The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity
Abstract
:1. Introduction
1.1. Arf6 and Its Relatives
1.2. Activation and Inactivation of Arf6 through GEFs and GAPs
1.3. Research Tools to Study Arf6
1.4. Roles and Mechanisms of Arf6
1.4.1. Arf6 Directly Activates Lipid Modifying Enzymes
1.4.2. Arf6 Stimulates Actin Polymerization
1.4.3. Arf6 and Rab35 Are Mutual Antagonists that Cooperate
1.4.4. Arf6 Can Direct Transport along Microtubules
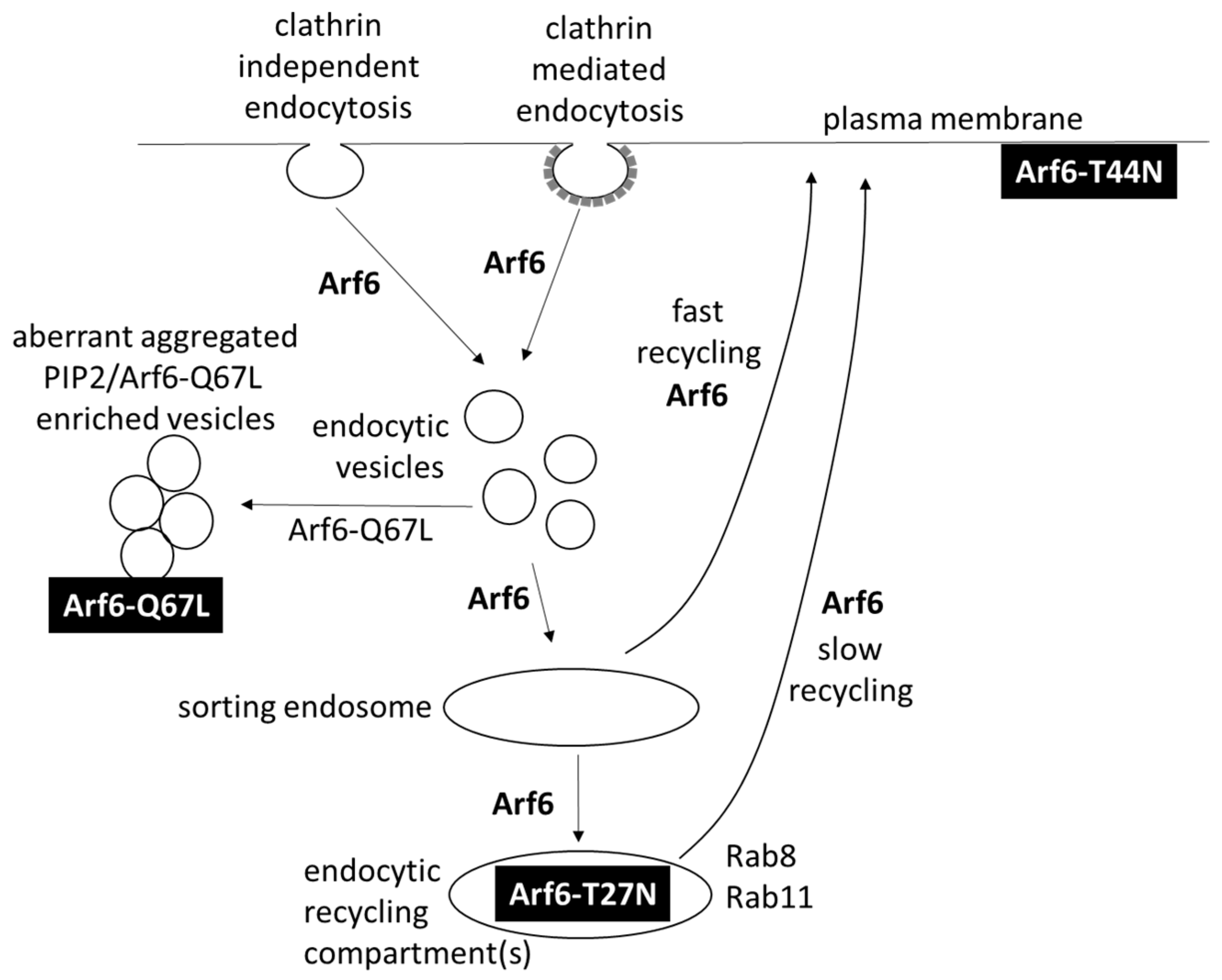
1.4.5. Arf6 Directs Transport after Clathrin-Mediated Endocytosis and Clathrin-Independent Endocytosis
1.4.6. Arf6 Assists Autophagy
1.5. Roles of Arf6 in Health and Disease
2. Arf6 Drives Phagocytosis through Multiple Mechanisms of Action, which Can Be Counteracted by Pathogens
3. Bacteria and Protozoa Hijack Arf6 for Invasion in Host Cells and Virulence
4. Viruses Take Advantage of Arf6
5. Arf6 Activation Precedes Reactive Oxygen Species (ROS) Production
6. The Crucial Role of Arf6 in TLR Signaling
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACAP2 | Arf GAP with coiled coil, ankyrin repeat and PH domains 2 |
| ADAP2 | ArfGAP With Dual PH Domains 2 |
| Arf | ADP-ribosylation factor |
| ARNO | ARF nucleotide binding site opener |
| ASAP2 | Arf-GAP with SH3 domain, ankyrin repeat and PH domain-containing protein 2 |
| BFA | Brefeldin A |
| BRAG2 | Brefeldin A-resistant Arf GEF 2 |
| CR | complement receptor |
| EFA6 | Exchange Factor for Arf6 |
| EHEC | enterohemorrhagic Escherichia coli |
| ELMOD | ELMO Domain Containing |
| EPEC | enteropathogenic Escherichia coli |
| ERK1/2 | extracellular signal-regulated protein kinase 1/2 |
| FcγR | Fcγ receptor |
| fMLP | formyl-methionine-leucine-phenylalanine |
| FRET | fluorescence resonance energy transfer |
| GAP | GTPase-activating proteins |
| GEF | guanine nucleotide exchange factor |
| Grp1 | General receptor for phosphoinositides isoform 1 |
| IFN-γ | interferon-γ |
| IL | interleukin |
| IRF3 | interferon-regulator factor 3 |
| LPS | lipopolysaccharide |
| Mal | MyD88-adaptor like |
| MHC | major histocompatibility complex |
| MyD88 | Myeloid differentiation factor 88 |
| NF-κB | nuclear factor kappa B |
| OCRL | OculoCerebroRenal syndrome of Lowe |
| PAMP | pathogen-associated molecular pattern |
| PI3K | phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PIP5K | phosphatidylinositol 4-phosphate 5-kinase |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| PLD | phospholipase D |
| PMA | phorbol-12-myristate 13-acetate |
| SCV | Salmonella-containing vacuole |
| TGN | trans-Golgi network |
| TIR | Toll/interleukin-1 receptor |
| TLR | Toll-like receptor |
| Tram | Trif-related adaptor molecule |
| Trif | TIR-domain-containing adaptor-inducing interferon-β |
References
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.A.; Gilman, A.G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J. Biol. Chem. 1984, 259, 6228–6234. [Google Scholar] [PubMed]
- Tsuchiya, M.; Price, S.R.; Tsai, S.C.; Moss, J.; Vaughan, M. Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells. J. Biol. Chem. 1991, 266, 2772–2777. [Google Scholar] [PubMed]
- D’Souza-Schorey, C.; Chavrier, P. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006, 7, 347–358. [Google Scholar] [CrossRef]
- Donaldson, J.G. Multiple Roles for Arf6: Sorting, Structuring, and Signaling at the Plasma Membrane. J. Biol. Chem. 2003, 278, 41573–41576. [Google Scholar] [CrossRef]
- Gaschet, J.; Hsu, V.W. Distribution of ARF6 between membrane and cytosol is regulated by its GTPase cycle. J. Biol. Chem. 1999, 274, 20040–20045. [Google Scholar] [PubMed]
- Renault, L.; Guibert, B.; Cherfils, J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 2003, 426, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J. Structural basis for activation of ARF GTPase: Mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 1998, 95, 237–248. [Google Scholar] [CrossRef]
- Mossessova, E.; Corpina, R.A.; Goldberg, J. Crystal Structure of ARF1•Sec7 Complexed with Brefeldin A and Its Implications for the Guanine Nucleotide Exchange Mechanism. Mol. Cell 2003, 12, 1403–1411. [Google Scholar] [CrossRef]
- Vetter, I.R.; Wittinghofer, A. The guanine nucleotide-binding switch in three dimensions. Science 2001, 294, 1299–1304. [Google Scholar] [CrossRef]
- Szafer, E.; Pick, E.; Rotman, M.; Zuck, S.; Huber, I.; Cassel, D. Role of coatomer and phospholipids in GTPase-activating protein-dependent hydrolysis of GTP by ADP-ribosylation factor-1. J. Biol. Chem. 2000, 275, 23615–23619. [Google Scholar] [CrossRef] [PubMed]
- Mandiyan, V.; Andreev, J.; Schlessinger, J.; Hubbard, S.R. Crystal structure of the ARF-GAP domain and ankyrin repeats of PYK2-associated protein beta. EMBO J. 1999, 18, 6890–6898. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.A.; East, M.P.; Yi, S.L.; Kahn, R.A. Characterization of recombinant ELMOD (cell engulfment and motility domain) proteins as GTPase-activating proteins (GAPs) for ARF family GTPases. J. Biol. Chem. 2014, 289, 11111–11121. [Google Scholar] [CrossRef]
- Hongu, T.; Kanaho, Y. Activation machinery of the small GTPase Arf6. Adv. Biol. Regul. 2013, 54, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Schorey, C.; Li, G.; Colombo, M.I.; Stahl, P.D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science 1995, 267, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Macia, E. The GDP-bound form of Arf6 is located at the plasma membrane. J. Cell Sci. 2004, 117, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Caumont, A.S.; Galas, M.C.; Vitale, N.; Aunis, D.; Bader, M.F. Regulated exocytosis in chromaffin cells: Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem. 1998, 273, 1373–1379. [Google Scholar] [CrossRef]
- Van Acker, T.; Eyckerman, S.; Vande Walle, L.; Gerlo, S.; Goethals, M.; Lamkanfi, M.; Bovijn, C.; Tavernier, J.; Peelman, F. The small GTPase Arf6 is essential for the Tram/Trif pathway in TLR4 signaling. J. Biol. Chem. 2014, 289, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C.; Medzhitov, R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 2006, 125, 943–955. [Google Scholar] [CrossRef]
- Shome, K.; Nie, Y.; Romero, G. ADP-ribosylation factor proteins mediate agonist-induced activation of phospholipase D. J. Biol. Chem. 1998, 273, 30836–30841. [Google Scholar] [CrossRef]
- Toda, K.; Nogami, M.; Murakami, K.; Kanaho, Y.; Nakayama, K. Colocalization of phospholipase D1 and GTP-binding-defective mutant of ADP-ribosylation factor 6 to endosomes and lysosomes. FEBS Lett. 1999, 442, 221–225. [Google Scholar] [CrossRef]
- Santy, L.C.; Casanova, J.E. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 2001, 154, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Melendez, A.J.; Harnett, M.M.; Allen, J.M. Crosstalk between ARF6 and protein kinase Calpha in FcγRI-mediated activation of phospholipase D1. Curr. Biol. 2001, 11, 869–874. [Google Scholar] [CrossRef]
- Cockcroft, S.; de Matteis, M.A. Inositol lipids as spatial regulators of membrane traffic. J. Membr. Biol. 2001, 180, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.-S.; Enjalbert, S.; Comunale, F.; Bodin, S.; Vitale, N.; Charrasse, S.; Gauthier-Rouvière, C. ADP-ribosylation factor 6 regulates mammalian myoblast fusion through phospholipase D1 and phosphatidylinositol 4,5-bisphosphate signaling pathways. Mol. Biol. Cell 2010, 21, 2412–2424. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-C.; Huang, T.-H.; Chang, C.-S.; Tsai, Y.-R.; Lin, R.-H.; Lee, P.-W.; Hsu, M.-F.; Huang, L.-J.; Wang, J.-P. Signaling mechanisms of inhibition of phospholipase D activation by CHS-111 in formyl peptide-stimulated neutrophils. Biochem. Pharmacol. 2011, 81, 269–278. [Google Scholar] [CrossRef]
- Honda, A.; Nogami, M.; Yokozeki, T.; Yamazaki, M.; Nakamura, H.; Watanabe, H.; Kawamoto, K.; Nakayama, K.; Morris, A.J.; Frohman, M.A.; et al. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 1999, 99, 521–532. [Google Scholar] [CrossRef]
- Humphreys, D.; Davidson, A.C.; Hume, P.J.; Makin, L.E.; Koronakis, V. Arf6 coordinates actin assembly through the WAVE complex, a mechanism usurped by Salmonella to invade host cells. Proc. Natl. Acad. Sci. USA 2013, 110, 16880–16885. [Google Scholar] [CrossRef] [PubMed]
- Koronakis, V.; Hume, P.J.; Humphreys, D.; Liu, T.; Horning, O.; Jensen, O.N.; McGhie, E.J. WAVE regulatory complex activation by cooperating GTPases Arf and Rac1. Proc. Natl. Acad. Sci. USA 2011, 108, 14449–14454. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Davidson, A.C.; Hume, P.J.; Humphreys, D.; Koronakis, V. Arf GTPase interplay with Rho GTPases in regulation of the actin cytoskeleton. Small GTPases 2017, 1–8. [Google Scholar] [CrossRef]
- Santy, L.C.; Ravichandran, K.S.; Casanova, J.E. The DOCK180/Elmo complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr. Biol. 2005, 15, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, N.; Scott, D.W.; Castle, J.D.; Casanova, J.E.; Schwartz, M.A. Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat. Cell Biol. 2007, 9, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.R.; Casanova, J.E. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 2008, 18, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Boshans, R.L.; Szanto, S.; van Aelst, L.; D’Souza-Schorey, C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol. Cell. Biol. 2000, 20, 3685–3694. [Google Scholar] [CrossRef]
- Frank, S.R.; Hansen, S.H. The PIX–GIT complex: A G protein signaling cassette in control of cell shape. Semin. Cell Dev. Biol. 2008, 19, 234–244. [Google Scholar] [CrossRef]
- Osmani, N.; Peglion, F.; Chavrier, P.; Etienne-Manneville, S. Cdc42 localization and cell polarity depend on membrane traffic. J. Cell Biol. 2010, 191, 1261–1269. [Google Scholar] [CrossRef]
- Chesneau, L.; Dambournet, D.; MacHicoane, M.; Kouranti, I.; Fukuda, M.; Goud, B.; Echard, A. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr. Biol. 2012, 22, 147–153. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuda, M. Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J. Cell Sci. 2012, 125, 2235–2243. [Google Scholar] [CrossRef]
- Dambournet, D.; MacHicoane, M.; Chesneau, L.; Sachse, M.; Rocancourt, M.; El Marjou, A.; Formstecher, E.; Salomon, R.; Goud, B.; Echard, A. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat. Cell Biol. 2011, 13, 981–988. [Google Scholar] [CrossRef]
- Cauvin, C.; Rosendale, M.; Gupta-Rossi, N.; Rocancourt, M.; Larraufie, P.; Salomon, R.; Perrais, D.; Echard, A. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr. Biol. 2016, 26, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, G.; Sibarita, J.B.; Loubéry, S.; Daviet, L.; Romao, M.; Raposo, G.; Chavrier, P. ARF6 Interacts with JIP4 to Control a Motor Switch Mechanism Regulating Endosome Traffic in Cytokinesis. Curr. Biol. 2009, 19, 184–195. [Google Scholar] [CrossRef]
- Fielding, A.B.; Schonteich, E.; Matheson, J.; Wilson, G.; Yu, X.; Hickson, G.R.X.; Srivastava, S.; Baldwin, S.A.; Prekeris, R.; Gould, G.W. Rab11-FIP3 and FIP4 interact with Arf6 and the Exocyst to control membrane traffic in cytokinesis. EMBO J. 2005, 24, 3389–3399. [Google Scholar] [CrossRef]
- Montagnac, G.; de Forges, H.; Smythe, E.; Gueudry, C.; Romao, M.; Salamero, J.; Chavrier, P. Decoupling of activation and effector binding underlies ARF6 priming of fast endocytic recycling. Curr. Biol. 2011, 21, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Marchesin, V.; Castro-Castro, A.; Lodillinsky, C.; Castagnino, A.; Cyrta, J.; Bonsang-Kitzis, H.; Fuhrmann, L.; Irondelle, M.; Infante, E.; Montagnac, G.; et al. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J. Cell Biol. 2015, 211, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.P.; Hanscom, S.R.; Jolly, R.S.; Futter, C.E.; McCaffrey, M.W. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J. Cell Sci. 2010, 123, 181–191. [Google Scholar] [CrossRef]
- Simon, G.C.; Prekeris, R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem. Soc. Trans. 2008, 36, 391–394. [Google Scholar] [CrossRef]
- Radhakrishna, H.; Donaldson, J.G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 1997, 139, 49–61. [Google Scholar] [CrossRef]
- Sugita, M.; Grant, E.P.; Van Donselaar, E.; Hsu, V.W.; Rogers, R.A.; Peters, P.J.; Brenner, M.B. Separate pathways for antigen presentation by CD1 molecules. Immunity 1999, 11, 743–752. [Google Scholar] [CrossRef]
- Palacios, F.; Schweitzer, J.K.; Boshans, R.L.; D’Souza-Schorey, C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 2002, 4, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.D.; Rozelle, A.L.; Yin, H.L.; Balla, T.; Donaldson, J.G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001, 154, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Donaldson, J.G. Sorting of Clathrin-Independent Cargo Proteins Depends on Rab35 Delivered by Clathrin-Mediated Endocytosis. Traffic 2015, 16, 994–1009. [Google Scholar] [CrossRef] [PubMed]
- Kouranti, I.; Sachse, M.; Arouche, N.; Goud, B.; Echard, A. Rab35 Regulates an Endocytic Recycling Pathway Essential for the Terminal Steps of Cytokinesis. Curr. Biol. 2006, 16, 1719–1725. [Google Scholar] [CrossRef]
- Goldenring, J.R. Recycling endosomes. Curr. Opin. Cell Biol. 2015, 35, 117–122. [Google Scholar] [CrossRef]
- Hattula, K.; Furuhjelm, J.; Tikkanen, J.; Tanhuanpaa, K.; Laakkonen, P.; Peranen, J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J. Cell Sci. 2006, 119, 4866–4877. [Google Scholar] [CrossRef]
- Wilson, G.M. The FIP3-Rab11 Protein Complex Regulates Recycling Endosome Targeting to the Cleavage Furrow during Late Cytokinesis. Mol. Biol. Cell 2004, 16, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Oshima, K.; Onishi, S.; Takeda, M.; Shinmyozu, K.; Yonemura, S.; Hayashi, S. IKKε Regulates Cell Elongation through Recycling Endosome Shuttling. Dev. Cell 2011, 20, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Prigent, M.; Dubois, T.; Raposo, G.; Derrien, V.; Tenza, D.; Rossé, C.; Camonis, J.; Chavrier, P. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 2003, 163, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.; Ravikumar, B.; Puri, C.; Rubinsztein, D.C. Arf6 promotes autophagosome formation via effects on phosphatidylinositol 4,5-bisphosphate and phospholipase D. J. Cell Biol. 2012, 196, 483–496. [Google Scholar] [CrossRef]
- George, A.A.; Hayden, S.; Stanton, G.R.; Brockerhoff, S.E. Arf6 and the 5’phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors. BioEssays 2016, 38, S119–S135. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, C.; Zhang, X.; Wu, Y.; Pan, S.; Xiao, Y. Roles of Arf6 in cancer cell invasion, metastasis and proliferation. Life Sci. 2017, 182, 80–84. [Google Scholar] [CrossRef]
- Schweitzer, J.K.; Sedgwick, A.E.; D’Souza-Schorey, C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin. Cell Dev. Biol. 2011, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Millar, C.A.; Powell, K.A.; Hickson, G.R.X.; Bader, M.F.; Gould, G.W. Evidence for a role for ADP-ribosylation factor 6 in insulin-stimulated glucose transporter-4 (GLUT4) trafficking in 3T3-L1 adipocytes. J. Biol. Chem. 1999, 274, 17619–17625. [Google Scholar] [CrossRef] [PubMed]
- Tagliatti, E.; Fadda, M.; Falace, A.; Benfenati, F.; Fassio, A. Arf6 regulates the cycling and the readily releasable pool of synaptic vesicles at hippocampal synapse. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, J.; Nakayama, K.; Nagao, T.; Ichijo, H.; Urushidani, T. Role of ADP-ribosylation factor 6 (ARF6) in gastric acid secretion. J. Biol. Chem. 2003, 278, 36470–36475. [Google Scholar] [CrossRef]
- Grossmann, A.H.; Yoo, J.H.; Clancy, J.; Sorensen, L.K.; Sedgwick, A.; Tong, Z.; Ostanin, K.; Rogers, A.; Grossmann, K.F.; Tripp, S.R.; et al. The small GTPase ARF6 stimulates β-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci. Signal. 2013, 6. [Google Scholar] [CrossRef]
- Knizhnik, A.V.; Kovaleva, O.V.; Komelkov, A.V.; Trukhanova, L.S.; Rybko, V.A.; Zborovskaya, I.B.; Tchevkina, E.M. Arf6 promotes cell proliferation via the PLD-mTORC1 and p38MAPK pathways. J. Cell. Biochem. 2012, 113, 360–371. [Google Scholar] [CrossRef]
- Egami, Y.; Fukuda, M.; Araki, N. Rab35 regulates phagosome formation through recruitment of ACAP2 in macrophages during FcγR-mediated phagocytosis. J. Cell Sci. 2011, 124, 3557–3567. [Google Scholar] [CrossRef]
- Rougerie, P.; Miskolci, V.; Cox, D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: Role and regulation of the actin cytoskeleton. Immunol. Rev. 2013, 256, 222–239. [Google Scholar] [CrossRef]
- Zhang, Q.; Cox, D.; Tseng, C.C.; Donaldson, J.G.; Greenberg, S. A requirement for ARF6 in Fcγ receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998, 273, 19977–19981. [Google Scholar] [CrossRef]
- Crozier, P.A. Quantitative elemental mapping of materials by energy-filtered imaging. Ultramicroscopy 1995, 58, 157–174. [Google Scholar] [CrossRef]
- Niedergang, F.; Colucci-Guyon, E.; Dubois, T.; Raposo, G.; Chavrier, P. ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 2003, 161, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Boulais, J.; Charriere, G.M.; Hennessy, E.J.; Brunet, S.; Jutras, I.; Goyette, G.; Rondeau, C.; Letarte, S.; Huang, H.; et al. A systems biology analysis of the Drosophila phagosome. Nature 2007, 445, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.J.; Teruel, M.; Dierckman, R.; Anderson, R.; Wells, A.; York, J.D.; Meyer, T.; Grinstein, S. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 2000, 151, 1353–1368. [Google Scholar] [CrossRef]
- Coppolino, M.G.; Dierckman, R.; Loijens, J.; Collins, R.F.; Pouladi, M.; Jongstra-Bilen, J.; Schreiber, A.D.; Trimble, W.S.; Anderson, R.; Grinstein, S. Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J. Biol. Chem. 2002, 277, 43849–43857. [Google Scholar] [CrossRef] [PubMed]
- Beemiller, P.; Hoppe, A.D.; Swanson, J.A. A phosphatidylinositol-3-kinase-dependent signal transition regulates ARF1 and ARF6 during Fcγ receptor-mediated phagocytosis. PLoS Biol. 2006, 4, e162. [Google Scholar] [CrossRef] [PubMed]
- Beemiller, P.; Zhang, Y.; Mohan, S.; Levinsohn, E.; Gaeta, I.; Hoppe, A.D.; Swanson, J.A. A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis. Mol. Biol. Cell 2010, 21, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Klarlund, J.K.; Tsiaras, W.; Holik, J.J.; Chawla, A.; Czech, M.P. Distinct polyphosphoinositide binding selectivities for pleckstrin homology domains of GRP1-like proteins based on diglycine versus triglycine motifs. J. Biol. Chem. 2000, 275, 32816–32821. [Google Scholar] [CrossRef]
- Someya, A.; Moss, J.; Nagaoka, I. The guanine nucleotide exchange protein for ADP-ribosylation factor 6, ARF-GEP100/BRAG2, regulates phagocytosis of monocytic phagocytes in an ARF6-dependent process. J. Biol. Chem. 2010, 285, 30698–30707. [Google Scholar] [CrossRef] [PubMed]
- El Azreq, M.-A.; Garceau, V.; Bourgoin, S.G. Cytohesin-1 regulates fMLF-mediated activation and functions of the β2 integrin Mac-1 in human neutrophils. J. Leukoc. Biol. 2011, 89, 823–836. [Google Scholar] [CrossRef]
- Uchida, H.; Kondo, A.; Yoshimura, Y.; Mazaki, Y.; Sabe, H. PAG3/Papalpha/KIAA0400, a GTPase-activating protein for ADP-ribosylation factor (ARF), regulates ARF6 in Fcγ receptor-mediated phagocytosis of macrophages. J. Exp. Med. 2001, 193, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.L.; Fredericks, G.J.; Huang, Z.; Fay, J.D.; Hoffmann, F.W.; Hoffmann, P.R. Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient FcγR-mediated phagocytosis. J. Leukoc. Biol. 2017, 101, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Egami, Y.; Fujii, M.; Kawai, K.; Ishikawa, Y.; Fukuda, M.; Araki, N. Activation-inactivation cycling of Rab35 and ARF6 is required for phagocytosis of zymosan in RAW264 macrophages. J. Immunol. Res. 2015, 2015, 429439. [Google Scholar] [CrossRef]
- Loovers, H.M.; Kortholt, A.; de Groote, H.; Whitty, L.; Nussbaum, R.L.; van Haastert, P.J.M. Regulation of phagocytosis in Dictyostelium by the inositol 5-phosphatase OCRL homolog Dd5P4. Traffic 2007, 8, 618–628. [Google Scholar] [CrossRef]
- Humphreys, D.; Singh, V.; Koronakis, V. Inhibition of WAVE Regulatory Complex Activation by a Bacterial Virulence Effector Counteracts Pathogen Phagocytosis. Cell Rep. 2016, 17, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Smyth, D.; Mckay, C.M.; Gulbransen, B.D.; Phan, V.C.; Wang, A.; Mckay, D.M. Interferon-γ signals via an ERK1/2-ARF6 pathway to promote bacterial internalization by gut epithelia. Cell. Microbiol. 2012, 14, 1257–1270. [Google Scholar] [CrossRef]
- Humphreys, D.; Davidson, A.; Hume, P.J.; Koronakis, V. Salmonella virulence effector SopE and Host GEF ARNO cooperate to recruit and activate WAVE to trigger bacterial invasion. Cell Host Microbe 2012, 11, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.C.; Humphreys, D.; Brooks, A.B.E.; Hume, P.J.; Koronakis, V. The Arf GTPase-activating protein family is exploited by salmonella enterica serovar typhimurium to invade nonphagocytic host cells. MBio 2015, 6. [Google Scholar] [CrossRef]
- Smith, A.C.; Cirulis, J.T.; Casanova, J.E.; Scidmore, M.A.; Brumell, J.H. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J. Biol. Chem. 2005, 280, 24634–24641. [Google Scholar] [CrossRef]
- Garza-Mayers, A.C.; Miller, K.A.; Russo, B.C.; Nagda, D.V.; Goldberg, M.B. Shigella flexneri regulation of ARF6 activation during bacterial entry via an IpgD-mediated positive feedback loop. MBio 2015, 6, e02584. [Google Scholar] [CrossRef] [PubMed]
- Kaasch, A.J.; Dinter, J.; Goeser, T.; Plum, G.; Seifert, H. Yersinia pseudotuberculosis bloodstream infection and septic arthritis: Case report and review of the literature. Infection 2012, 40, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-W.; Isberg, R.R. Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for β1 integrin-mediated bacterial uptake. J. Exp. Med. 2003, 198, 603–614. [Google Scholar] [CrossRef]
- Dowd, G.C.; Bhalla, M.; Kean, B.; Thomas, R.; Ireton, K. Role of host type IA phosphoinositide 3-kinase pathway components in invasin-mediated internalization of Yersinia enterocolitica. Infect. Immun. 2016, 84, 1826–1841. [Google Scholar] [CrossRef]
- Balana, M.E.; Niedergang, F.; Subtil, A.; Alcover, A.; Chavrier, P.; Dautry-Varsat, A. ARF6 GTPase controls bacterial invasion by actin remodelling. J. Cell Sci. 2005, 118, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Gavicherla, B.; Ritchey, L.; Gianfelice, A.; Kolokoltsov, A.A.; Davey, R.A.; Ireton, K. Critical role for the host GTPase-activating protein ARAP2 in InlB-mediated entry of Listeria monocytogenes. Infect. Immun. 2010, 78, 4532–4541. [Google Scholar] [CrossRef]
- Rennoll-Bankert, K.E.; Rahman, M.S.; Guillotte, M.L.; Lehman, S.S.; Beier-Sexton, M.; Gillespie, J.J.; Azad, A.F. RalF-mediated activation of Arf6 controls Rickettsia typhi invasion by co-opting phosphoinositol metabolism. Infect. Immun. 2016, 84, 3496–3506. [Google Scholar] [CrossRef] [PubMed]
- Rennoll-Bankert, K.E.; Rahman, M.S.; Gillespie, J.J.; Guillotte, M.L.; Kaur, S.J.; Lehman, S.S.; Beier-Sexton, M.; Azad, A.F. Which Way In? The RalF Arf-GEF Orchestrates Rickettsia Host Cell Invasion. PLoS Pathog. 2015, 11, e1005115. [Google Scholar] [CrossRef]
- Selyunin, A.S.; Sutton, S.E.; Weigele, B.A.; Reddick, L.E.; Orchard, R.C.; Bresson, S.M.; Tomchick, D.R.; Alto, N.M. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature 2011, 469, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zhu, Y.; Lu, Q.; Hu, L.; Zheng, Y.; Shao, F. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell 2012, 150, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Furniss, R.C.D.; Slater, S.; Frankel, G.; Clements, A. Enterohaemorrhagic E. coli modulates an ARF6:Rab35 signaling axis to prevent recycling endosome maturation during infection. J. Mol. Biol. 2016, 428, 3399–3407. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.V.; da Silva, E.A.; Cruz, M.C.; Chavrier, P.; Mortara, R.A. ARF6, PI3-kinase and host cell actin cytoskeleton in Toxoplasma gondii cell invasion. Biochem. Biophys. Res. Commun. 2009, 378, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.L.; Cruz, L.; Mortara, R.A.; Da Silva, C.V. Revealing Annexin A2 and ARF-6 enrollment during Trypanosoma cruzi extracellular amastigote–host cell interaction. Parasites and Vectors 2015, 8, 493. [Google Scholar] [CrossRef]
- Lodge, R.; Descoteaux, A. Phagocytosis of Leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of NADPH oxidase activation. Eur. J. Immunol. 2006, 36, 2735–2744. [Google Scholar] [CrossRef]
- García-Expósito, L.; Barroso-González, J.; Puigdomènech, I.; Machado, J.-D.; Blanco, J.; Valenzuela-Fernández, A. HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes. Mol. Biol. Cell 2011, 22, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Ablan, S.D.; Lockett, S.J.; Nagashima, K.; Freed, E.O. Phosphatidylinositol(4,5)bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA 2004, 101, 14889–14894. [Google Scholar] [CrossRef]
- Vidricaire, G.; Tremblay, M.J. Rab5 and Rab7, but not ARF6, govern the early events of HIV-1 infection in polarized human placental cells. J. Immunol. 2005, 175, 6517–6530. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef]
- Naslavsky, N.; Weigert, R.; Donaldson, J.G. Characterization of a nonclathrin endocytic pathway: Membrane cargo and lipid requirements. Mol. Biol. Cell 2004, 15, 3542–3552. [Google Scholar] [CrossRef]
- Klein, S.; Franco, M.; Chardin, P.; Luton, F. Role of the Arf6 GDP/GTP cycle and Arf6 GTPase-activating proteins in actin remodeling and intracellular transport. J. Biol. Chem. 2006, 281, 12352–12361. [Google Scholar] [CrossRef]
- Walseng, E.; Bakke, O.; Roche, P.A. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J. Biol. Chem. 2008, 283, 14717–14727. [Google Scholar] [CrossRef]
- Blagoveshchenskaya, A.D.; Thomas, L.; Feliciangeli, S.F.; Hung, C.H.; Thomas, G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 2002, 111, 853–866. [Google Scholar] [CrossRef]
- Larsen, J.E. HIV Nef-mediated Major Histocompatibility Complex Class I Down-Modulation Is Independent of Arf6 Activity. Mol. Biol. Cell 2003, 15, 323–331. [Google Scholar] [CrossRef]
- Wonderlich, E.R.; Leonard, J.A.; Kulpa, D.A.; Leopold, K.E.; Norman, J.M.; Collins, K.L. ADP Ribosylation Factor 1 Activity Is Required To Recruit AP-1 to the Major Histocompatibility Complex Class I (MHC-I) Cytoplasmic Tail and Disrupt MHC-I Trafficking in HIV-1-Infected Primary T Cells. J. Virol. 2011, 85, 12216–12226. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Rosales, T.; Rose, J.J.; Chaudhury, B.; Knutson, J.R.; Venkatesan, S. HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking. J. Biol. Chem. 2010, 285, 30884–30905. [Google Scholar] [CrossRef] [PubMed]
- Heikkila, O.; Susi, P.; Tevaluoto, T.; Harma, H.; Marjomaki, V.; Hyypia, T.; Kiljunen, S. Internalization of Coxsackievirus A9 Is Mediated by 2-Microglobulin, Dynamin, and Arf6 but Not by Caveolin-1 or Clathrin. J. Virol. 2010, 84, 3666–3681. [Google Scholar] [CrossRef]
- Huttunen, M.; Waris, M.; Kajander, R.; Hyypia, T.; Marjomaki, V. Coxsackievirus A9 Infects Cells via Nonacidic Multivesicular Bodies. J. Virol. 2014, 88, 5138–5151. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.; Sall, A.; Si, X.; Abraham, T.; Wu, W.; Luo, Z.; Petersen, T.; Hegele, R.G.; McManus, B.M. ERK MAP kinase-activated Arf6 trafficking directs coxsackievirus type B3 into an unproductive compartment during virus host–cell entry. J. Gen. Virol. 2009, 90, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Lennemann, N.J.; Sarkar, S.N.; Sadovsky, Y.; Coyne, C.B. ADAP2 Is an Interferon Stimulated Gene That Restricts RNA Virus Entry. PLoS Pathog. 2015, 11, e1005150. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer, S.; Zeltzer, C.A.; Igarashi, S.; Wilson, J.; Donaldson, J.G.; Goodrum, F. Virus Control of Trafficking from Sorting Endosomes. MBio 2018, 9, e00683-18. [Google Scholar] [CrossRef] [PubMed]
- Karleuša, L.; Mahmutefendić, H.; Tomaš, M.I.; Zagorac, G.B.; Lučin, P. Landmarks of endosomal remodeling in the early phase of cytomegalovirus infection. Virology 2018, 515, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Krzyzaniak, M.A.; Mach, M.; Britt, W.J. HCMV-encoded glycoprotein M (UL100) interacts with rab11 effector protein FIP4. Traffic 2009, 10, 1439–1457. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science (80-. ). 2008, 320, 531–535. [Google Scholar] [CrossRef]
- Hsiao, J.-C.; Chu, L.-W.; Lo, Y.-T.; Lee, S.-P.; Chen, T.-J.; Huang, C.-Y.; Ping, Y.-H.; Chang, W. Intracellular Transport of Vaccinia Virus in HeLa Cells Requires WASH-VPEF/FAM21-Retromer Complexes and Recycling Molecules Rab11 and Rab22. J. Virol. 2015, 89, 8365–8382. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Terajima, M. Vaccinia virus K1L protein mediates host-range function in RK-13 cells via ankyrin repeat and may interact with a cellular GTPase-activating protein. Virus Res. 2005, 114, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xiang, Y. Vaccinia virus K1L protein supports viral replication in human and rabbit cells through a cell-type-specific set of its ankyrin repeat residues that are distinct from its binding site for ACAP2. Virology 2006, 353, 220–233. [Google Scholar] [CrossRef]
- Tugizov, S.M.; Herrera, R.; Palefsky, J.M. Epstein-Barr Virus Transcytosis through Polarized Oral Epithelial Cells. J. Virol. 2013, 87, 8179–8194. [Google Scholar] [CrossRef] [PubMed]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Dana, R.R.; Eigsti, C.; Holmes, K.L.; Leto, T.L. A regulatory role for ADP-ribosylation factor 6 (ARF6) in activation of the phagocyte NADPH oxidase. J. Biol. Chem. 2000, 275, 32566–32571. [Google Scholar] [CrossRef]
- Tsai, Y.-R.; Huang, L.-J.; Lin, H.-Y.; Hung, Y.-J.; Lee, M.-R.; Kuo, S.-C.; Hsu, M.-F.; Wang, J.-P. Inhibition of formyl peptide-stimulated phospholipase D activation by Fal-002-2 via blockade of the Arf6, RhoA and protein kinase C signaling pathways in rat neutrophils. Naunyn. Schmiedebergs. Arch. Pharmacol. 2013, 386, 507–519. [Google Scholar] [CrossRef]
- El Azreq, M.-A.A.; Garceau, V.; Harbour, D.; Pivot-Pajot, C.; Bourgoin, S.G.; Azreq, M. El Cytohesin-1 regulates the Arf6-phospholipase D signaling axis in human neutrophils: Impact on superoxide anion production and secretion. J. Immunol. 2010, 184, 637–649. [Google Scholar] [CrossRef]
- Melendez, A.J.; Bruetschy, L.; Andres Floto, R.; Harnett, M.M.; Allen, J.M. Functional coupling of FcγRI to nicotinamide adenine dinucleotide phosphate (reduced form) oxidative burst and immune complex trafficking requires the activation of phospholipase D1. Blood 2001, 98, 3421–3428. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Husebye, H.; Aune, M.H.; Stenvik, J.; Samstad, E.; Skjeldal, F.; Halaas, Ø.; Nilsen, N.J.; Stenmark, H.; Latz, E.; Lien, E.; et al. The Rab11a GTPase controls toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 2010, 33, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Svensson, H.G.; West, M.A.; Mollahan, P.; Prescott, A.R.; Zaru, R.; Watts, C. A role for ARF6 in dendritic cell podosome formation and migration. Eur J. Immunol 2008, 38, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Horng, T.; Barton, G.M.; Flavell, R.A.; Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002, 420, 329–333. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Mori, K.; Hoshino, K.; Takeuchi, O.; Takeda, K.; Akira, S. Cutting edge: A novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 2002, 169, 6668–6672. [Google Scholar] [CrossRef]
- Wu, J.Y.; Kuo, C.C. Pivotal role of ADP-ribosylation factor 6 in Toll-like receptor 9-mediated immune signaling. J. Biol. Chem. 2012, 287, 4323–4334. [Google Scholar] [CrossRef]
- Wu, J.Y.; Kuo, C.C. TLR9-mediated ARF6 activation is involved in advancing CpG ODN cellular uptake. Commun. Integr. Biol. 2012, 5, 316–318. [Google Scholar] [CrossRef]
- Murase, M.; Kawasaki, T.; Hakozaki, R.; Sueyoshi, T.; Putri, D.D.P.; Kitai, Y.; Sato, S.; Ikawa, M.; Kawai, T. Intravesicular Acidification Regulates Lipopolysaccharide Inflammation and Tolerance through TLR4 Trafficking. J. Immunol. 2018, 200, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Lorenzo, A.; Skinner, M.; El Annan, J.; Futai, M.; Sun-Wada, G.H.; Bourgoin, S.; Casanova, J.; Wildeman, A.; Bechoua, S.; Ausiello, D.A.; et al. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat. Cell Biol. 2006, 8, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Merkulova, M.; Hurtado-Lorenzo, A.; Hosokawa, H.; Zhuang, Z.; Brown, D.; Ausiello, D.A.; Marshansky, V. Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution. Am. J. Physiol. Cell Physiol. 2011, 300, C1442–C1455. [Google Scholar] [CrossRef] [PubMed]
- Van Weert, A.W.M.; Dunn, K.W.; Geuze, H.J.; Maxfield, F.R.; Stoorvogel, W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol. 1995, 130, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Liberman, R.; Bond, S.; Shainheit, M.G.; Stadecker, M.J.; Forgac, M. Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-Kinase/mTOR-dependent pathway. J. Biol. Chem. 2014, 289, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, Q.; Wu, N.; He, M.; Liu, N.; Huang, J.; Lu, B.; Yao, Q.; Yang, Y.; Hu, R. miR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine 2018, 60, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; London, N.R.; Gibson, C.C.; Davis, C.T.; Tong, Z.; Sorensen, L.K.; Shi, D.S.; Guo, J.; Smith, M.C.; Grossmann, A.H.; et al. Interleukin receptor activates a MYD88-ARNO-ARF6 cascade to disrupt vascular stability. Nature 2012, 492, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.T.; Zhu, W.; Gibson, C.C.; Bowman-Kirigin, J.A.; Sorensen, L.; Ling, J.; Sun, H.; Navankasattusas, S.; Li, D.Y. ARF6 Inhibition Stabilizes the Vasculature and Enhances Survival during Endotoxic Shock. J. Immunol. 2014, 192, 6045–6052. [Google Scholar] [CrossRef]
- Nair-Gupta, P.; Baccarini, A.; Tung, N.; Seyffer, F.; Florey, O.; Huang, Y.; Banerjee, M.; Overholtzer, M.; Roche, P.A.; Tampé, R.; et al. TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell 2014, 158, 506–521. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.G.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Acker, T.; Tavernier, J.; Peelman, F. The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity. Int. J. Mol. Sci. 2019, 20, 2209. https://doi.org/10.3390/ijms20092209
Van Acker T, Tavernier J, Peelman F. The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity. International Journal of Molecular Sciences. 2019; 20(9):2209. https://doi.org/10.3390/ijms20092209
Chicago/Turabian StyleVan Acker, Tim, Jan Tavernier, and Frank Peelman. 2019. "The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host–Pathogen Interactions and Innate Immunity" International Journal of Molecular Sciences 20, no. 9: 2209. https://doi.org/10.3390/ijms20092209



