | 2.44 |
| 22.195 | C16:0 (Palmitic, SFA) | 22.66 |
| 25.755 | C18:3n6 (γ-Linolenic, PUFA) | 14.05 |
| 25.89 | C18:4n3 (Stearidonic, PUFA) | 6.04 |
| 26.135 | C18:2n6c (Linoleic, PUFA) | 16.90 |
| 26.29 | C18:3n3 (α-Linolenic, PUFA) | 30.37 |
| 26.89 | C18:0 (Stearic, SFA) | 3.14 |
| 31.2 | C20:0 (Arachidic, SFA) | 0.84 |
| 35.185 | C22:0 (Behenic, SFA) | 1.12 |
| 40.12 | C24:0 (Lignoceric, SFA) | 2.44 |
| Total SFAs | 32.64 | |
| Total PUFAs | 67.36 | |
| PUFAs: SFAs | 2.06 | |
| Total lipids (% DW) | 10.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.Y.; Kim, D.H.; Saini, R.K.; Gopal, J.; Keum, Y.-S.; Sivanesan, I. Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray. Int. J. Mol. Sci. 2019, 20, 2141. https://doi.org/10.3390/ijms20092141
Park HY, Kim DH, Saini RK, Gopal J, Keum Y-S, Sivanesan I. Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray. International Journal of Molecular Sciences. 2019; 20(9):2141. https://doi.org/10.3390/ijms20092141
Chicago/Turabian StylePark, Han Yong, Doo Hwan Kim, Ramesh Kumar Saini, Judy Gopal, Young-Soo Keum, and Iyyakkannu Sivanesan. 2019. "Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray" International Journal of Molecular Sciences 20, no. 9: 2141. https://doi.org/10.3390/ijms20092141
APA StylePark, H. Y., Kim, D. H., Saini, R. K., Gopal, J., Keum, Y.-S., & Sivanesan, I. (2019). Micropropagation and Quantification of Bioactive Compounds in Mertensia maritima (L.) Gray. International Journal of Molecular Sciences, 20(9), 2141. https://doi.org/10.3390/ijms20092141







