Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice
Abstract
1. Introduction
2. Results
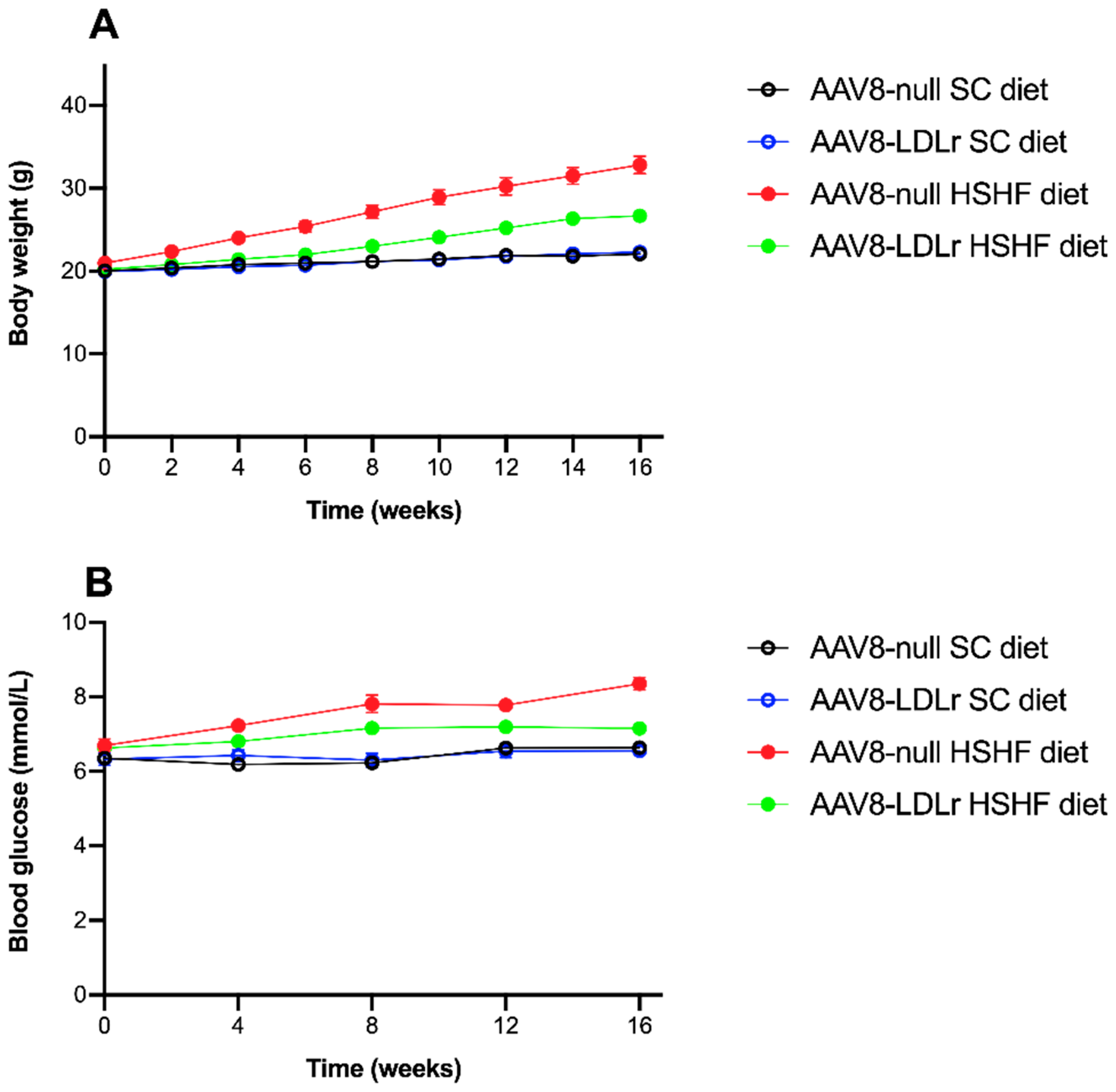
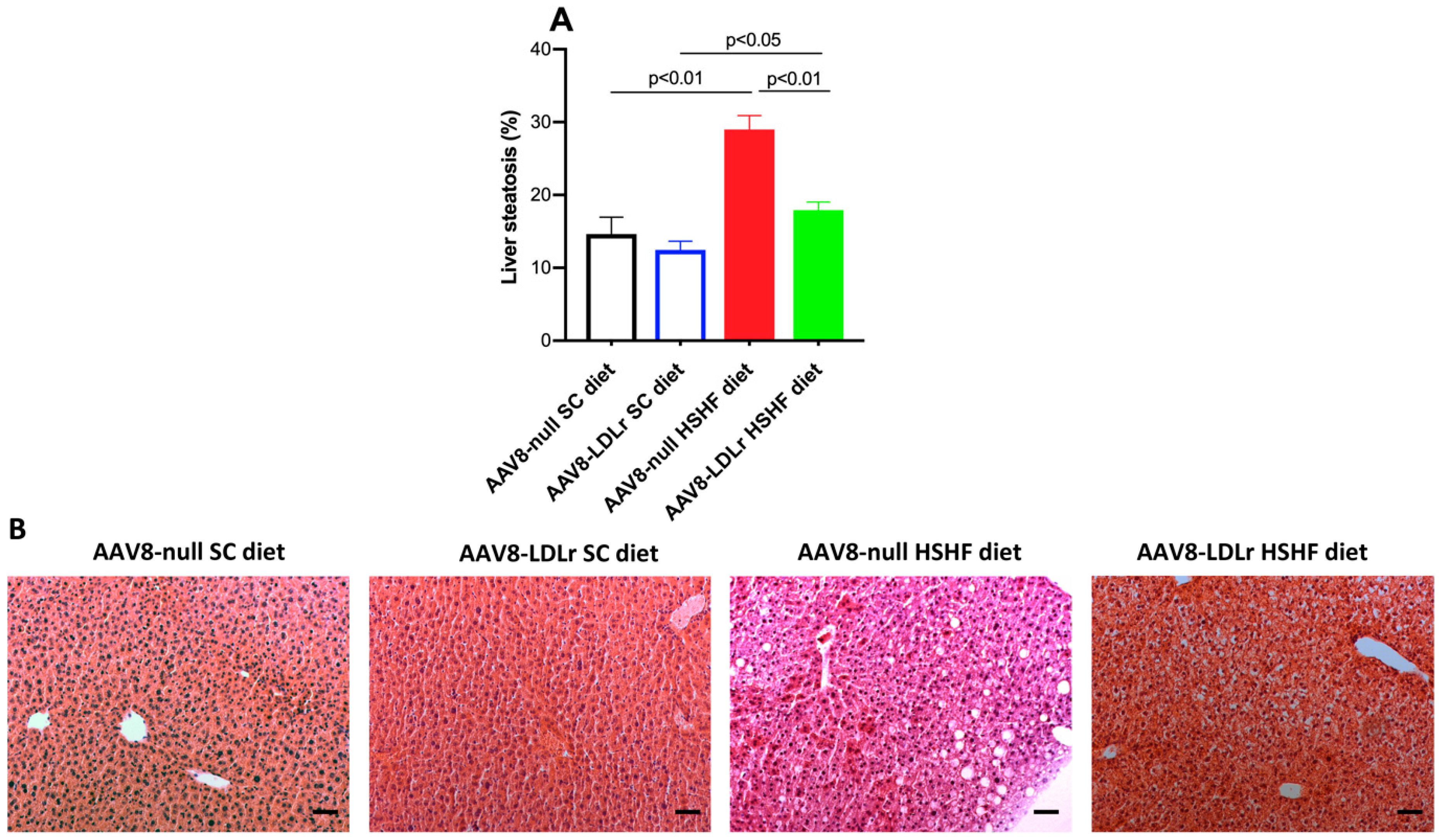
2.1. The HSHF Diet Induces Obesity and Type 2 Diabetes Mellitus in Female C57BL/6J LDLr−/− Mice
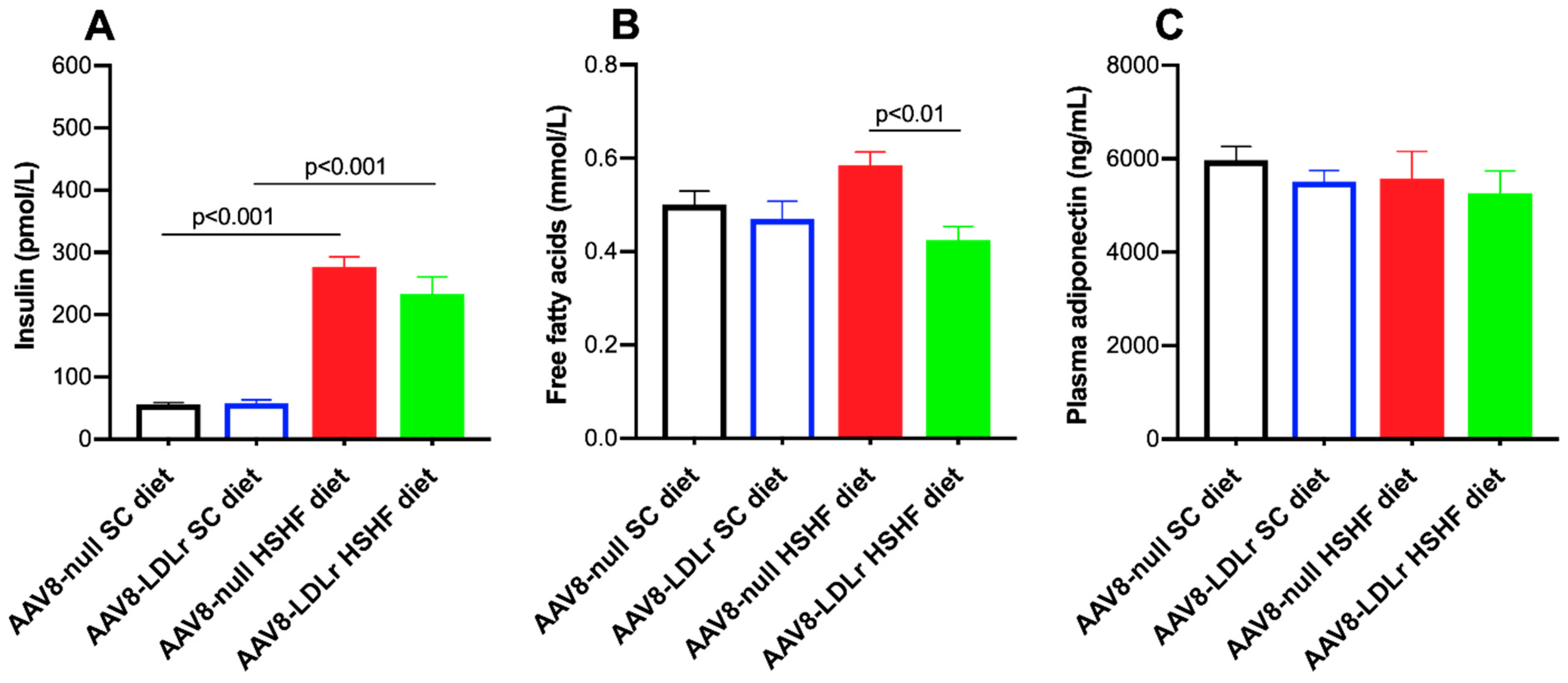
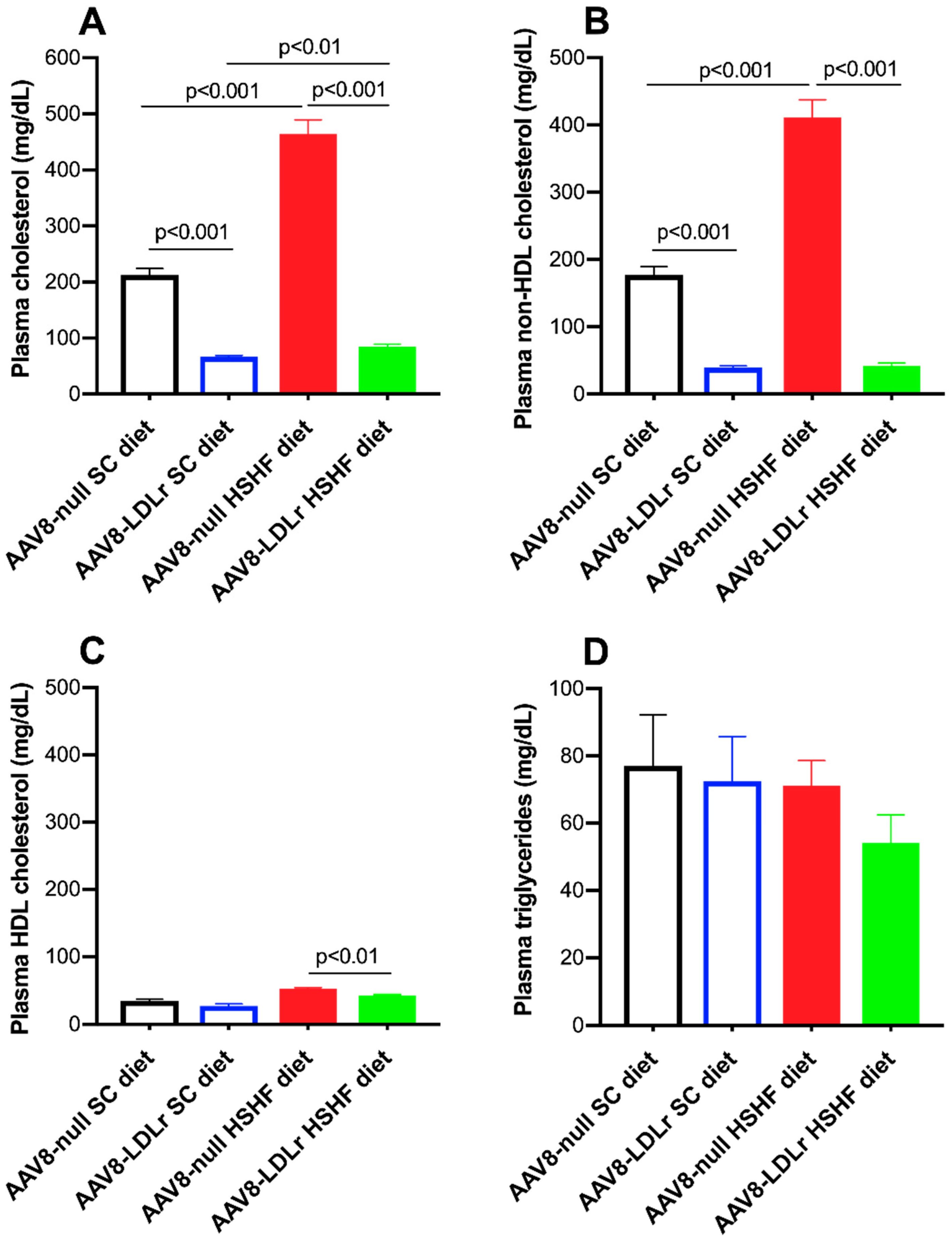
2.2. Effect of AAV8-LDLr Gene Transfer on Metabolic Parameters in SC diet and in HSHF Diet Mice
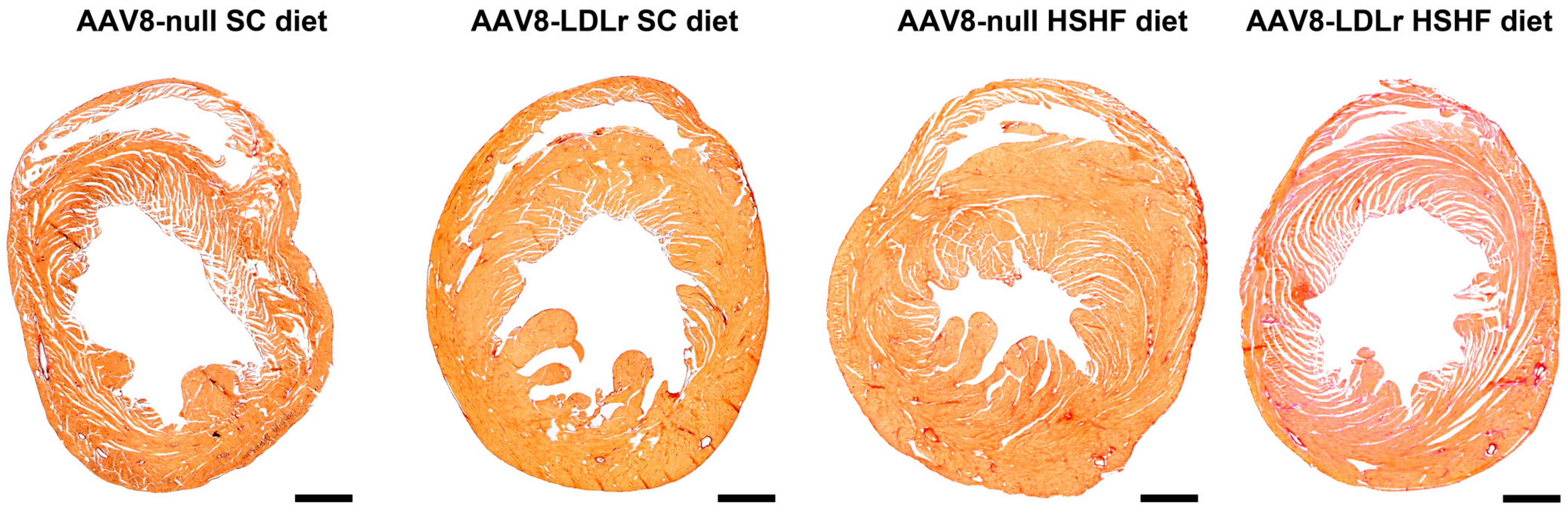
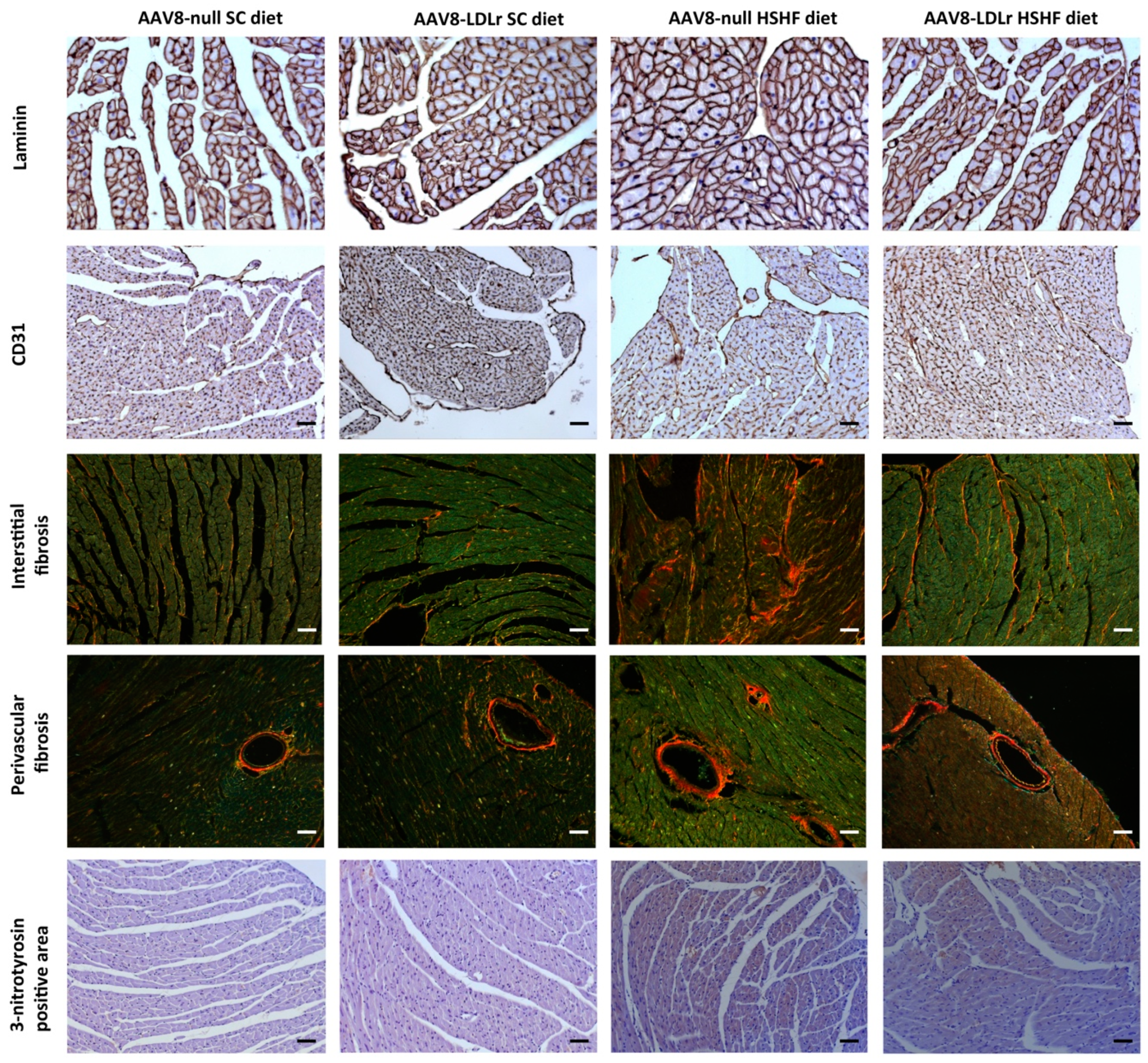
2.3. AAV8-Gene Transfer Potently Counteracts Cardiac Hypertrophy and Pathological Remodeling in Female C57BL/6J LDLr−/− Mice Fed the HSHF Diet
2.4. Cardiac Dysfunction in C57BL/6J LDLr−/− Mice Fed the HSHF Diet Is Consistent with HFpEF, whereas AAV8-LDLr Gene Transfer Completely Normalizes Cardiac Function
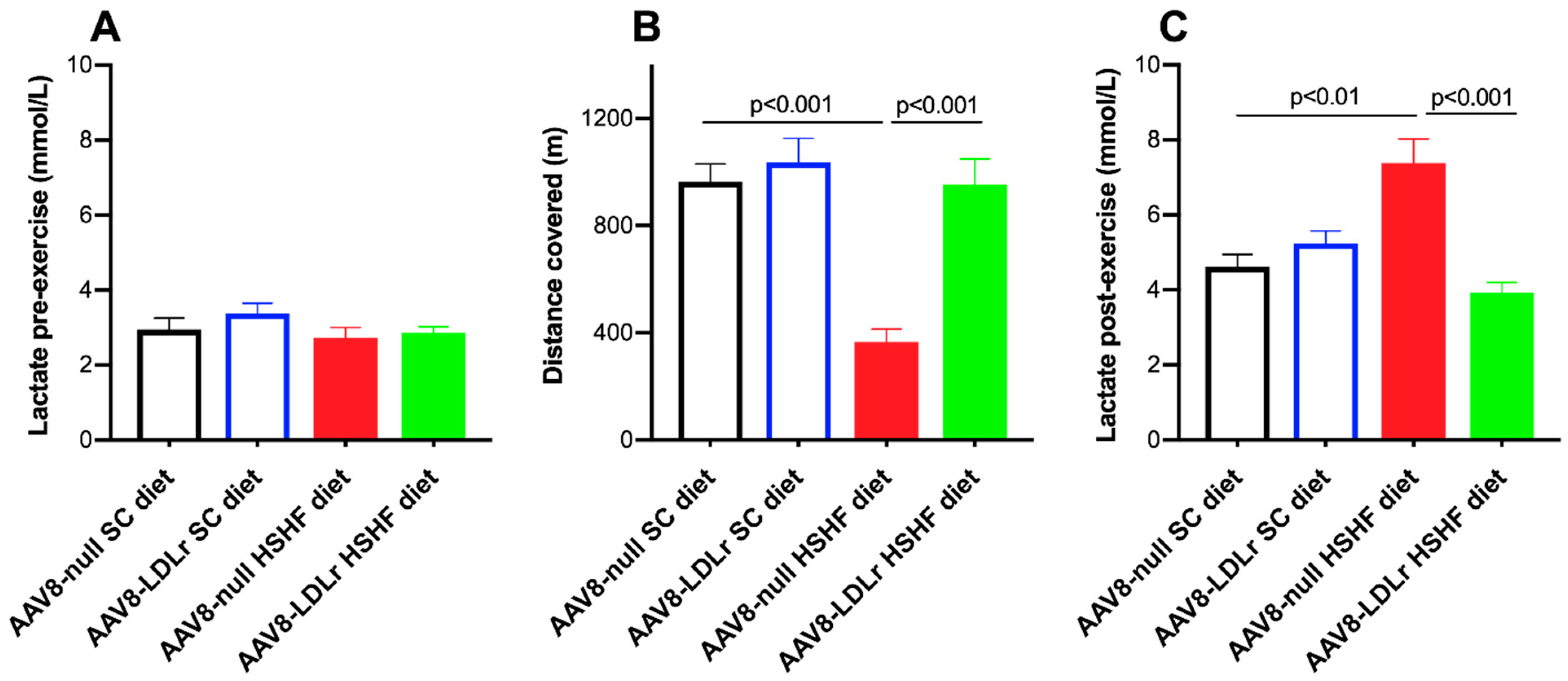
2.5. Exercise Capacity Is Severely Limited in C57BL/6J LDLr−/− Mice Fed the HSHF Diet and Is Completely Normalized by AAV8-LDLr
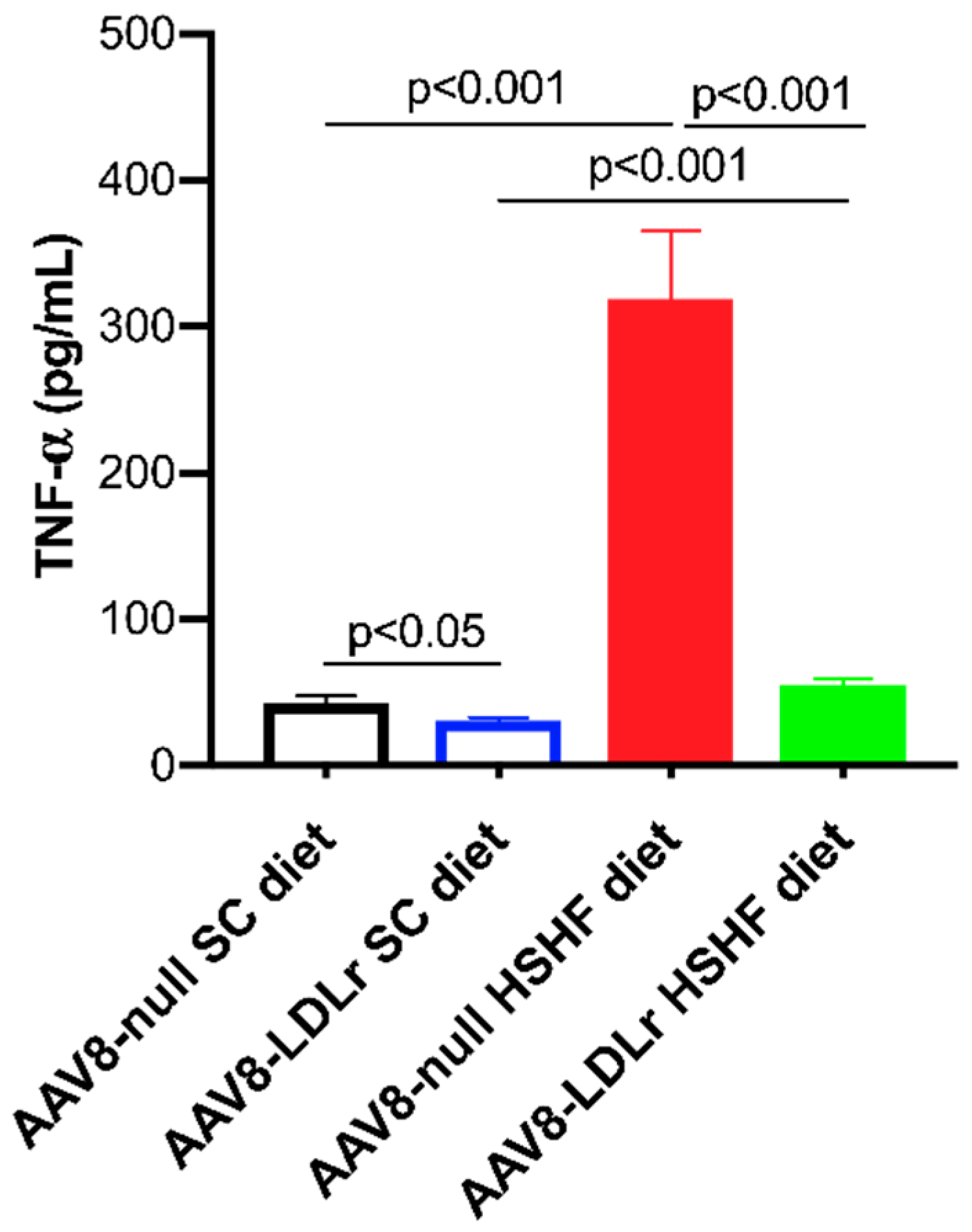
2.6. Pronounced Increase of Tumor Necrosis Factor (TNF)-α Levels in C57BL/6J LDLr−/− Mice Fed the HSHF Diet Is Completely Abrogated by AAV8-LDLr Gene Transfer
3. Discussion
4. Materials and Methods
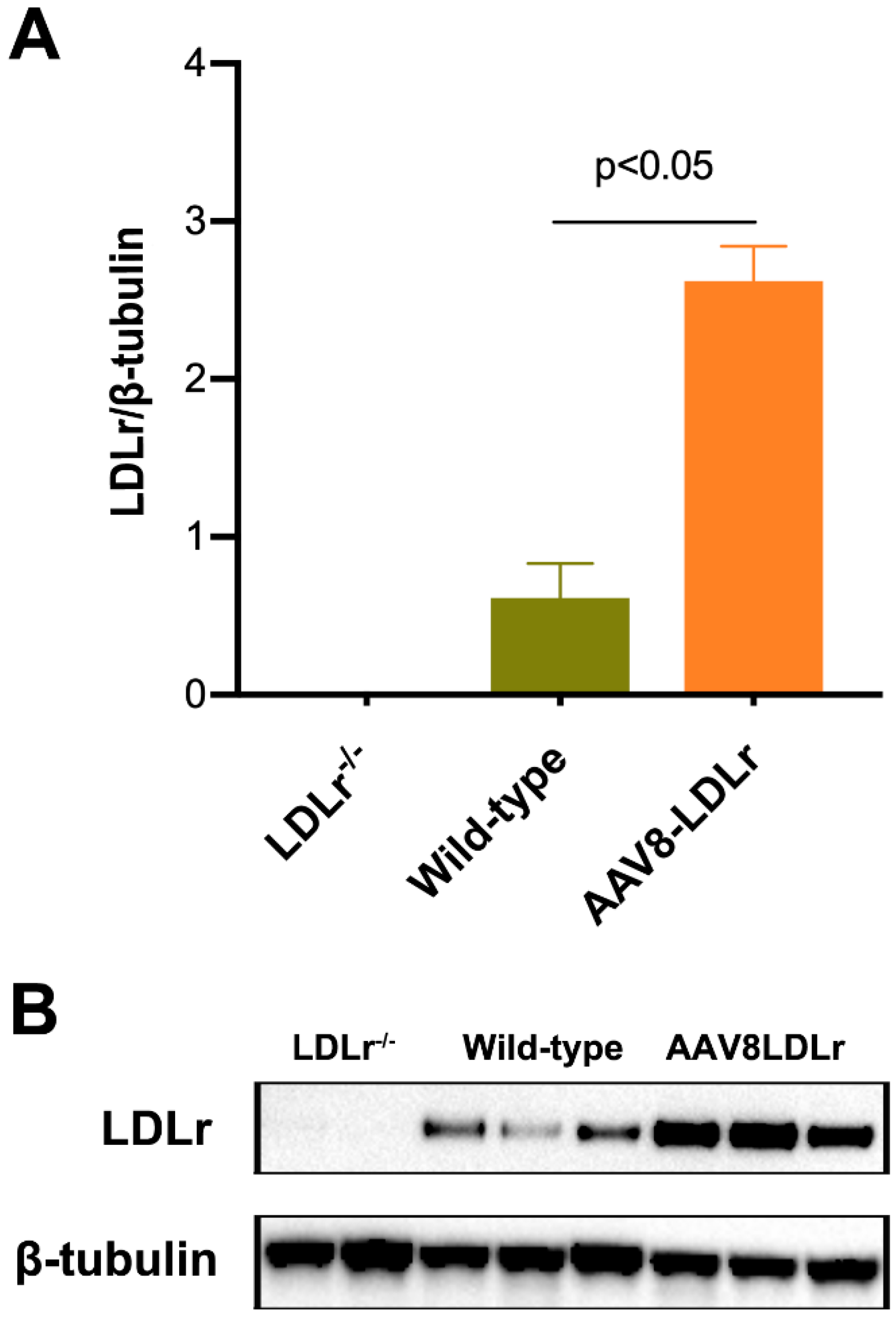
4.1. Gene Therapy
4.2. In Vivo Experiments and Study Design
4.3. Quantification of Murine LDLr Expression in the Liver by Western Blot
4.4. In Vivo Hemodynamic Pressure–Volume Loop Measurements
4.5. Quantification of Plasma Lipid Levels and Lipoprotein Cholesterol
4.6. Quantification of Plasma Free Fatty Acid Levels
4.7. Determination of Plasma Levels of Insulin, Adiponectin, and Tumor Necrosis Factor-α
4.8. Histological Analyses of the Myocardium
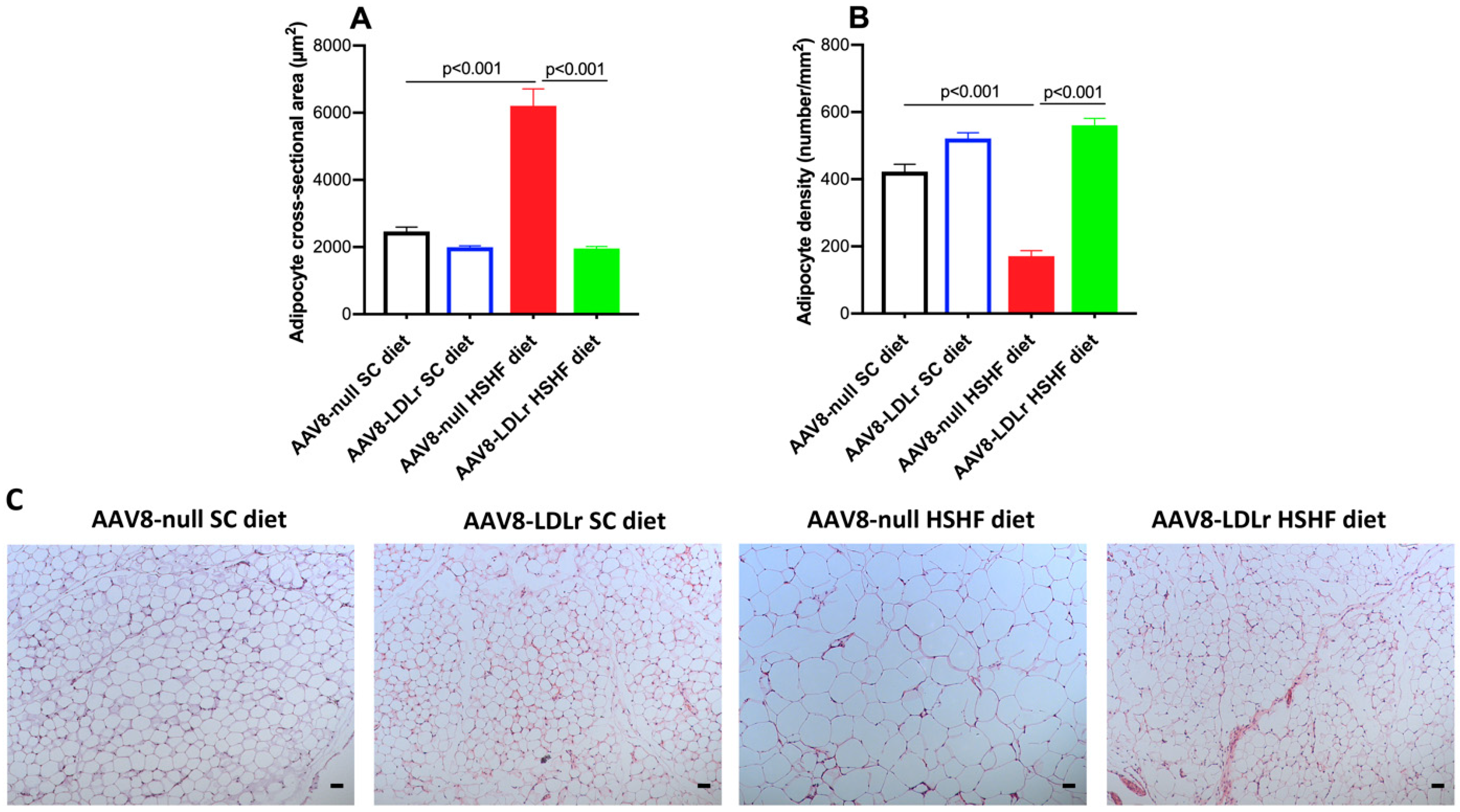
4.9. Histological Analysis of Gonadal Fat Pad
4.10. Quantification of Liver Steatosis
4.11. Exercise Treadmill Testing
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SC | Standard chow |
| HSHF | High-sucrose/high-fat |
| HFpEF | Heart failure with preserved ejection |
| LDLr | Low-density lipoprotein receptor |
| AAV8 | Adeno-associated viral serotype 8 |
| FFA | Free fatty acids |
| TNF-α | Tumor necrosis factor-α |
| VLDL | Very low-density lipoprotein |
| NAFLD | Non-alcoholic fatty liver disease |
References
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef]
- Packer, M. Are the effects of drugs to prevent and to treat heart failure always concordant? The statin paradox and its implications for understanding the actions of antidiabetic medications. Eur. J. Heart Fail. 2018, 20, 1100–1105. [Google Scholar] [CrossRef]
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Paulus, W.J.; Dal Canto, E. Distinct Myocardial Targets for Diabetes Therapy in Heart Failure with Preserved or Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fukuda, S.; Tanaka, A.; Otsuka, K.; Taguchi, H.; Shimada, K. Relationships Between Periventricular Epicardial Adipose Tissue Accumulation, Coronary Microcirculation, and Left Ventricular Diastolic Dysfunction. Can. J. Cardiol. 2017, 33, 1489–1497. [Google Scholar] [CrossRef]
- Velagaleti, R.S.; Massaro, J.; Vasan, R.S.; Robins, S.J.; Kannel, W.B.; Levy, D. Relations of lipid concentrations to heart failure incidence: The Framingham Heart Study. Circulation 2009, 120, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Benson, L.; Edner, M.; Dahlstrom, U.; Lund, L.H. Association Between Use of Statins and Mortality in Patients with Heart Failure and Ejection Fraction of ≥50. Circ. Heart Fail. 2015, 8, 862–870. [Google Scholar] [CrossRef]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Aboumsallem, J.P.; Dresselaers, T.; Himmelreich, U.; Van Veldhoven, P.P.; Gheysens, O.; Jacobs, F.; et al. Cholesterol-Lowering Gene Therapy Counteracts the Development of Non-ischemic Cardiomyopathy in Mice. Mol. Ther. 2017, 25, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Castilho, R.F.; Vercesi, A.E. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001, 495, 12–15. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Cosso, R.G.; Alberici, L.C.; Maciel, E.N.; Salerno, A.G.; Dorighello, G.G.; Velho, J.A.; de Faria, E.C.; Vercesi, A.E. Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J. 2005, 19, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Hemnani, T.; Parihar, M.S. Reactive oxygen species and oxidative DNA damage. Ind. J. Physiol. Pharmacol. 1998, 42, 440–452. [Google Scholar]
- Suematsu, N.; Tsutsui, H.; Wen, J.; Kang, D.; Ikeuchi, M.; Ide, T.; Hayashidani, S.; Shiomi, T.; Kubota, T.; Hamasaki, N.; et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 2003, 107, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Cesselli, D.; Jakoniuk, I.; Barlucchi, L.; Beltrami, A.P.; Hintze, T.H.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001, 89, 279–286. [Google Scholar] [CrossRef]
- Chiamvimonvat, N.; O’Rourke, B.; Kamp, T.J.; Kallen, R.G.; Hofmann, F.; Flockerzi, V.; Marban, E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ. Res. 1995, 76, 325–334. [Google Scholar] [CrossRef]
- Wilson, A.J.; Gill, E.K.; Abudalo, R.A.; Edgar, K.S.; Watson, C.J.; Grieve, D.J. Reactive oxygen species signalling in the diabetic heart: Emerging prospect for therapeutic targeting. Heart 2018, 104, 293–299. [Google Scholar] [CrossRef]
- Westermann, D.; Van Linthout, S.; Dhayat, S.; Dhayat, N.; Schmidt, A.; Noutsias, M.; Song, X.Y.; Spillmann, F.; Riad, A.; Schultheiss, H.P.; et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500–507. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Prabhu, S.D. Cytokine-induced modulation of cardiac function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Targher, G.; Chonchol, M.; Bertolini, L.; Rodella, S.; Zenari, L.; Lippi, G.; Franchini, M.; Zoppini, G.; Muggeo, M. Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J. Am. Soc. Nephrol. 2008, 19, 1564–1570. [Google Scholar] [CrossRef]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; McMahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef]
- Perumpail, B.J.; Khan, M.A.; Yoo, E.R.; Cholankeril, G.; Kim, D.; Ahmed, A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8263–8276. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Bonapace, S.; Perseghin, G.; Molon, G.; Canali, G.; Bertolini, L.; Zoppini, G.; Barbieri, E.; Targher, G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care 2012, 35, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kypreos, K.E.; Karagiannides, I.; Fotiadou, E.H.; Karavia, E.A.; Brinkmeier, M.S.; Giakoumi, S.M.; Tsompanidi, E.M. Mechanisms of obesity and related pathologies: Role of apolipoprotein E in the development of obesity. FEBS J. 2009, 276, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Karagiannides, I.; Abdou, R.; Tzortzopoulou, A.; Voshol, P.J.; Kypreos, K.E. Apolipoprotein E predisposes to obesity and related metabolic dysfunctions in mice. FEBS J. 2008, 275, 4796–4809. [Google Scholar] [CrossRef]
- Hofmann, S.M.; Perez-Tilve, D.; Greer, T.M.; Coburn, B.A.; Grant, E.; Basford, J.E.; Tschop, M.H.; Hui, D.Y. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes 2008, 57, 5–12. [Google Scholar] [CrossRef]
- Hofmann, S.M.; Zhou, L.; Perez-Tilve, D.; Greer, T.; Grant, E.; Wancata, L.; Thomas, A.; Pfluger, P.T.; Basford, J.E.; Gilham, D.; et al. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J. Clin. Investig. 2007, 117, 3271–3282. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Laane, C.; Park, B.; Sztalryd, C. Low-density lipoprotein receptors in rat adipocytes: Regulation with fasting. Am. J. Physiol. 1994, 266, E26–E32. [Google Scholar] [CrossRef]
- Herz, J.; Gotthardt, M.; Willnow, T.E. Cellular signalling by lipoprotein receptors. Curr. Opin. Lipidol. 2000, 11, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Willnow, T.E. The low-density lipoprotein receptor gene family: A cellular Swiss army knife? Trends Cell. Biol. 2002, 12, 273–280. [Google Scholar] [CrossRef]
- Skogsberg, J.; Dicker, A.; Ryden, M.; Astrom, G.; Nilsson, R.; Bhuiyan, H.; Vitols, S.; Mairal, A.; Langin, D.; Alberts, P.; et al. ApoB100-LDL acts as a metabolic signal from liver to peripheral fat causing inhibition of lipolysis in adipocytes. PLoS ONE 2008, 3, e3771. [Google Scholar] [CrossRef] [PubMed]
- Besseling, J.; Kastelein, J.J.; Defesche, J.C.; Hutten, B.A.; Hovingh, G.K. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 2015, 313, 1029–1036. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Preiss, D.; Seshasai, S.R.; Welsh, P.; Murphy, S.A.; Ho, J.E.; Waters, D.D.; DeMicco, D.A.; Barter, P.; Cannon, C.P.; Sabatine, M.S.; et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011, 305, 2556–2564. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Preiss, D.; Kuchenbaecker, K.B.; Holmes, M.V.; Engmann, J.E.; Shah, T.; Sofat, R.; Stender, S.; Johnson, P.C.; Scott, R.A.; et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 2015, 385, 351–361. [Google Scholar] [CrossRef]
- Preiss, D.; Campbell, R.T.; Murray, H.M.; Ford, I.; Packard, C.J.; Sattar, N.; Rahimi, K.; Colhoun, H.M.; Waters, D.D.; LaRosa, J.C.; et al. The effect of statin therapy on heart failure events: A collaborative meta-analysis of unpublished data from major randomized trials. Eur. Heart J. 2015, 36, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- McConnachie, A.; Walker, A.; Robertson, M.; Marchbank, L.; Peacock, J.; Packard, C.J.; Cobbe, S.M.; Ford, I. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: A record linkage study. Eur. Heart J. 2014, 35, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, J.; Apetrei, E.; Barrios, V.; Bohm, M.; Cleland, J.G.; Cornel, J.H.; Dunselman, P.; Fonseca, C.; Goudev, A.; Grande, P.; et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 2007, 357, 2248–2261. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef]
- Felker, G.M. Coenzyme Q10 and statins in heart failure: The dog that didn’t bark. J. Am. Coll. Cardiol. 2010, 56, 1205–1206. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef]
- Jacobs, F.; Snoeys, J.; Feng, Y.; Van Craeyveld, E.; Lievens, J.; Armentano, D.; Cheng, S.H.; De Geest, B. Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Ther. 2008, 15, 594–603. [Google Scholar] [CrossRef]
- Lock, M.; Alvira, M.; Vandenberghe, L.H.; Samanta, A.; Toelen, J.; Debyser, Z.; Wilson, J.M. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum. Gene Ther. 2010, 21, 1259–1271. [Google Scholar] [CrossRef]
- Mishra, M.; Muthuramu, I.; Aboumsallem, J.P.; Kempen, H.; De Geest, B. Reconstituted HDL (Milano) Treatment Efficaciously Reverses Heart Failure with Preserved Ejection Fraction in Mice. Int. J. Mol. Sci. 2018, 19, 3399. [Google Scholar] [CrossRef]
- Aboumsallem, J.; Muthuramu, I.; Mishra, M.; Kempen, H.; De Geest, B. Effective Treatment of Diabetic Cardiomyopathy and Heart Failure with Reconstituted HDL (Milano) in Mice. Int. J. Mol. Sci. 2019, 20, 1273. [Google Scholar] [CrossRef]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Jacobs, F.; Gheysens, O.; Van Veldhoven, P.P.; De Geest, B. Coconut Oil Aggravates Pressure Overload-Induced Cardiomyopathy without Inducing Obesity, Systemic Insulin Resistance, or Cardiac Steatosis. Int. J. Mol. Sci. 2017, 18, 1565. [Google Scholar] [CrossRef]
- Muthuramu, I.; Singh, N.; Amin, R.; Nefyodova, E.; Debasse, M.; Van Horenbeeck, I.; Jacobs, F.; De Geest, B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J. Mol. Med. 2015, 93, 609–618. [Google Scholar] [CrossRef]
- Aboumsallem, J.P.; Mishra, M.; Amin, R.; Muthuramu, I.; Kempen, H.; De Geest, B. Successful treatment of established heart failure in mice with recombinant HDL (Milano). Br. J. Pharmacol. 2018, 175, 4167–4182. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T.; Toko, H.; Okada, S.; Ikeda, H.; Yasuda, N.; Tateno, K.; Moriya, J.; Yokoyama, M.; Nojima, A.; et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J. Clin. Investig. 2010, 120, 1506–1514. [Google Scholar] [CrossRef]
- Van Craeyveld, E.; Jacobs, F.; Gordts, S.C.; De Geest, B. Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther. 2012, 19, 860–871. [Google Scholar] [CrossRef]
- Gordts, S.C.; Muthuramu, I.; Nefyodova, E.; Jacobs, F.; Van Craeyveld, E.; De Geest, B. Beneficial effects of selective HDL-raising gene transfer on survival, cardiac remodelling and cardiac function after myocardial infarction in mice. Gene Ther. 2013, 20, 1053–1061. [Google Scholar] [CrossRef]
- Bayat, H.; Swaney, J.S.; Ander, A.N.; Dalton, N.; Kennedy, B.P.; Hammond, H.K.; Roth, D.M. Progressive heart failure after myocardial infarction in mice. Basic Res. Cardiol. 2002, 97, 206–213. [Google Scholar] [CrossRef] [PubMed]
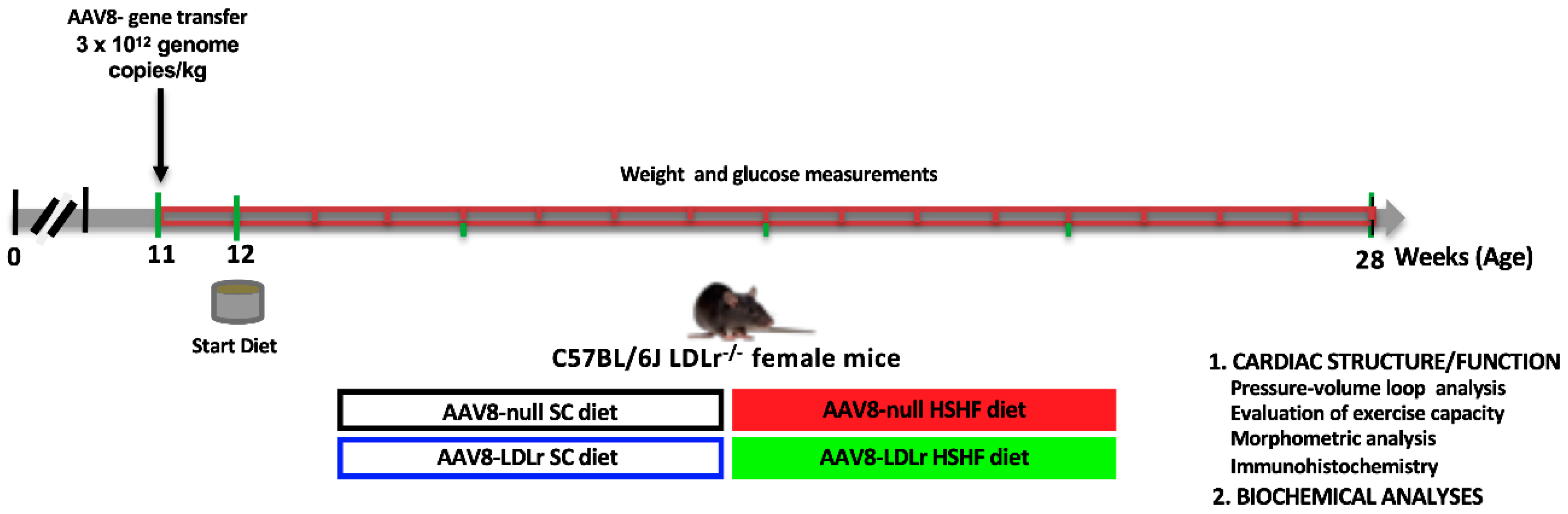
| AAV8-null SC Diet (n = 14) | AAV8-LDLr SC Diet (n = 12) | AAV8-null HSHF Diet (n = 15) | AAV8-LDLr HSHF Diet (n = 23) | |
|---|---|---|---|---|
| Heart weight (mg) | 118 ± 3 | 114 ± 2 | 140 ± 4 §§§ | 118 ± 2 *** |
| Tibia length (mm) | 17.4 ± 0.1 | 17.3 ± 0.1 | 17.5 ± 0.1 | 17.4 ± 0.1 |
| Heart weight/tibia length (mg/mm) | 6.82 ± 0.15 | 6.58 ± 0.08 | 8.01 ± 0.21 §§§ | 6.79 ± 0.10 *** |
| Left ventricular weight (mg) | 78.8 ± 1.8 | 75.5 ± 1.0 | 97.2 ± 2.5 §§§ | 81.0 ± 1.1 §§*** |
| Right ventricular weight (mg) | 22.0 ± 0.9 | 24.3 ± 1.0 | 29.6 ± 2.4 §§ | 22.5 ± 0.7 ** |
| Lung weight (mg) | 140 ± 6 | 140 ± 2 | 166 ± 4 §§§ | 145 ± 3 *** |
| Liver weight (mg) | 917 ± 21 | 896 ± 22 | 1230 ± 50 §§§ | 1060 ± 40 §** |
| Kidney weight(mg) | 245 ± 5 | 257 ± 8 | 300 ± 8 §§§ | 259 ± 3 *** |
| Spleen weight (mg) | 69.2 ± 1.6 | 75.9 ± 1.9 | 95.0 ± 2.9 §§§ | 77.8 ± 1.9 *** |
| AAV8-null SC Diet (n = 11) | AAV8-LDLr SC Diet (n = 11) | AAV8-null HSHF Diet (n = 20) | AAV8-LDLr HSHF Diet (n = 21) | |
|---|---|---|---|---|
| Left ventricular wall area (mm2) | 9.28 ± 0.28 | 8.41 ± 0.33 | 11.5 ± 0.3 §§§ | 9.92 ± 0.33 §§** |
| Anterior wall thickness (µm) | 1060 ± 40 | 980 ± 33 | 1340 ± 40 §§§ | 1130 ± 30 §§*** |
| Cardiomyocyte cross-sectional area (µm2) | 189 ± 7 | 197 ± 8 | 326 ±17 §§§ | 219 ± 9 *** |
| Cardiomyocyte density (number/mm2) | 3770 ± 120 | 3540 ± 110 | 2460 ± 120 §§§ | 3490 ± 150 *** |
| Capillary density (number/mm2) | 6040 ± 300 | 6580 ± 130 | 4770 ± 250 §§ | 5790 ± 200 §** |
| Relative vascularity (µm−2) | 0.00864 ± 0.00058 | 0.00965 ± 0.00032 | 0.00633 ± 0.00041 §§ | 0.00784 ± 0.00029 §§** |
| Interstitial fibrosis (%) | 1.86 ± 0.18 | 2.01 ± 0.20 | 3.81 ± 0.31 §§§ | 2.38 ± 0.17 *** |
| Perivascular fibrosis (ratio) | 0.456 ± 0.048 | 0.436 ± 0.048 | 0.522 ± 0.023 | 0.408 ± 0.044 * |
| 3-nitrotyrosine positive area (%) | 1.51 ± 0.15 | 1.90 ± 0.16 | 5.11 ± 0.45 §§§ | 3.55 ± 0.36 §§* |
| AAV8-null SC Diet (n = 10) | AAV8-LDLr SC Diet (n = 7) | AAV8-null HSHF Diet (n = 14) | AAV8-LDLr HSHF Diet (n = 13) | |
|---|---|---|---|---|
| Heart rate (bpm) | 607 ± 12 | 580 ± 15 | 600 ± 16 | 580 ± 17 |
| Pmax (mm Hg) | 99.9 ± 1.9 | 102 ± 1 | 87.7 ± 2.4 §§ | 97.5 ± 3.0 * |
| Pes (mm Hg) | 95.2 ± 2.4 | 94.3 ± 1.6 | 80.2 ± 2.5 §§ | 90.5 ± 3.4 * |
| dP/dtmax (mmHg/ms) | 11.9 ± 0.3 | 14.2 ± 0.4 °° | 8.46 ± 0.35 §§§ | 11.4 ± 1.2 * |
| PRSW (mm Hg) | 74.0 ±1.6 | 77.8 ± 5.1 | 59.4 ± 4.1 § | 84.5 ± 5.6 ** |
| Ees (mmHg/µl) | 6.67 ± 0.63 | 8.16 ± 0.82 | 4.69 ± 0.55 § | 6.40 ± 0.47 * |
| Pmin (mm Hg) | 1.82 ± 0.44 | 0.415 ± 0.494 | 1.99 ± 0.57 | 1.47 ± 0.43 |
| Ped (mm Hg) | 5.02 ± 0.37 | 4.23 ± 0.44 | 5.24 ± 0.53 | 5.36 ± 0.70 |
| dP/dtmin (mmHg/ms) | −9.93 ± 0.55 | −10.4 ± 0.5 | −7.93 ± 0.36 §§ | −9.65 ± 0.51 * |
| Tau (ms) | 5.64 ± 0.16 | 5.45 ± 0.21 | 7.14 ± 0.22 §§§ | 5.74 ± 0.22 *** |
| Slope EDPVR (mmHg/µl) | 0.523 ± 0.070 | 0.487 ± 0.155 | 0.932 ± 0.103 §§ | 0.276 ± 0.081 *** |
| EDV (µl) | 31.5 ± 1.5 | 27.6 ± 2.0 | 25.1 ± 1.4 § | 29.2 ± 2.1 |
| ESV (µl) | 13.6 ± 1.0 | 9.15 ± 1.00 ° | 12.2 ± 1.2 | 12.7 ± 1.2 |
| Stroke volume (µl) | 17.9 ± 0.9 | 18.4 ± 1.3 | 12.9 ± 0.7 §§§ | 16.5 ± 1.2 * |
| Ejection fraction (%) | 57.2 ± 2.1 | 67.0 ± 2.0 °° | 52.1 ± 2.5 | 56.9 ± 1.8 §§ |
| Cardiac output (ml/min) | 10.9 ± 0.6 | 10.7 ± 0.8 | 7.76 ± 0.46 §§§ | 9.69 ± 0.72 * |
| Stroke work (mmHg.µl) | 1420 ± 70 | 1540 ± 110 | 898 ± 46 §§§ | 1310 ± 120 ** |
| dV/dtmax (µl/s) | 712 ± 22 | 839 ± 86 | 509 ± 52 §§ | 554 ± 51 § |
| dV/dtmin (µl/s) | −790 ± 44 | −992 ± 70 ° | −587 ± 46 §§ | −672 ± 40 §§ |
| Ea (mmHg/µl) | 5.42 ± 0.38 | 5.19 ± 0.40 | 6.55 ± 0.52 | 5.75 ± 0.36 |
| Ea/Ees | 0.865 ± 0.086 | 0.687 ± 0.101 | 1.79 ± 0.35 § | 0.966 ± 0.081 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboumsallem, J.P.; Muthuramu, I.; Mishra, M.; De Geest, B. Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 2222. https://doi.org/10.3390/ijms20092222
Aboumsallem JP, Muthuramu I, Mishra M, De Geest B. Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice. International Journal of Molecular Sciences. 2019; 20(9):2222. https://doi.org/10.3390/ijms20092222
Chicago/Turabian StyleAboumsallem, Joseph Pierre, Ilayaraja Muthuramu, Mudit Mishra, and Bart De Geest. 2019. "Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice" International Journal of Molecular Sciences 20, no. 9: 2222. https://doi.org/10.3390/ijms20092222
APA StyleAboumsallem, J. P., Muthuramu, I., Mishra, M., & De Geest, B. (2019). Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice. International Journal of Molecular Sciences, 20(9), 2222. https://doi.org/10.3390/ijms20092222






