Synthesis and Anticancer Activity of Novel 9-O-Substituted Berberine Derivatives
Abstract
:1. Introduction
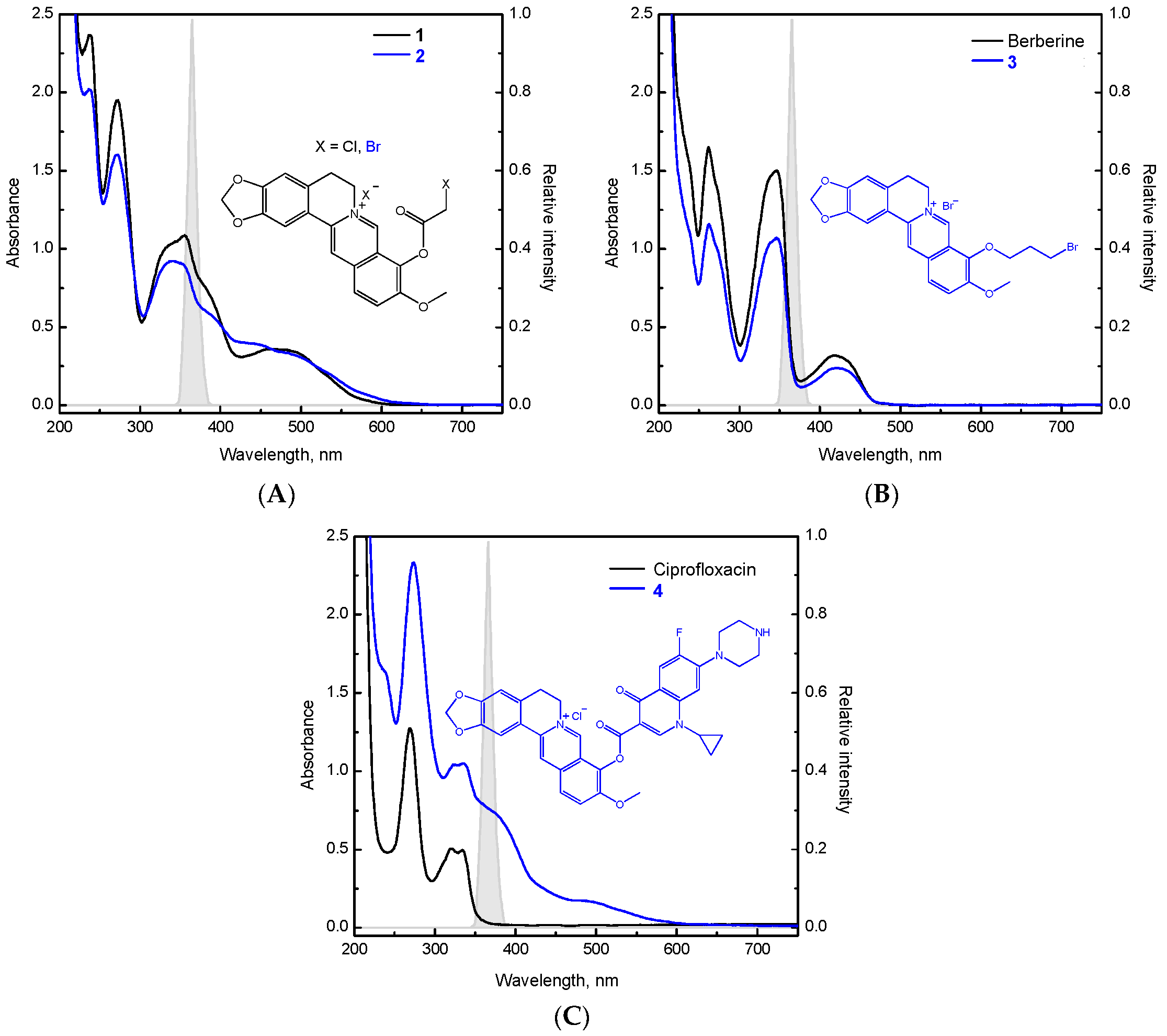
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Methods
3.1.1. Chemistry
General
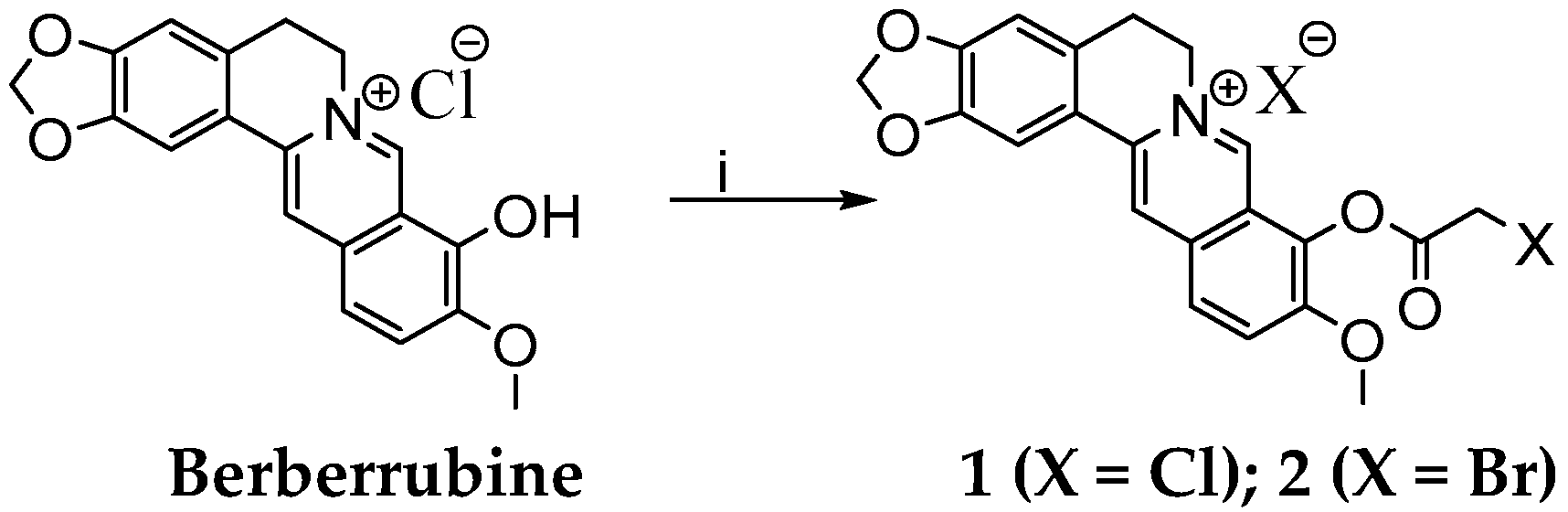
Selective Demethylation of Berberine on Its C9 Position
Synthesis of 9-(2-Chloro- or Bromo-acetoxy)-10-metoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a] isoquinoline-7-ylium Chloride or Bromide
Synthesis of 9-(3-Bromopropoxy)-10-methoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a] isoquinolin-7-ylium Bromide
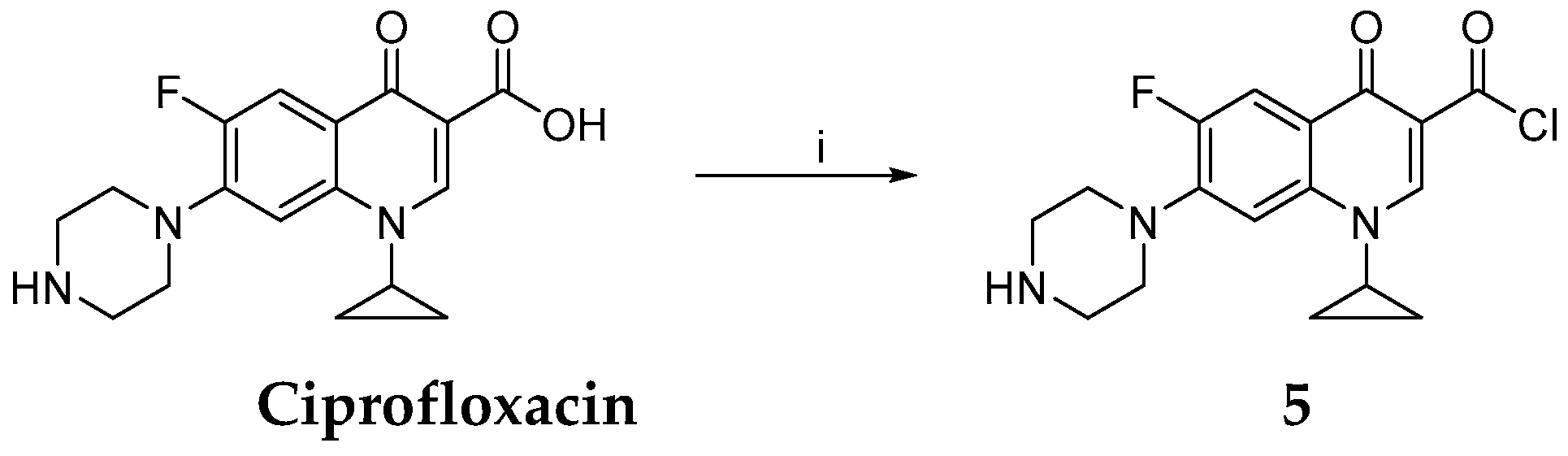
Synthesis of 1-Cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carbonyl Chloride
Synthesis of 9-(1-Cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carbonyloxy)-10-methoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium Chloride
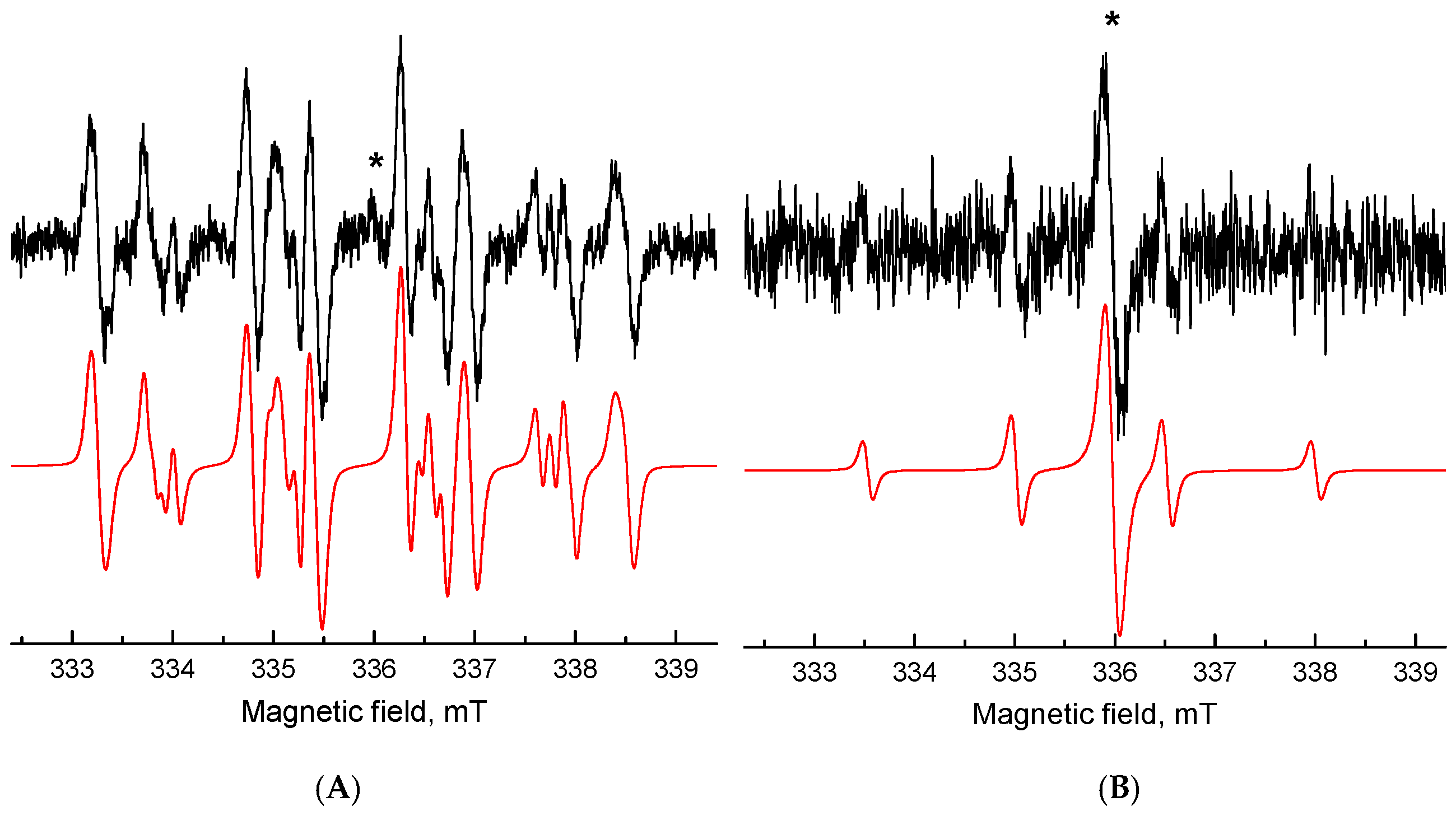
3.1.2. Electron Paramagnetic Resonance (EPR) Spectroscopy Studies of Berberine Derivatives
3.1.3. Evaluation of Antiproliferative Activities of Berberine Derivatives
Cell Cultures and Determination of Cell Viability and Morphology
Detection of Induction of Apoptosis by DNA Laddering Assay
Detection of Apoptosis by Caspase-3 Assay
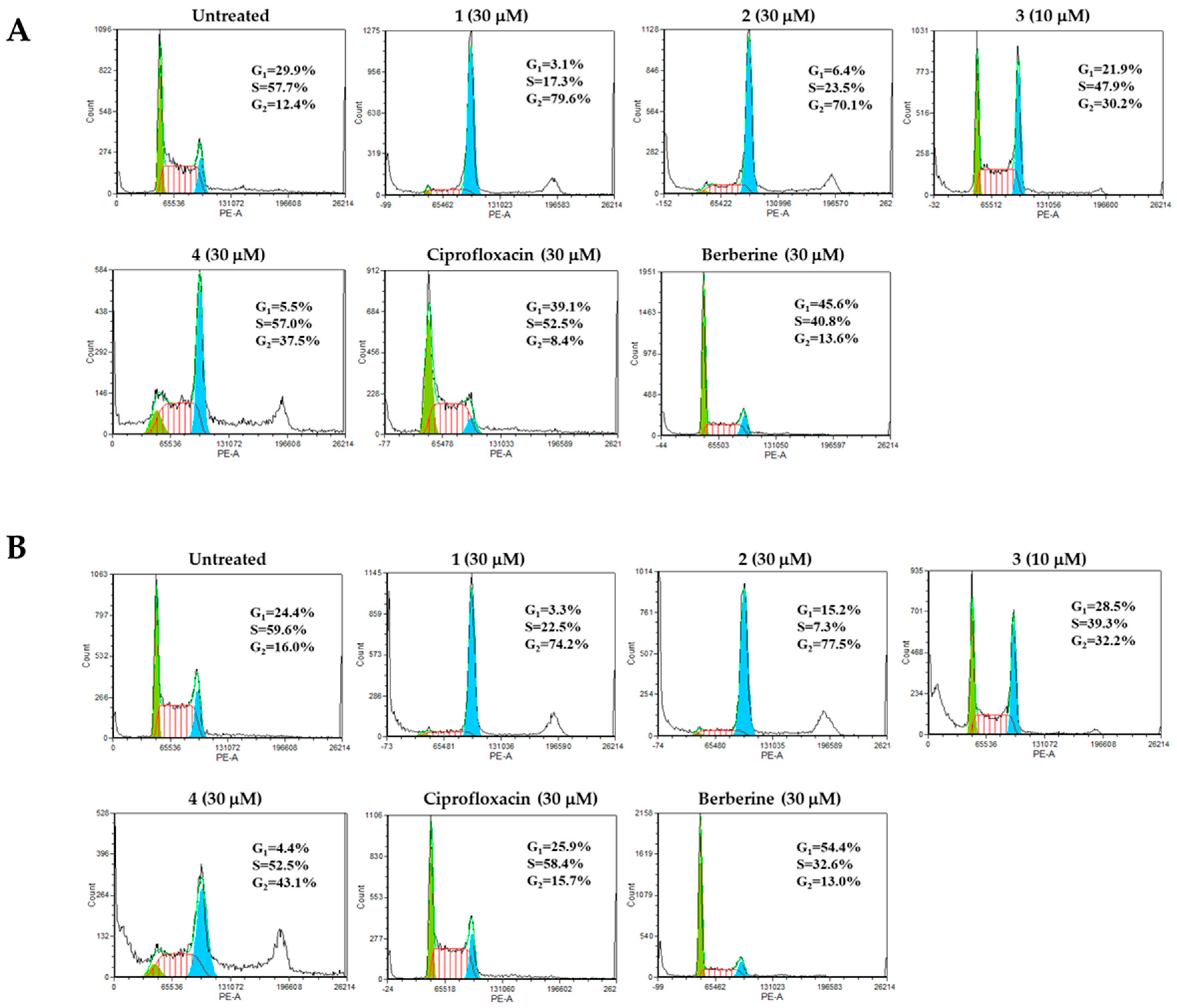
Determination of Cell Cycle Distribution
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DBP | 1,3-Dibromopropane |
| DMF | Dimethylformamide |
| DMPO | 5,5-Dimethyl-1-pyrroline N-oxide |
| DMSO | Dimethyl Sulfoxide |
| DTT | Dithiothreitol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | Phosphate buffer saline |
| PI | Propidium iodide |
| ROS | Reactive Oxygen Species |
| RT | Room temperature |
| Tempone | 4-Oxo-2,2,6,6-tetramethylpiperidine N-oxyl |
| THF | Tetrahydrofuran |
| TMPO | 4-Oxo-2,2,6,6-tetramethylpiperidine |
References
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A natural isoquinoline alkaloid with antitumor activity: Studies of the biological activities of berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Freile, M.L.; Giannini, F.; Pucci, G.; Sturniolo, A.; Rodero, L.; Pucci, O.; Balzareti, V.; Enriz, R.D. Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Fitoterapia 2003, 74, 702–705. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Huang, G.R.; Wu, M.H.; Tang, H.Y.; Huang, Z.S.; Zhou, X.H.; Yu, W.Q.; Su, J.W.; Mo, X.Q.; Chen, B.P.; et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. 2015, 21, 4225–4231. [Google Scholar] [CrossRef]
- Guamán-Ortiz, L.M.; Croce, A.L.; Aredia, F.; Sapienza, S.; Fiorillo, G.; Syeda, T.M.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Effect of new berberine derivatives on colon cancer cells. Acta Biochim. Biophys. Sin. 2015, 47, 824–833. [Google Scholar] [CrossRef]
- Jantova, S.; Cipak, L.; Letasiova, S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol. In Vitro 2007, 21, 25–31. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, L.; Wang, Y.; Zhang, H.; Xu, D.; Zhao, X.; Li, Y.; Li, J. Berberine inhibits androgen synthesis by interaction with aldo-keto reductase 1C3 in 22Rν1 prostate cancer cells. Asian J. Androl. 2016, 18, 607–612. [Google Scholar]
- Lu, J.J.; Fu, L.; Tang, Z.; Zhang, C.; Qin, L.; Wang, J.; Yu, Z.; Shi, D.; Xiao, X.; Xie, F.; et al. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 2016, 7, 2985–3001. [Google Scholar]
- Wang, J.; Qi, Q.; Feng, Z.; Zhang, X.; Huang, B.; Chen, A.; Prestegarden, L.; Li, X.; Wang, J. Berberine induces autophagy in glioblastoma by targeting the AMPK/mTOR/ULK1-pathway. Oncotarget 2016, 7, 66944–66958. [Google Scholar] [CrossRef]
- Mishan, M.A.; Ahmadiankia, N.; Matin, M.M.; Heirani-Tabasi, A.; Shahriyari, M.; Bidkhori, H.R.; Naderi-Meshkin, H.; Bahrami, A.R. Role of Berberine on molecular markers involved in migration of esophageal cancer cells. Cell. Mol. Biol. 2015, 61, 37–43. [Google Scholar]
- Chen, Q.; Qin, R.; Fang, Y.; Li, H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR93/PTEN/Akt signaling pathway. Cell Physiol. Biochem. 2015, 36, 956–965. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Geng, F.; Zhang, Z.; Li, J.; Yang, M.; Dong, L.; Gao, F. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis 2014, 19, 946–957. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Sharvan, R.; Gao, J.; Qu, S. Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed. Pharmacother. 2017, 95, 1225–1231. [Google Scholar] [CrossRef]
- Li, F.; Dong, X.; Lin, P.; Jiang, J. Regulation of Akt/FoxO3a/Skp2 Axis is critically involved in berberine-induced cell cycle arrest in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018, 19, 327. [Google Scholar]
- Eo, S.H.; Kim, J.H.; Kim, S.J. Induction of G₂/M arrest by berberine via activation of PI3K/Akt and p38 in human chondrosarcoma cell line. Oncol. Res. 2014, 22, 147–157. [Google Scholar] [CrossRef]
- Park, S.H.; Sung, J.H.; Kim, E.J.; Chung, N. Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Braz. J. Med. Biol. Res. 2015, 48, 111–119. [Google Scholar] [CrossRef]
- Gu, M.; Xu, J.; Han, C.; Kang, Y.; Liu, T.; He, Y.; Huang, Y.; Liu, C. Effects of berberine on cell cycle, DNA, reactive oxygen species, and apoptosis in L929 murine fibroblast cells. Evid. Based Complement. Alternat. Med. 2015, 2015, 796306. [Google Scholar] [CrossRef]
- Jantova, S.; Letasiova, S.; Brezova, V.; Cipak, L.; Labaj, J. Photochemical and phototoxic activity of berberine on murine fibroblast NIH-3T3 and Ehrlich ascites carcinoma cells. J. Photochem. Photobiol. B 2006, 85, 163–176. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Shi, Y.; Cao, H.; Chaturvedi, R.; Calcutt, M.W.; Hu, T.; Ren, X.; Wilson, K.T.; Polk, D.B.; et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS ONE 2012, 7, e36418. [Google Scholar] [CrossRef]
- Jung-Mu, H.; Mee-Sun, H.; Sang-Yong, L.; Woo-Yiel, L.; Dongho, K. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J. Cell. Biochem. 2009, 107, 955–964. [Google Scholar]
- Tong, N.; Zhang, J.; Chen, Y.; Zhubo, L.I.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes mutliple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Li, Y.; Mao, W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, J.; Tang, Q.; Xiao, Q.; Wu, W.; Hann, S.S. The enhancement of combination of berberine and metformin in inhibition of DNMT1 gene expression through interplay of SP1 and PDPK1. J. Cell Mol. Med. 2018, 22, 600–612. [Google Scholar] [CrossRef]
- Halicka, H.D.; Garcia, J.; Li, J.; Zhao, H.; Darzynkiewicz, Z. Synergy of 2-deoxy-d-glucose combined with berberine in inducing the lysosome/autophagy and transglutaminase activation-facilitated apoptosis. Apoptosis 2017, 22, 229–238. [Google Scholar] [CrossRef]
- Pandey, A.; Vishnoi, K.; Mahata, S.; Tripathi, S.C.; Misra, S.P.; Misra, V.; Mehrotra, R.; Dwivedi, M.; Bharti, A.C. Berberine and curcumin target survivin and STAT3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr. Cancer 2015, 67, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Munoz, A.R.; Pingali, S.; Payton-Stewart, F.; Chan, D.E.; Freeman, J.W.; Ghosh, R.; Kumar, A.P. Downregulation of STAT3/NF-kappaB potentiates gemcitabine activity in pancreatic cancer cells. Mol. Carcinog. 2017, 56, 402–411. [Google Scholar] [CrossRef]
- Yang, X.L.; Huang, N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol. Med. Rep. 2013, 8, 505–510. [Google Scholar] [CrossRef]
- Liu, B.; Wang, G.S.; Yang, J.; Pan, X.D.; Yang, Z.C.; Zang, L.Q. Berberine inhibits human hepatoma cell invasion without cytotoxicity in healthy hepatocytes. PLoS ONE 2011, 6, e21416. [Google Scholar] [CrossRef]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K.; Guo, Y. Preparation and evaluation of antidiabetic agents of berberine organic acid salts for enhancing the bioavailability. Molecules 2018, 24, 103. [Google Scholar] [CrossRef]
- Hsu, H.K.; Hsu, K.H.; Cheng, Y.M.; Suen, H.Y.; Peng, S.F. Development and in vitro evaluation of linear PEI-shelled heparin/berberine nanoparticles in human osteosarcoma U-2 OS cells. Molecules 2018, 23, 3122. [Google Scholar] [CrossRef]
- Huang, Z.J.; Zeng, Y.; Lan, P.; Sun, P.H.; Chen, W.M. Advances in structural modifications and biological activities of berberine: An active compound in traditional Chinese medicine. Mini Rev. Med. Chem. 2011, 11, 1122–1129. [Google Scholar] [CrossRef]
- Lo, C.Y.; Hsu, L.C.; Chen, M.S.; Lin, Y.J.; Chen, L.G.; Kuo, C.D.; Wu, J.Y. Synthesis and anticancer activity of a novel series of 9-O-substituted berberine derivatives: A lipophilic substitute role. Bioorg. Med. Chem. Lett. 2013, 23, 305–309. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, X.; Yin, W.; Liu, Z.; Zhou, M.; Xiao, D.; Liu, Y.; Peng, D. Synthesis and hypoglycemic activity of 9-O-(lipophilic group substituted) berberine derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 4799–4803. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; He, Y.; Hai, L.; Wu, Y. Synthesis and in vitro evaluation of 12-(substituted aminomethyl) berberrubine derivatives as anti-diabetics. Bioorg. Med. Chem. Lett. 2014, 24, 1762–1765. [Google Scholar] [CrossRef]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Jin, X.; Song, X.; Cao, Y.B.; Jiang, Y.Y.; Sun, Q.Y. Research progress in structural modification and pharmacological activities of berberine. J. Pharma Prac. 2014, 32, 171–175. [Google Scholar]
- Xiao, D.; He, F.; Peng, D.; Zou, M.; Peng, J.; Liu, P.; Liu, Y.; Liu, Z. Synthesis and anticancer activity of 9-O-pyrazole alkyl substituted berberine derivatives. Anticancer Agents Med. Chem. 2018, 18, 1639–1648. [Google Scholar] [CrossRef]
- Xiong, Y.-X.; Su, H.F.; Lv, P.; Ma, Y.; Wang, S.K.; Miao, H.; Huang, Z.S. A newly identified berberine derivative induces cancer cell senescence by stabilizing endogenous G-quadruplexes and sparking a DNA damage response at the telomere region. Oncotarget 2015, 6, 35625–35635. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, X.; Zhang, H.; Zhang, S.; Li, Y.; Liu, Y.; Peng, D. Synthesis and anti-inflammatory effects of a series of novel 9-O-substituted berberine derivatives. Med. Chem. Res. 2017, 26, 672–679. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Z.; Zhang, S.; Zhou, M.; He, F.; Zou, M.; Peng, J.; Xie, X.; Liu, Y.; Peng, D. Berberine derivatives with different pharmacological activities via structural modifications. Mini Rev. Med. Chem. 2018, 18, 1424–1441. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Khadka, D.B.; Cho, W.J. Pharmacological effects of berberine and its derivatives: A patent update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kłosińska-Szmurło, E.; Pluciński, F.A.; Grudzień, M.; Betlejewska-Kielak, K.; Biernacka, J.; Mazurek, A.P. Experimental and theoretical studies on the molecular properties of ciprofloxacin, norfloxacin, pefloxacin, sparfloxacin, and gatifloxacin in determining bioavailability. J. Biol. Phys. 2014, 40, 335–345. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Ye, X.G.; He, L.T.; Zhang, S.R.; Wang, R.L.; Zhou, J.; He, Z.S. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumoniae. J. Antibiot. 2016, 69, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Potacek, M.; Necas, M.; Zak, Z.; Dostal, J. Molecular and crystal structures of three berberine derivatives. Molecules 2001, 6, 433–441. [Google Scholar] [CrossRef]
- Das, B.; Srinivas, K.V.N.S. Conversion of berberine into berberrubine by selective demethylation under microwave irradiation. Synth. Commun. 2002, 32, 3027–3029. [Google Scholar] [CrossRef]
- Delgado-Camon, A.; Jarne, C.; Cebolla, V.L.; Larrañaga, O.; de Cozar, A.; Cossio, F.P.; Vara, Y.; Dominguez, A.; Membrado, L.; Galban, J.; et al. Resonance driven regioselective demethylation of berberine. Microwave assisted synthesis of berberrubine and its assessment as fluorescent chemosensor for alkanes. Tetrahedron 2015, 71, 6148–6154. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Patel, N.B.; Rajani, D. Antimycobacterial and antimicrobial studies of newly synthesized 3-(4-(6-methylbenzo[d]thiazol-2-yl) phenyl)quinazolin-4(3H)-ones. Indian J. Res. Pharm. Biotechnol. 2014, 2, 935–942. [Google Scholar]
- Tomisic, Z.B.; Kujundzic, N.K.; Bukvic Krajacic, M.; Visnjevac, A.; Kojic-Prodic, B. Molecular structures of new ciprofloxacin derivatives. J. Mol. Struct. 2002, 611, 73–81. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Harakeh, S.; Sakkaf, K.A.; Denetiu, I. Progress in microwave-aided chemical synthesis. Australian J. Chem. 2012, 65, 1647–1654. [Google Scholar] [CrossRef]
- Han, X.; Shao, K.; Hu, W. Synthesis of 9-substituted berberine derivatives with microwave irradiation. Chem. Res. Chin. Univ. 2018, 34, 571–577. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Kukielczak, B.M.; Bilski, P.; Sandvik, S.L.; Chignell, C.F. Photochemistry and photocytotoxicity of alkaloids from Goldenseal (Hydrastis canadensis L.) 1. Berberine. Chem. Res. Toxicol. 2001, 14, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K.; Hirano, T.; Nishimura, Y.; Arai, T.; Nosaka, Y. Dynamics of singlet oxygen generation by DNA-binding photosensitizers. J. Phys. Chem. B 2012, 116, 3037–3044. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K.; Hirano, T. The microenvironment of DNA switches the activity of singlet oxygen generation photosensitized by berberine and palmatine. Photochem. Photobiol. 2008, 84, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zalibera, M.; Rapta, P.; Stasko, A.; Brindzova, L.; Brezova, V. Thermal generation of stable spin trap adducts with super-hyperfine structure in their EPR spectra: An alternative EPR spin trapping assay for radical scavenging capacity determination in dimethylsulphoxide. Free Radic. Res. 2009, 43, 457–469. [Google Scholar] [CrossRef]
- Landolt, H.; Börnstein, R.; Fischer, H.; Madelung, O.; Deuschle, G. Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology; Springer: Berlin/Heidelberg, Germany, 1987; Volume 17, pp. 35–249. [Google Scholar]
- Marshall, D.L.; Christian, M.L.; Gryn’ova, G.; Coote, M.L.; Barker, P.J.; Blanksby, S.J. Oxidation of 4-substituted TEMPO derivatives reveals modifications at the 1- and 4-positions. Org. Biomol. Chem. 2011, 9, 4936–4947. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Fujita, K.; Iwama, H.; Oura, K.; Tadokoro, T.; Samukawa, E.; Sakamoto, T.; Nomura, T.; Tani, J.; Yoneyama, H.; Morishita, A.; et al. Cancer therapy due to apoptosis: Galectin-9. Int. J. Mol. Sci. 2017, 18, 74. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Jantova, S.; Paulovicova, E.; Paulovicova, L.; Topolska, D.; Panik, M.; Milata, V. Assessment of immunomodulatory activities and in vitro toxicity of new quinolone 7-ethyl-9-ethyl-6-oxo-6,9-dihydro[1,2,5]selenadiazolo[3,4-h]quinoline-7-carboxylate. Immunol. Investig. 2017, 46, 341–360. [Google Scholar] [CrossRef]
- Cipak, L.; Rauko, P.; Miadokova, E.; Cipakova, I.; Novotny, L. Effects of flavonoids on cisplatin-induced apoptosis of HL-60 and L1210 leukemia cells. Leuk. Res. 2003, 27, 65–72. [Google Scholar] [CrossRef]
- Cipak, L.; Novotny, L.; Cipakova, I.; Rauko, P. Differential modulation of cisplatin and doxorubicin efficacies in leukemia cells by flavonoids. Nutr. Res. 2003, 8, 1045–1057. [Google Scholar] [CrossRef]





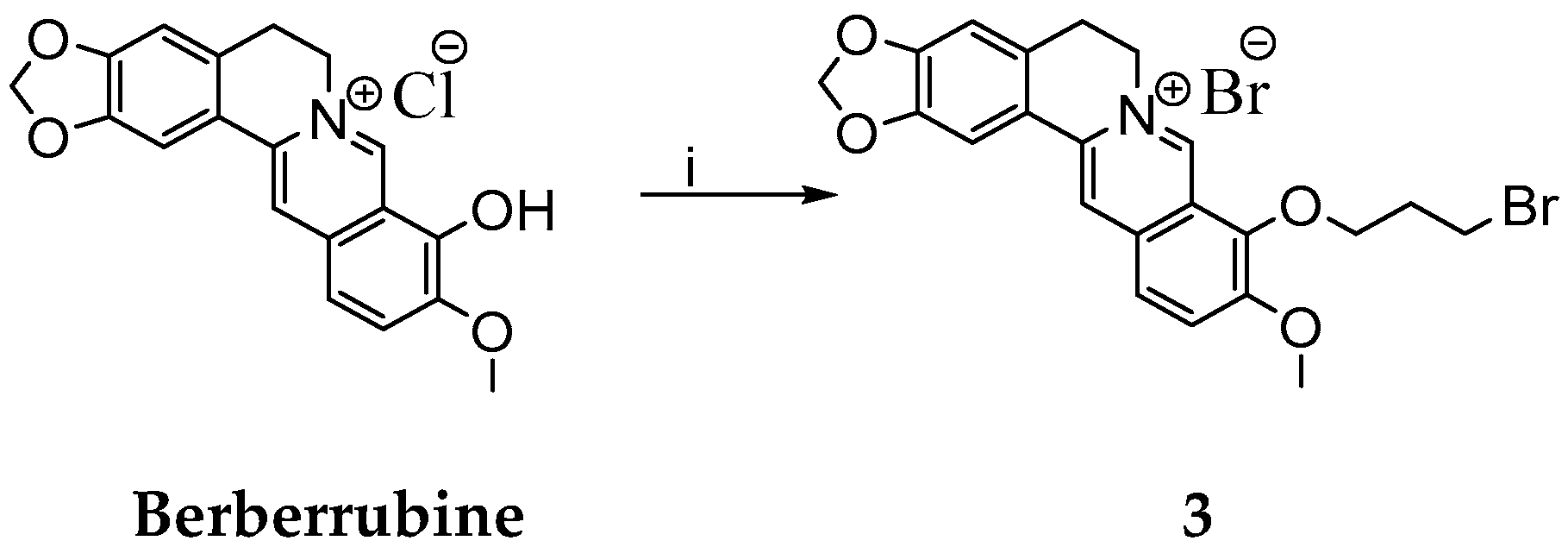

| Reaction | DBP (Equivalents) | Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| R1 | 3 | CH3CN | 60 | 2 | 48 |
| R2 | 10 | CH3CN | 60 | 2 | 55 |
| R3 | 10 | DMF | 80 | 2 | 59 |
| R4 | 58 | DMF | 80 | 2 | 74 |
| Cell Line | Time (h) | Berberine | Ciprofloxacin | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|---|
| HeLa | 24 | ˃200 | ˃200 | ˃200 | ˃200 | 58 ± 2.6 | ˃200 |
| 48 | 126 ± 7.8 | 175 ± 8.7 | 115 ± 7.4 | 95 ± 4.8 | 36 ± 1.9 | ˃200 | |
| HL-60 | 24 | 43.7 ± 1.1 | ˃200 | 23.4 ± 2.6 | 14.6 ± 1.7 | 2.7 ± 0.9 | 19.3 ± 1.3 |
| 48 | 27.1 ± 2.6 | 142 ± 5.7 | 16.7 ± 0.8 | 11.2 ± 1.6 | 0.7 ± 0.2 | 15.7 ± 1.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milata, V.; Svedova, A.; Barbierikova, Z.; Holubkova, E.; Cipakova, I.; Cholujova, D.; Jakubikova, J.; Panik, M.; Jantova, S.; Brezova, V.; et al. Synthesis and Anticancer Activity of Novel 9-O-Substituted Berberine Derivatives. Int. J. Mol. Sci. 2019, 20, 2169. https://doi.org/10.3390/ijms20092169
Milata V, Svedova A, Barbierikova Z, Holubkova E, Cipakova I, Cholujova D, Jakubikova J, Panik M, Jantova S, Brezova V, et al. Synthesis and Anticancer Activity of Novel 9-O-Substituted Berberine Derivatives. International Journal of Molecular Sciences. 2019; 20(9):2169. https://doi.org/10.3390/ijms20092169
Chicago/Turabian StyleMilata, Viktor, Alexandra Svedova, Zuzana Barbierikova, Eva Holubkova, Ingrid Cipakova, Dana Cholujova, Jana Jakubikova, Miroslav Panik, Sona Jantova, Vlasta Brezova, and et al. 2019. "Synthesis and Anticancer Activity of Novel 9-O-Substituted Berberine Derivatives" International Journal of Molecular Sciences 20, no. 9: 2169. https://doi.org/10.3390/ijms20092169
APA StyleMilata, V., Svedova, A., Barbierikova, Z., Holubkova, E., Cipakova, I., Cholujova, D., Jakubikova, J., Panik, M., Jantova, S., Brezova, V., & Cipak, L. (2019). Synthesis and Anticancer Activity of Novel 9-O-Substituted Berberine Derivatives. International Journal of Molecular Sciences, 20(9), 2169. https://doi.org/10.3390/ijms20092169





