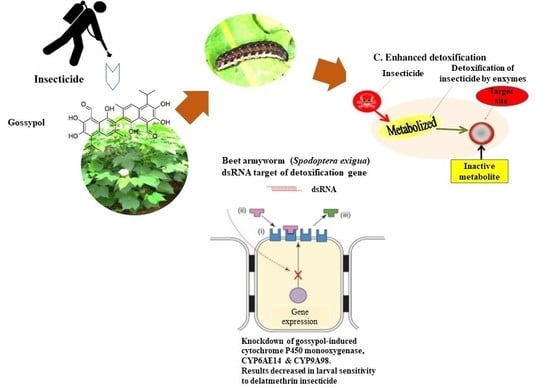
Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner)
Abstract
1. Introduction
2. Results
2.1. Induced Effect of Gossypol to Deltamethrin Tolerance and Synergism Assessment
2.2. Effect of Gossypol Diet on Larval Body Weight
2.3. Effect of Gossypol on Midgut P450 Activity
2.4. Effect of Gossypol, Flavone, and Deltamethrin on Expression Response of P450 Genes
2.5. Effect of dsCYP6AB14 and dsCYP9A98 on Larval Mortality
2.6. The Combined Effect of Target dsCYP6AB14+dsCYP9A98 Genes on Larval Mortality
2.7. Effect of Silencing by dsRNA
3. Discussion
4. Materials and Methods
4.1. Insect Culture
4.2. Chemicals
4.3. Preparation of Treatment Diets
4.4. Toxicological Analysis of Deltamethrin Tolerance in Larvae
4.5. Effect of PBO on the Toxicity of Insecticides
4.6. The Effect of 0.1% Gossypol Diet on Bodyweight
4.7. Samples Preparation for P450 Enzyme Activity
4.8. Measurement of P450 Enzyme Activity
4.9. Sample Preparation
4.10. RNA Extraction and cDNA Synthesis
4.11. Quantitative Real-Time PCR
4.12. Preparation of dsRNA
4.13. Administration of dsRNA by Droplet-Feeding
4.14. Combined Effects of dsRNA on Mortality
4.15. Analysis of the Silencing Effect
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Taggar, G.K.; Gill, R.S. Host plant resistance in Vigna sp. towards whitefly, Bemisia tabaci (Gennadius): A review. Entomol. Gen. 2016, 36, 1–24. [Google Scholar] [CrossRef]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore- and elicitor-induced resistance in groundnut to Asian armyworm, Spodoptera litura (Fab.) (lepidoptera: Noctuidae). Plant Signal. Behav. 2011, 6, 1769–1777. [Google Scholar] [CrossRef]
- Lattanzio, V.; Arpaia, S.; Cardinali, A.; Di Venere, D.; Linsalata, V. Endogenous flavonoids in resistance mechanism of Vigna role of to aphids. J. Agric. Food Chem. 2000, 48, 5316–5320. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kotkar, H.M.; Mendki, P.S.; Upasani, S.M.; Maheshwari, V.L. Efficacy of flavonoids in controlling Callosobruchus chinensis (L.) (Coleoptera: Bruchidae), a post-harvest pest of grain legumes. Crop Prot. 2005, 24, 888–893. [Google Scholar] [CrossRef]
- Duisembecova, B.A.; Dubovskiyb, I.M.; Glupov, V.V. Effect of plant secondary metabolites on susceptibility of insects to entomopathogenic microorganisms. Contemp. Probl. Ecol. 2017, 10, 286–292. [Google Scholar] [CrossRef]
- Wang, X.; Howell, C.P.; Chen, F.; Yin, J.; Jiang, Y. Gossypol-A polyphenolic compound from cotton plant. Adv. Food Nutr. Res. 2009, 58, 215–263. [Google Scholar]
- Krempl, C.; Heidel-Fischer, H.M.; Jiménez-Alemán, G.H.; Reichelt, M.; Menezes, R.C.; Boland, W.; Vogel, H.; Heckel, D.G.; Joußen, N. Gossypol toxicity and detoxification in Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 78, 69–77. [Google Scholar] [CrossRef]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect Cytochrome P450. In Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–77. ISBN 0-44-451524-0. [Google Scholar]
- Chen, C.; Liu, Y.; Shi, X.; Desneux, N.; Han, P.; Gao, X. Elevated carboxylesterase activity contributes to the lambda-cyhalothrin insensitivity in quercetin fed Helicoverpa armigera (Hübner). PLoS ONE 2017, e0183111. [Google Scholar] [CrossRef]
- Mao, Y.B.; Tao, X.Y.; Xue, X.Y.; Wang, L.J.; Chen, X.Y. Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res. 2011, 20, 665–673. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular Mechanisms of Metabolic Resistance to Synthetic and Natural Xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Schuler, M.A. P450s in plant-insect interactions. Biochim. Biophys. Acta - Proteins Proteomics 2011, 1814, 36–45. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, S.H.; Ren, M.M.; Tian, X.R.; Wei, Q.; Mburu, D.K.; Su, J.Y. The expression of Spodoptera exigua P450 and UGT genes: Tissue specificity and response to insecticides. Insect Sci. 2017, 26, 199–216. [Google Scholar] [CrossRef]
- Tao, X.Y.; Xue, X.Y.; Huang, Y.P.; Chen, X.Y.; Mao, Y.B. Gossypol-enhanced P450 gene pool contributes to cotton bollworm tolerance to a pyrethroid insecticide. Mol. Ecol. 2012, 21, 4371–4385. [Google Scholar] [CrossRef]
- Mao, W.; Rupasinghe, S.G.; Johnson, R.M.; Zangerl, A.R.; Schuler, M.A.; Berenbaum, M.R. Quercetin-metabolizing CYP6AS enzymes of the pollinator Apis mellifera (Hymenoptera: Apidae). Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2009, 154, 427–434. [Google Scholar] [CrossRef]
- Wee, C.W.; Lee, S.F.; Robin, C.; Heckel, D.G. Identification of candidate genes for fenvalerate resistance in Helicoverpa armigera using cDNA-AFLP. Insect Mol. Biol. 2008, 17, 351–360. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, T.; Feng, X.; Qiu, L. Cloning and characterisation of NADPH-dependent cytochrome P450 reductase gene in the cotton bollworm, Helicoverpa armigera. Pest Manag. Sci. 2014, 555, 262–270. [Google Scholar] [CrossRef]
- Li, X.; Berenbaum, M.R.; Schuler, M.A. Molecular cloning and expression of CYP6B8: A xanthotoxin-inducible cytochrome P450 cDNA from Helicoverpa zea. Insect Biochem. Mol. Biol. 2000, 30, 75–84. [Google Scholar] [CrossRef]
- Xie, W.; Wang, S.; Wu, Q.; Feng, Y.; Pan, H.; Jiao, X.; Zhou, L.; Yang, X.; Fu, W.; Teng, H.; et al. Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2011, 67, 87–93. [Google Scholar] [CrossRef]
- Uǧurlu Karaaǧaç, S.; Konuş, M.; Büyük, M. Determination of susceptibility levels of Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera) strains collected from different regions to some insecticides in Turkey. J. Entomol. Res. Soc. 2013, 15, 37–45. [Google Scholar]
- Qiu, L.; Hou, L.; Zhang, B.; Liu, L.; Li, B.; Deng, P.; Ma, W.; Wang, X.; Fabrick, J.A.; Chen, L.; et al. Cadherin is involved in the action of Bacillus thuringiensis toxins Cry1Ac and Cry2Aa in the beet armyworm, Spodoptera exigua. J. Invertebr. Pathol. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Gong, C.; Yao, X.; Jiang, C.; Yang, Q. Molecular identification of four novel cytochrome P450 genes related to the development of resistance of Spodoptera exigua (Lepidoptera: Noctuidae) to chlorantraniliprole. Pest Manag. Sci. 2018. [Google Scholar] [CrossRef]
- Lai, T.; Li, J.; Su, J. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Tian, X.; Sun, X.; Su, J. Biochemical mechanisms for metaflumizone resistance in beet armyworm, Spodoptera exigua. Pestic. Biochem. Physiol. 2014, 113, 8–14. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, X.; Yu, H.; Liu, S.; Yin, Y.; Cui, P.; Wu, Y.; Yang, J.; Jiang, C.; Yang, Q. Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2018, 146, 71–89. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Saleem, M.A.; Razaq, M. Monitoring of resistance in Spodoptera exigua (Lepidoptera: Noctuidae) from four districts of the Southern Punjab, Pakistan to four conventional and six new chemistry insecticides. Crop Prot. 2012, 33, 13–20. [Google Scholar] [CrossRef]
- Tian, H.; Peng, H.; Yao, Q.; Chen, H.; Xie, Q.; Tang, B.; Zhang, W. Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 2009, 4, e6225. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.A.; Poelman, E.H.; Dicke, M. Plant Interactions with Multiple Insect Herbivores: From Community to Genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Holm Freiesleben, S.; Jäger, A.K. Correlation between plant secondary metabolites and their antifungal mechanisms—A review. Med. Aromat. Plants 2014. [Google Scholar] [CrossRef]
- Rani, P.U.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Bos, N.; Lefèvre, T.; Jensen, A.B.; d’Ettorre, P.; Schluns, H.; Crozier, R.H.; Moret, Y.; Schmid-Hempel, P.; Tsakas, S.M.V.J.; Zhang, J.; et al. Herbivores: Their Interactions with Secondary Plant Metabolites: The Chemical Participants. Evolution (N. Y). 2012, 7, 481. [Google Scholar]
- Chen, C.; Han, P.; Yan, W.; Wang, S.; Shi, X.; Zhou, X.; Desneux, N.; Gao, X. Uptake of quercetin reduces larval sensitivity to lambda-cyhalothrin in Helicoverpa armigera. J. Pest Sci. 2018, 91, 919–926. [Google Scholar] [CrossRef]
- Li, X.C.; Zangerl, A.R.; Schuler, M.A.; Berenbaum, B.M. Cross-resistance to α-cypermethrin after xanthotoxin ingestion in Helicoverpa zea (Lepidoptera: Noctuidae). J. Econ. Entomol. 2000, 93, 18–25. [Google Scholar] [CrossRef]
- Chen, S.; Elzaki, M.E.A.; Ding, C.; Li, Z.F.; Wang, J.; Zeng, R.S.; Song, Y.Y. Plant allelochemicals affect tolerance of polyphagous lepidopteran pest Helicoverpa armigera (Hübner) against insecticides. Pestic. Biochem. Physiol. 2019, 154, 32–38. [Google Scholar] [CrossRef]
- Johnson, R.M.; Mao, W.; Pollock, H.S.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis Mellifera. PLoS ONE 2012, 7, e31051. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2018, 75, 683–693. [Google Scholar] [CrossRef]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004. [Google Scholar] [CrossRef]
- Wang, R.L.; Liu, S.W.; Baerson, S.R.; Qin, Z.; Ma, Z.H.; Su, Y.J.; Zhang, J.E. Identification and functional analysis of a novel cytochrome P450 gene CYP9A105 associated with pyrethroid detoxification in Spodoptera exigua hübner. Int. J. Mol. Sci. 2018, 19, 737. [Google Scholar] [CrossRef]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Wang, R.L.; Li, J.; Staehelin, C.; Xin, X.W.; Su, Y.J.; Zeng, R. Sen Expression Analysis of Two P450 Monooxygenase Genes of the Tobacco Cutworm Moth (Spodoptera litura) at Different Developmental Stages and in Response to Plant Allelochemicals. J. Chem. Ecol. 2014, 41, 111–119. [Google Scholar] [CrossRef]
- Wang, R.L.; Xia, Q.Q.; Baerson, S.R.; Ren, Y.; Wang, J.; Su, Y.J.; Zheng, S.C.; Zeng, R. Sen A novel cytochrome P450 CYP6AB14 gene in Spodoptera litura (Lepidoptera: Noctuidae) and its potential role in plant allelochemical detoxification. J. Insect Physiol. 2015, 75, 54–62. [Google Scholar] [CrossRef]
- Wang, R.-L.; Staehelin, C.; Xia, Q.-Q.; Su, Y.-J.; Zeng, R.-S. Identification and Characterization of CYP9A40 from the Tobacco Cutworm Moth (Spodoptera litura), a Cytochrome P450 Gene Induced by Plant Allelochemicals and Insecticides. Int. J. Mol. Sci. 2015, 16, 22606–22620. [Google Scholar] [CrossRef]
- Wang, R.-L.; He, Y.-N.; Staehelin, C.; Liu, S.-W.; Su, Y.-J.; Zhang, J.-E. Identification of Two Cytochrome Monooxygenase P450 Genes, CYP321A7 and CYP321A9, from the Tobacco Cutworm Moth (Spodoptera Litura) and Their Expression in Response to Plant Allelochemicals. Int. J. Mol. Sci. 2017, 18, 2278. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Shen, G.; Xu, Z.; Xu, Q.; He, L. Collaborative contribution of six cytochrome P450 monooxygenase genes to fenpropathrin resistance in Tetranychus cinnabarinus (Boisduval). Insect Mol. Biol. 2016. [Google Scholar] [CrossRef]
- Jan, S.; Liu, S.; Hafeez, M.; Zhang, X.; Dawar, F.U.; Guo, J.; Gao, C.; Wang, M. Isolation and functional identification of three cuticle protein genes during metamorphosis of the beet armyworm, Spodoptera exigua. Sci. Rep. 2017. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, R.; Wu, H.; Zhang, X.; Liu, Y.; Zhu, K.Y.; Zhang, J.; Ma, E. Identification and characterization of two CYP9A genes associated with pyrethroid detoxification in Locusta migratoria. Pestic. Biochem. Physiol. 2016, 132, 65–71. [Google Scholar] [CrossRef]
- Wang, R.L.; Zhu-Salzman, K.; Baerson, S.R.; Xin, X.W.; Li, J.; Su, Y.J.; Zeng, R. Sen Identification of a novel cytochrome P450 CYP321B1 gene from tobacco cutworm (Spodoptera litura) and RNA interference to evaluate its role in commonly used insecticides. Insect Sci. 2017, 24, 235–247. [Google Scholar] [CrossRef]
- Liu, X.; Liang, P.; Gao, X.; Shi, X. Induction of the cytochrome P450 activity by plant allelochemicals in the cotton bollworm, Helicoverpa armigera (Hübner). Pestic. Biochem. Physiol. 2006, 84, 127–134. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 84, 127–134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bautista, M.A.M.; Miyata, T.; Miura, K.; Tanaka, T. RNA interference-mediated knockdown of a cytochrome P450, CYP6BG1, from the diamondback moth, Plutella xylostella, reduces larval resistance to permethrin. Insect Biochem. Mol. Biol. 2009, 39, 38–46. [Google Scholar] [CrossRef]






| Treatment | N | LC50 (mg a.i./L) | 95% CL | Slope ± SE | df | χ2 | SR |
|---|---|---|---|---|---|---|---|
| Control | 420 | 0.887 | 0.75 ± 1.03 | 1.56 ± 0.14 | 4 | 1.66 | 1.7 |
| Control + PBO | 420 | 0.681 | 0.58 ± 0.79 | 1.61 ± 0.14 | 4 | 0.79 | ---- |
| Gossypol | 420 | 1.704 | 1.46 ± 1.98 | 1.71 ± 0.15 | 4 | 1.24 | ---- |
| Gossypol + PBO | 420 | 0.735 | 0.61 ± 0.87 | 1.47 ± 0.14 | 4 | 1.979 | 2.3 |
| Function | Primer Name | Primer Sequence (5′-3′) |
|---|---|---|
| Real-Time PCR | ||
| CYP6AB14 | CYP6AB14-F | TCTTGATGCTGACTCGCTCA |
| CYP6AB14-R | TACAGGCTTCCGGGAACATT | |
| CYP9A98 | CYP9A98-F | CTACCAGCATCTGCGTCAC |
| CYP9A98-R | TTAGCCTACACCTTAACCAAT | |
| β-actin | β-actin-F | ATCCTCCGTCTGGACTTGG |
| β-actin-R | GCACGATTTCCCTCTCA | |
| dsRNA synthesis | ||
| CYP6AB14 | T7CYP6AB14-F1 | ggatcctaatacgactcactataggATGGGCTTTTCCAATCTTTC |
| CYP6AB14-R1 | GCTTAAACGTGCACAAGACAG | |
| CYP6AB14-F2 | ATGGGCTTTTCCAATCTTTC | |
| T7CYP6AB14-R2 | GCTTAAACGTGCACAAGACAGggatcctaatacgactcactatagg | |
| CYP9A98 | T7CYP9A98-F1 | ggatcctaatacgactcactataggGAGAACTTCCTCAACCATCCTAA |
| CYP9A98-R1 | TGATTCCGCTAAGTATCTTTCCC | |
| CYP9A98-F2 | GAGAACTTCCTCAACCATCCTAA | |
| T7CYP9A98-R2 | TGATTCCGCTAAGTATCTTTCCCggatcctaatacgactcactatagg | |
| dsRED | T7dsRED-F1 | ggatcctaatacgactcactataggGCAAGCTATGCATCCAACGCGTTGGG |
| dsRED-R1 | CAAGCTATGCATCCAACGCGTTGGGAG | |
| dsRED-F2 | GCAAGCTATGCATCCAACGCGTTGGG | |
| T7dsRED-R2 | CAAGCTATGCATCCAACGCGTTGGGAGggatcctaatacgactcactatagg | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, M.; Liu, S.; Jan, S.; Shi, L.; Fernández-Grandon, G.M.; Gulzar, A.; Ali, B.; Rehman, M.; Wang, M. Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner). Int. J. Mol. Sci. 2019, 20, 2248. https://doi.org/10.3390/ijms20092248
Hafeez M, Liu S, Jan S, Shi L, Fernández-Grandon GM, Gulzar A, Ali B, Rehman M, Wang M. Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner). International Journal of Molecular Sciences. 2019; 20(9):2248. https://doi.org/10.3390/ijms20092248
Chicago/Turabian StyleHafeez, Muhammad, Sisi Liu, Saad Jan, Le Shi, G. Mandela Fernández-Grandon, Asim Gulzar, Bahar Ali, Muzammal Rehman, and Mo Wang. 2019. "Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner)" International Journal of Molecular Sciences 20, no. 9: 2248. https://doi.org/10.3390/ijms20092248
APA StyleHafeez, M., Liu, S., Jan, S., Shi, L., Fernández-Grandon, G. M., Gulzar, A., Ali, B., Rehman, M., & Wang, M. (2019). Knock-Down of Gossypol-Inducing Cytochrome P450 Genes Reduced Deltamethrin Sensitivity in Spodoptera exigua (Hübner). International Journal of Molecular Sciences, 20(9), 2248. https://doi.org/10.3390/ijms20092248