Resveratrol and Vascular Function
Abstract
:1. Resveratrol and its Molecular Targets
2. Effects of Resveratrol on Endothelial Cells
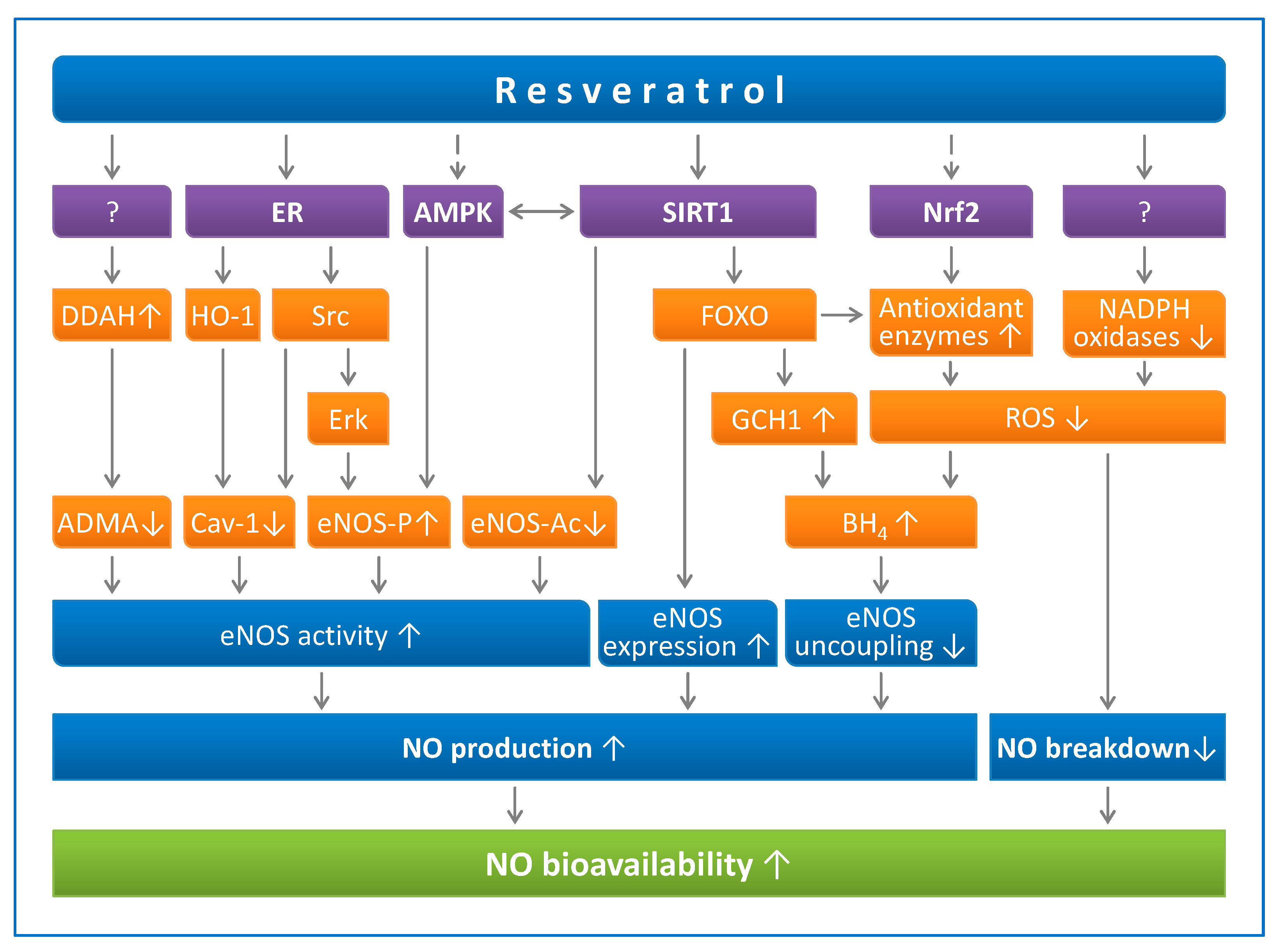
2.1. Resveratrol Enhances Endothelial NO Production
2.1.1. Resveratrol Upregulates eNOS Expression
2.1.2. Resveratrol Increases eNOS Activity
2.1.3. Resveratrol Prevents eNOS Uncoupling
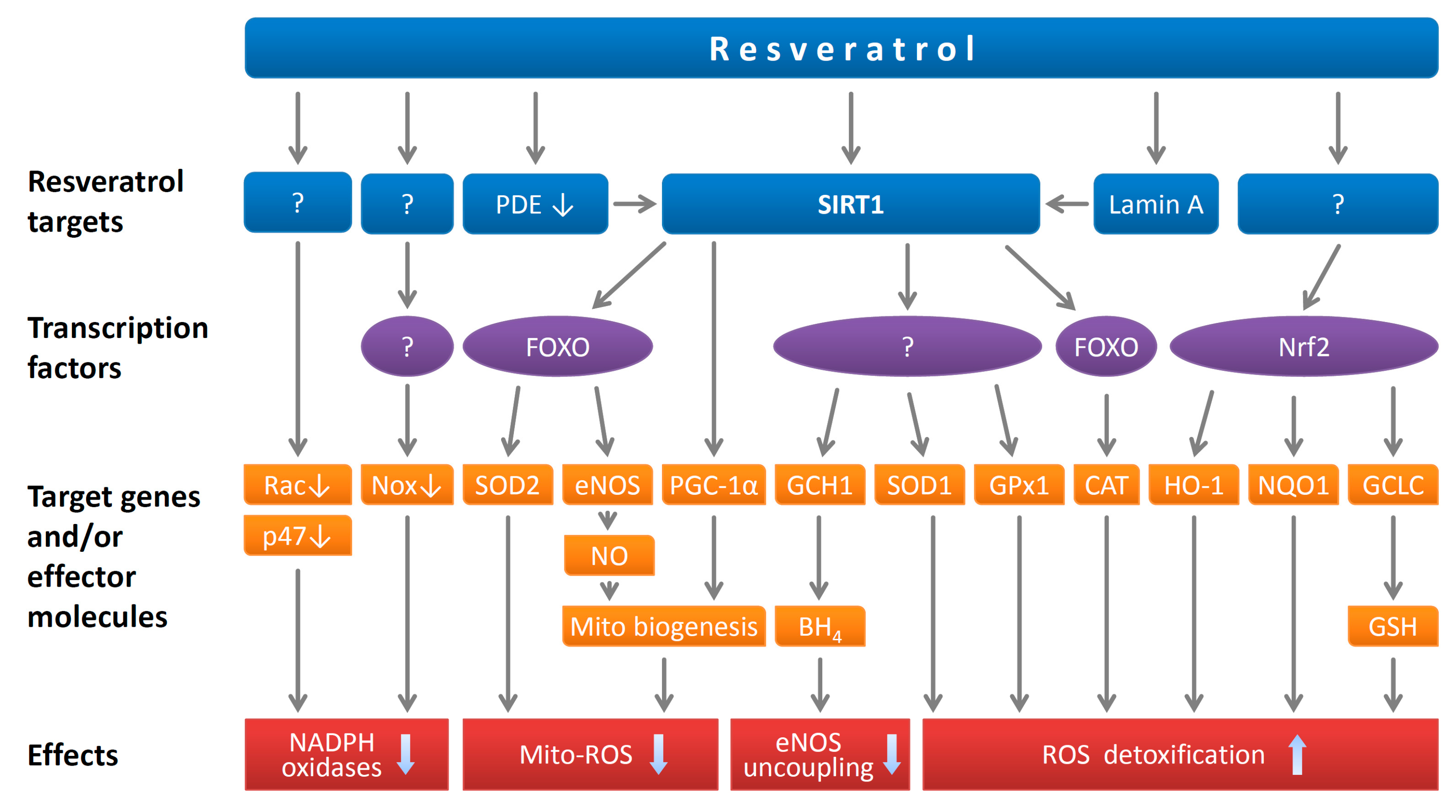
2.2. Resveratrol Reduces Endothelial Oxidative Stress
2.3. Resveratrol Reduces Endothelin-1 Synthesis
3. Effects of Resveratrol on Vascular Smooth Muscle Cells
3.1. Resveratrol Reduces Oxidative Stress in Smooth Muscle Cells
3.2. Resveratrol Inhibits Smooth Muscle Cell Proliferation
3.3. Resveratrol Prevents Arterial Stiffness and Vascular Remodeling
4. Effects of Resveratrol on Immune Cells
5. Effects of Resveratrol on PVAT
6. Effects of Resveratrol on Vascular Function and Blood Pressure in Vivo
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kannappan, R.; Reuter, S.; Kim, J.H.; Aggarwal, B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Pirola, L.; Frojdo, S. Resveratrol: One molecule, many targets. IUBMB Life 2008, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle 2008, 7, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Forstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br. J. Pharmacol. 2015, 172, 6024–6109. [Google Scholar] [CrossRef]
- Park, S.J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ghosh, S.; Yang, X.; Zheng, H.; Liu, X.; Wang, Z.; Jin, G.; Zheng, B.; Kennedy, B.K.; Suh, Y.; et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012, 16, 738–750. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Pinto, J.T.; Ballabh, P.; Zhang, H.; Losonczy, G.; Pearson, K.; de Cabo, R.; Pacher, P.; Zhang, C.; et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H13–H20. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Forstermann, U.; Li, H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef]
- Yu, H.P.; Hwang, T.L.; Hwang, T.L.; Yen, C.H.; Lau, Y.T. Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Crit. Care Med. 2010, 38, 1147–1154. [Google Scholar] [CrossRef]
- Nagaoka, T.; Hein, T.W.; Yoshida, A.; Kuo, L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: Role of nitric oxide and potassium channels. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4232–4239. [Google Scholar] [CrossRef]
- Gojkovic-Bukarica, L.; Novakovic, A.; Kanjuh, V.; Bumbasirevic, M.; Lesic, A.; Heinle, H. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J. Pharmacol. Sci. 2008, 108, 124–130. [Google Scholar] [CrossRef]
- Protic, D.; Radunovic, N.; Spremovic-Radenovic, S.; Zivanovic, V.; Heinle, H.; Petrovic, A.; Gojkovic-Bukarica, L. The Role of Potassium Channels in the Vasodilatation Induced by Resveratrol and Naringenin in Isolated Human Umbilical Vein. Drug. Dev. Res. 2015, 76, 17–23. [Google Scholar] [CrossRef]
- Kim, T.T.; Parajuli, N.; Sung, M.M.; Bairwa, S.C.; Levasseur, J.; Soltys, C.M.; Wishart, D.S.; Madsen, K.; Schertzer, J.D.; Dyck, J.R.B. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E511–E519. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Park, I.; Lee, Y.; Kim, H.D.; Kim, K. Effect of Resveratrol, a SIRT1 Activator, on the Interactions of the CLOCK/BMAL1 Complex. Endocrinol. Metab. 2014, 29, 379–387. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008, 67, 42–47. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Li, H.; Wallerath, T.; Forstermann, U. Physiological mechanisms regulating the expression of endothelial-type NO synthase. Nitric Oxide 2002, 7, 132–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef]
- Huang, P.L.; Huang, Z.; Mashimo, H.; Bloch, K.D.; Moskowitz, M.A.; Bevan, J.A.; Fishman, M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 1995, 377, 239–242. [Google Scholar] [CrossRef]
- Kuhlencordt, P.J.; Gyurko, R.; Han, F.; Scherrer-Crosbie, M.; Aretz, T.H.; Hajjar, R.; Picard, M.H.; Huang, P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation 2001, 104, 448–454. [Google Scholar] [CrossRef]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef]
- Duplain, H.; Burcelin, R.; Sartori, C.; Cook, S.; Egli, M.; Lepori, M.; Vollenweider, P.; Pedrazzini, T.; Nicod, P.; Thorens, B.; et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001, 104, 342–345. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Cummins, T.D.; Tang, Y.; Hellmann, J.; Holden, C.R.; Harbeson, M.A.; Chen, Y.; Patel, R.P.; Spite, M.; Bhatnagar, A.; et al. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ. Res. 2012, 111, 1176–1189. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef]
- Li, H.; Wallerath, T.; Munzel, T.; Forstermann, U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide 2002, 7, 149–164. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Forstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef]
- Wallerath, T.; Poleo, D.; Li, H.; Forstermann, U. Red wine increases the expression of human endothelial nitric oxide synthase: A mechanism that may contribute to its beneficial cardiovascular effects. J. Am. Coll. Cardiol. 2003, 41, 471–478. [Google Scholar] [CrossRef]
- Wallerath, T.; Li, H.; Godtel-Ambrust, U.; Schwarz, P.M.; Forstermann, U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide 2005, 12, 97–104. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, Z.; Chen, H.Z.; Zhou, S.; Zheng, W.; Liu, G.; Wei, Y.S.; Cai, H.; Liu, D.P.; Liang, C.C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2008, 80, 191–199. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef]
- Heiss, E.H.; Dirsch, V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharm. Des. 2014, 20, 3503–3513. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hao, X.; Yang, Q.; Si, L. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Biochem. Biophys. Res. Commun. 2009, 388, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.; Boger, R.; Luneburg, N. ADMA and the role of the genes: Lessons from genetically modified animals and human gene polymorphisms. Pharmacol. Res. 2009, 60, 475–480. [Google Scholar] [CrossRef]
- Frombaum, M.; Therond, P.; Djelidi, R.; Beaudeux, J.L.; Bonnefont-Rousselot, D.; Borderie, D. Piceatannol is more effective than resveratrol in restoring endothelial cell dimethylarginine dimethylaminohydrolase expression and activity after high-glucose oxidative stress. Free Radic. Res. 2011, 45, 293–302. [Google Scholar] [CrossRef]
- Feron, O.; Dessy, C.; Moniotte, S.; Desager, J.P.; Balligand, J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999, 103, 897–905. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, R.; Ye, X.; Zhang, C.; Jin, X.; Yamori, Y.; Hao, L.; Sun, X.; Ying, C. Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes Nutr. 2013, 8, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Penumathsa, S.V.; Koneru, S.; Samuel, S.M.; Maulik, G.; Bagchi, D.; Yet, S.F.; Menon, V.P.; Maulik, N. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: Role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic. Biol. Med. 2008, 45, 1027–1034. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Zhan, L.; Maulik, G.; Menon, V.P.; Bagchi, D.; Maulik, N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008, 12, 2350–2361. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Pharmacological Prevention of eNOS Uncoupling. Curr. Pharm. Des. 2014, 20, 3595–3606. [Google Scholar] [CrossRef]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013, 13, 161–167. [Google Scholar] [CrossRef]
- Laursen, J.B.; Somers, M.; Kurz, S.; McCann, L.; Warnholtz, A.; Freeman, B.A.; Tarpey, M.; Fukai, T.; Harrison, D.G. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 2001, 103, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Alp, N.J.; McAteer, M.A.; Khoo, J.; Choudhury, R.P.; Channon, K.M. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Shi, C.; Cohen, R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Investig. 2002, 109, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Habermeier, A.; Closs, E.I.; Thum, T.; Spanier, G.; Lu, Q.; Oelze, M.; Torzewski, M.; Lackner, K.J.; et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010, 335, 149–154. [Google Scholar] [CrossRef]
- Liu, J.C.; Chen, J.J.; Chan, P.; Cheng, C.F.; Cheng, T.H. Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension 2003, 42, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60 (Suppl. 4), 111–116. [Google Scholar]
- Ungvari, Z.; Orosz, Z.; Rivera, A.; Labinskyy, N.; Xiangmin, Z.; Olson, S.; Podlutsky, A.; Csiszar, A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2417–H2424. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol as a gene regulator in the vasculature. Curr. Pharm. Biotechnol. 2014, 15, 401–408. [Google Scholar] [CrossRef]
- Corder, R.; Douthwaite, J.A.; Lees, D.M.; Khan, N.Q.; Viseu Dos Santos, A.C.; Wood, E.G.; Carrier, M.J. Endothelin-1 synthesis reduced by red wine. Nature 2001, 414, 863–864. [Google Scholar] [CrossRef]
- Zou, J.G.; Wang, Z.R.; Huang, Y.Z.; Cao, K.J.; Wu, J.M. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int. J. Mol. Med. 2003, 11, 317–320. [Google Scholar] [CrossRef]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2010, 103, 1398–1403. [Google Scholar] [CrossRef]
- Coppa, T.; Lazze, M.C.; Cazzalini, O.; Perucca, P.; Pizzala, R.; Bianchi, L.; Stivala, L.A.; Forti, L.; Maccario, C.; Vannini, V.; et al. Structure-activity relationship of resveratrol and its analogue, 4,4’-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J. Med. Food 2011, 14, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Ruef, J.; Moser, M.; Kubler, W.; Bode, C. Induction of endothelin-1 expression by oxidative stress in vascular smooth muscle cells. Cardiovasc. Pathol. 2001, 10, 311–315. [Google Scholar] [CrossRef]
- Chao, H.H.; Juan, S.H.; Liu, J.C.; Yang, H.Y.; Yang, E.; Cheng, T.H.; Shyu, K.G. Resveratrol inhibits angiotensin II-induced endothelin-1 gene expression and subsequent proliferation in rat aortic smooth muscle cells. Eur. J. Pharmacol. 2005, 515, 1–9. [Google Scholar] [CrossRef]
- Juan, S.H.; Cheng, T.H.; Lin, H.C.; Chu, Y.L.; Lee, W.S. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem. Pharmacol. 2005, 69, 41–48. [Google Scholar] [CrossRef]
- Kim, J.W.; Lim, S.C.; Lee, M.Y.; Lee, J.W.; Oh, W.K.; Kim, S.K.; Kang, K.W. Inhibition of neointimal formation by trans-resveratrol: Role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food Res. 2010, 54, 1497–1505. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Zhu, H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006, 53, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Martin, K.A.; Rzucidlo, E.M. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS ONE 2014, 9, e85495. [Google Scholar] [CrossRef]
- Wang, D.; Uhrin, P.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, L.; Starzynski, R.R.; Tzvetkov, N.T.; et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: Molecular targets and pathways. Biotechnol. Adv. 2018, 36, 1586–1607. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.T.; Hwang, T.L.; Huang, Y.L.; Lin, C.F.; Wu, W.B. Vitisin B, a resveratrol tetramer, inhibits migration through inhibition of PDGF signaling and enhancement of cell adhesiveness in cultured vascular smooth muscle cells. Toxicol. Appl. Pharmacol. 2011, 256, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Lim, Y.; Hong, J.T.; Yoo, H.S.; Lee, C.K.; Pyo, M.Y.; Yun, Y.P. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul. Pharmacol. 2010, 53, 61–67. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Hebert, V.Y.; Kleinedler, J.J.; Rogers, L.K.; Ullevig, S.L.; Asmis, R.; Shi, R.; Dugas, T.R. Resveratrol and quercetin interact to inhibit neointimal hyperplasia in mice with a carotid injury. J. Nutr. 2012, 142, 1487–1494. [Google Scholar] [CrossRef]
- Orozco-Sevilla, V.; Naftalovich, R.; Hoffmann, T.; London, D.; Czernizer, E.; Yang, C.; Dardik, A.; Dardik, H. Epigallocatechin-3-gallate is a potent phytochemical inhibitor of intimal hyperplasia in the wire-injured carotid artery. J. Vasc. Surg. 2013, 58, 1360–1365. [Google Scholar] [CrossRef]
- Kleinedler, J.J.; Foley, J.D.; Orchard, E.A.; Dugas, T.R. Novel nanocomposite stent coating releasing resveratrol and quercetin reduces neointimal hyperplasia and promotes re-endothelialization. J. Control. Release 2012, 159, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Takayama, T.; Wang, B.; Kent, A.; Zhang, M.; Binder, B.Y.; Urabe, G.; Shi, Y.; DiRenzo, D.; Goel, S.A.; et al. Restenosis Inhibition and Re-differentiation of TGFbeta/Smad3-activated Smooth Muscle Cells by Resveratrol. Sci. Rep. 2017, 7, 41916. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, Y.; Chen, Q.; Wang, N.; Cao, K.; Hsieh, T.C.; Wu, J.M. Suppression of mitogenesis and regulation of cell cycle traverse by resveratrol in cultured smooth muscle cells. Int. J. Oncol. 1999, 15, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Yamori, Y. Resveratrol inhibits AGEs-induced proliferation and collagen synthesis activity in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2000, 274, 61–67. [Google Scholar] [CrossRef]
- Haider, U.G.; Sorescu, D.; Griendling, K.K.; Vollmar, A.M.; Dirsch, V.M. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol. Pharmacol. 2002, 62, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Mnjoyan, Z.H.; Fujise, K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: A role of p53-p21(WAF1/CIP1) pathway. Biochem. Biophys. Res. Commun. 2003, 311, 546–552. [Google Scholar] [CrossRef]
- Haider, U.G.; Sorescu, D.; Griendling, K.K.; Vollmar, A.M.; Dirsch, V.M. Resveratrol increases serine15-phosphorylated but transcriptionally impaired p53 and induces a reversible DNA replication block in serum-activated vascular smooth muscle cells. Mol. Pharmacol. 2003, 63, 925–932. [Google Scholar] [CrossRef]
- Poussier, B.; Cordova, A.C.; Becquemin, J.P.; Sumpio, B.E. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J. Vasc. Surg. 2005, 42, 1190–1197. [Google Scholar] [CrossRef]
- Lee, B.; Moon, S.K. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J. Nutr. 2005, 135, 2767–2773. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Labinskyy, N.; Hsieh, T.C.; Ungvari, Z.; Wu, J.M. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem. Biophys. Res. Commun. 2006, 346, 367–376. [Google Scholar] [CrossRef]
- Brito, P.M.; Devillard, R.; Negre-Salvayre, A.; Almeida, L.M.; Dinis, T.C.; Salvayre, R.; Auge, N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis 2009, 205, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kim, J.E.; Song, N.R.; Son, J.E.; Hwang, M.K.; Byun, S.; Kim, J.H.; Lee, K.W.; Lee, H.J. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc. Res. 2010, 85, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.L.; Al Sayah, L.; Weisbrod, R.M.; Van Roy, I.; Weng, X.; Cohen, R.A.; Bachschmid, M.M.; Seta, F. Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic Stiffness. Hypertension 2016, 68, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, R.; Ichiki, T.; Hashimoto, T.; Inanaga, K.; Imayama, I.; Sadoshima, J.; Sunagawa, K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 2018, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, M.Y.; Lim, J.H.; Kim, Y.; Shin, S.J.; Park, C.W.; Kim, Y.S.; Chang, Y.S.; Yoon, H.E.; Choi, B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis 2018, 270, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Cerletti, C.; Guglielmini, G.; Pignatelli, P.; de Gaetano, G.; Violi, F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011, 22, 201–211. [Google Scholar] [CrossRef]
- Rotondo, S.; Rajtar, G.; Manarini, S.; Celardo, A.; Rotillo, D.; de Gaetano, G.; Evangelista, V.; Cerletti, C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J. Pharmacol. 1998, 123, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.P.; Morrow, D.; Jin, Y.; von Offenberg Sweeney, N.; Sitzmann, J.V.; Cahill, P.A.; Redmond, E.M. Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis 2007, 195, e125–e133. [Google Scholar] [CrossRef]
- Li, Q.; Huyan, T.; Ye, L.J.; Li, J.; Shi, J.L.; Huang, Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014, 62, 10928–10935. [Google Scholar] [CrossRef]
- Pendurthi, U.R.; Williams, J.T.; Rao, L.V. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells: A possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 419–426. [Google Scholar] [CrossRef]
- Ferrero, M.E.; Bertelli, A.E.; Fulgenzi, A.; Pellegatta, F.; Corsi, M.M.; Bonfrate, M.; Ferrara, F.; De Caterina, R.; Giovannini, L.; Bertelli, A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am. J. Clin. Nutr. 1998, 68, 1208–1214. [Google Scholar] [CrossRef]
- Guo, R.; Liu, B.; Wang, K.; Zhou, S.; Li, W.; Xu, Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab. Vasc. Dis. Res. 2014, 11, 92–102. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y. Acad. Sci. 2017, 1403, 132–141. [Google Scholar] [CrossRef]
- Xia, N.; Horke, S.; Habermeier, A.; Closs, E.I.; Reifenberg, G.; Gericke, A.; Mikhed, Y.; Munzel, T.; Daiber, A.; Forstermann, U.; et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 78–85. [Google Scholar] [CrossRef]
- Xia, N.; Weisenburger, S.; Koch, E.; Burkart, M.; Reifenberg, G.; Forstermann, U.; Li, H. Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight. Br. J. Pharmacol. 2017, 174, 3443–3453. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xiao, N.; Wang, M.; Kou, J.; Qi, L.; Huang, F.; Liu, B.; Liu, K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol. Res. 2014, 89, 19–28. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Zhang, Y.; Liu, K.; Huang, F.; Liu, B.; Kou, J. Diosgenin regulates adipokine expression in perivascular adipose tissue and ameliorates endothelial dysfunction via regulation of AMPK. J. Steroid Biochem. Mol. Biol. 2016, 155 Pt A, 155–165. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta 2015, 1852, 1071–1113. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, Y.; Zhang, X.; Liu, Z.; Zhang, W.; Mao, W.; Wang, W.; Cui, W.; Zhang, X.; Jia, X.; et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin. Exp. Pharmacol. Physiol. 2005, 32, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Sehirli, O.; Ersahin, M.; Suleymanoglu, S.; Yiginer, O.; Emekli-Alturfan, E.; Yarat, A.; Yegen, B.C.; Sener, G. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J. Pharm. Pharmacol. 2010, 62, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Fenning, A.; Iyer, A.; Hoey, A.; Brown, L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011, 12, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef]
- Aubin, M.C.; Lajoie, C.; Clement, R.; Gosselin, H.; Calderone, A.; Perrault, L.P. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: Therapeutic potential of resveratrol. J. Pharmacol. Exp. Ther. 2008, 325, 961–968. [Google Scholar] [CrossRef]
- Miatello, R.; Vazquez, M.; Renna, N.; Cruzado, M.; Zumino, A.P.; Risler, N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am. J. Hypertens. 2005, 18, 864–870. [Google Scholar] [CrossRef]
- Akar, F.; Uludag, O.; Aydin, A.; Aytekin, Y.A.; Elbeg, S.; Tuzcu, M.; Sahin, K. High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats: Protective effect of resveratrol. Food Chem. Toxicol. 2012, 50, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Moron, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2000, 46, 78–83. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 10–100 µM | eNOS↑; NO↑ | [48] |
| EA.hy 926 | 10–100 µM | eNOS (via SIRT1/FOXO)↑; NO↑ | [14,48] |
| HCAEC | 1–100 µM | eNOS (via SIRT1)↑ | [13] |
| HUVEC | 0.1 µM | eNOS↑; VEGF↑; ET-1↓ | [33] |
| BAEC, HUVEC | 1–100 nM | p-eNOS↑ (via ERα & Erk1/2) | [24] |
| HUVEC | 1–100 µM | p-eNOS↑ (via AMPK) | [54] |
| STA | 50 µM | p-eNOS↑ (via AMPK) | [55] |
| RAEC | 100 µM | Ac-eNOS↓ | [56] |
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 1–100 µM | NADPH oxidase activity↓ | [72] |
| HUVEC | 10–100 µM | SOD1↑; GPx1↑; Nox4↓ | [73] |
| EA.hy 926 | 100 µM | SOD1↑; SOD2↑; SOD3↑; GPx1↑ catalase↑ | [71] |
| RAS | 1–100 µM | GPx1↑ catalase↑ | [74] |
| HCAEC | 1–10 µM | SOD2↑; SIRT1↑; GSH↑; mtROS↓ | [75] |
| HCAEC | 0.1–100 µM | Nrf2↑; NQO1↑; GCLC↑; HO-1↑ | [16] |
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 1–100 µM | ROS↓; p-Erk1/2↓; strain-induced ET-1↓ | [72] |
| HUVEC | 0.1 µM | ET-1↓; eNOS↑; VEGF↑ | [79] |
| HUVEC | 30 µM | ET-1↓; ECE-1↓ | [80] |
| HASMC | 100 µM | H2O2-induced ET-1↓; | [81] |
| RASMC | 10–100 µM | AngII-induced ET-1↓; proliferation↓ | [82] |
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| VSMC | 50–100 µM | Serum- and PDGF-induced proliferation↓ | [94] |
| RASMC | 0.1–1 µM | AGEs-stimulated proliferation↓ | [95] |
| RASMC | 25–50 µM | AngII-induced proliferation↓; p-Akt↓ | [96] |
| HASMC | 1–100 µM | Proliferation↓; p53↑; cell cycle arrest without apoptosis at 6.25–12.5 µM; apoptosis at 25 µM | [97] |
| RASMC | 50–100 µM | Serum-induced proliferation↓; cell cycle arrest | [98] |
| RASMC | 10–100 µM | AngII-induced proliferation↓; ET-1↓ | [82] |
| BASMC | 10–100 µM | Serum-induced proliferation↓; cell cycle arrest | [99] |
| HASMC | 20–100 µM | TNF-α-induced proliferation↓; cell cycle arrest | [100] |
| HASMC | 10–50 µM | Proliferation↓; p53↑; HSP27↑ | [101] |
| RFSMC | 25–50 µM | oxLDL-induced proliferation↓; PI3K/Akt/mTOR/p70S6K↓ | [102] |
| HASMC | 5–20 µM | PI3K activity↓; proliferation↓ | [103] |
| RASMC | 3–100 µM | Nrf2↑, HO-1↑; cyclin D↓, proliferation↓ | [84] |
| HVSMC | 3–100 µM | Differentiation of de-differentiated VSMC to the contractile phenotype | [86] |
| RASMC | 50 µM | TGF-β-stimulated SMC de-differentiation↓; p-Akt↓; p-mTOR↓; KLF5↓ | [93] |
| Model | Resveratrol dose | Effects | Reference |
|---|---|---|---|
| SHR | 5 mg/kg (50 mg/L in drinking water) for 10 weeks | BP↓; ROS↓; 3-NT↓; EF↑; eNOS↑; eNOS uncoupling↓ | [123] |
| SHR | 146 mg/kg (4 g/kg mixed in chow) for 5 weeks | BP↓; FMD↑; p-AMPK↑; p-eNOS↑; 4-HNE↓ | [124] |
| AngII-infused mouse | 320 mg/kg (4 g/kg mixed in chow) for 2 weeks | BP↓; FMD↑; p-AMPK↑; p-eNOS↑; 4-HNE↓ | [124] |
| Partially nephrectomized rats | 50 mg/kg/day mixed in diet for 4 weeks | BP↓; NO↑; ET-1↓; AngII↓ | [125] |
| Two-kidney, one-clip rats | 10 mg/kg i.p. for 6 weeks | BP↓; EF↑; plasma TAC ↑; NO↑; tissue SOD↑, catalase↑, GSH↑, MDA↓ cardiac hypertrophy↓ | [126] |
| DOCA salt | 1 mg/kg by gavage for 32 days | BP↓; EF↑ | [127] |
| Zucker rats | 10 mg/kg by gavage for 8 weeks | BP↓; eNOS↑; TG↓; TC↓; insulin↓; leptin↓ | [132] |
| HFD-fed female rats | 20 mg/kg/day mixed with diet for 8 weeks | BP↓; EF↑ | [129] |
| Fructose-fed rats | 10 mg/kg by gavage for 45 days | BP↓; cardiac hypertrophy↓ eNOS↑; TBARS↓ | [130] |
| HFCS-induced MetS in rats | 5 mg/day (50 mg/L in drinking water) for 10 weeks | BP↓; TG↓; EF↑; p-eNOS↑; ROS↓ | [131] |
| Ovariectomized rats | 5 mg/kg by gavage for 3 weeks | BP↓; EF↑ | [133] |
| Obese rats programmed by early weaning | 30 mg/kg/day for 30 days | BP↓; TG↓; LDL↓; plasma MDA↓, SOD↑, catalase↑ | [128] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. https://doi.org/10.3390/ijms20092155
Li H, Xia N, Hasselwander S, Daiber A. Resveratrol and Vascular Function. International Journal of Molecular Sciences. 2019; 20(9):2155. https://doi.org/10.3390/ijms20092155
Chicago/Turabian StyleLi, Huige, Ning Xia, Solveig Hasselwander, and Andreas Daiber. 2019. "Resveratrol and Vascular Function" International Journal of Molecular Sciences 20, no. 9: 2155. https://doi.org/10.3390/ijms20092155
APA StyleLi, H., Xia, N., Hasselwander, S., & Daiber, A. (2019). Resveratrol and Vascular Function. International Journal of Molecular Sciences, 20(9), 2155. https://doi.org/10.3390/ijms20092155






