Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells
Abstract
1. Introduction
2. Results
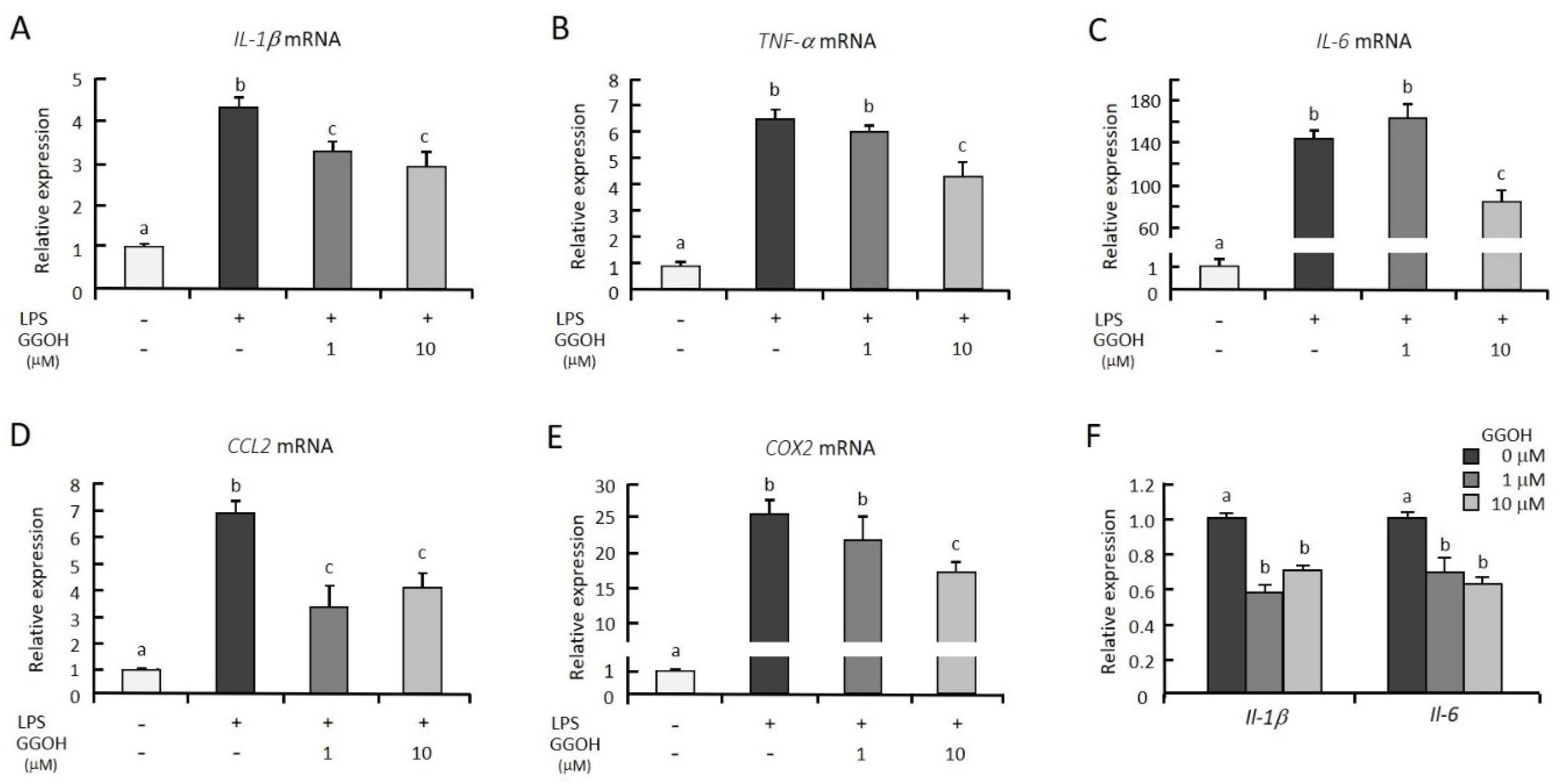
2.1. GGOH Suppresses LPS-Induced Inflammatory Genes in Macrophagic THP-1 Cells
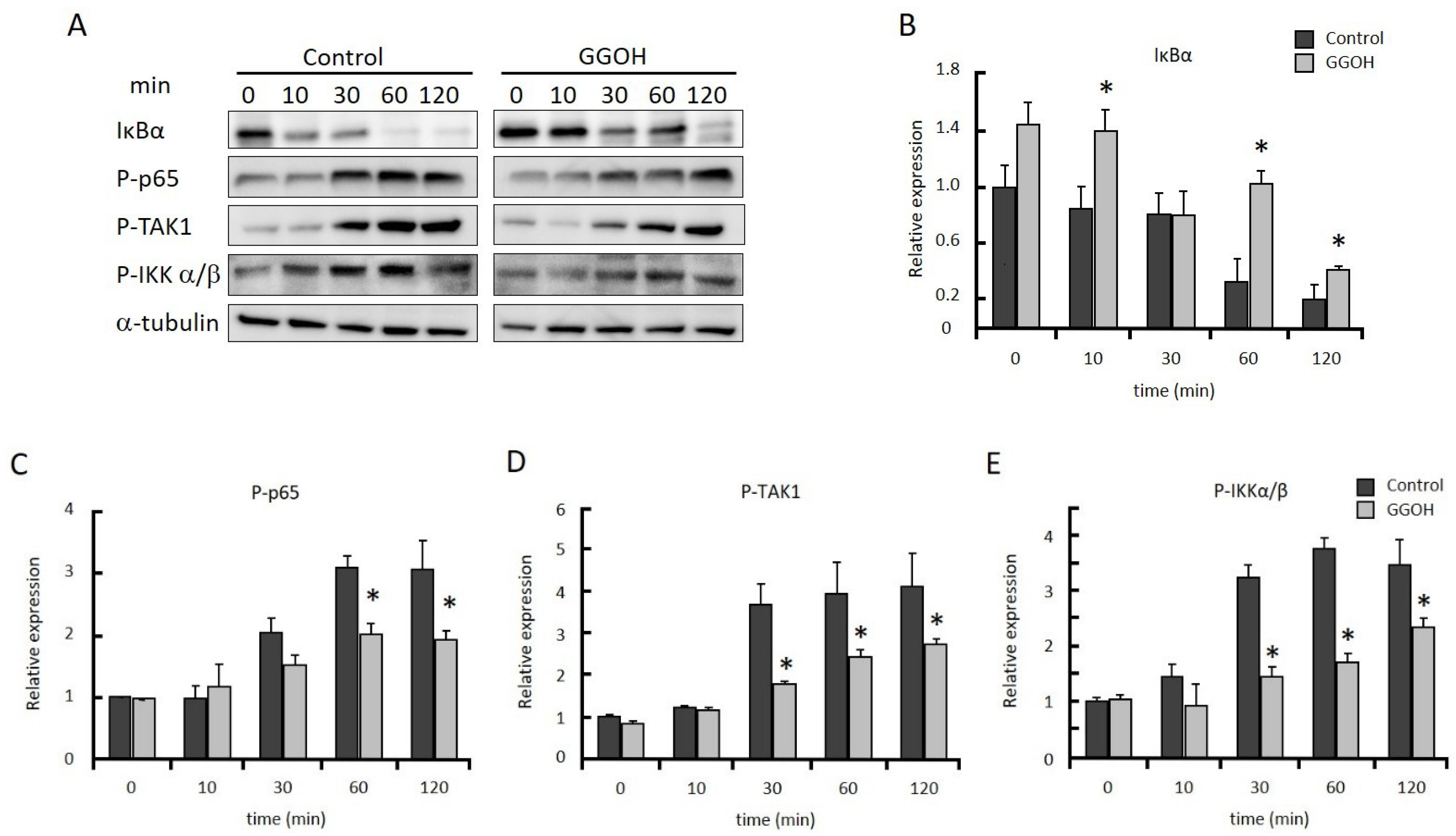
2.2. GGOH Suppresses NFκB Activation
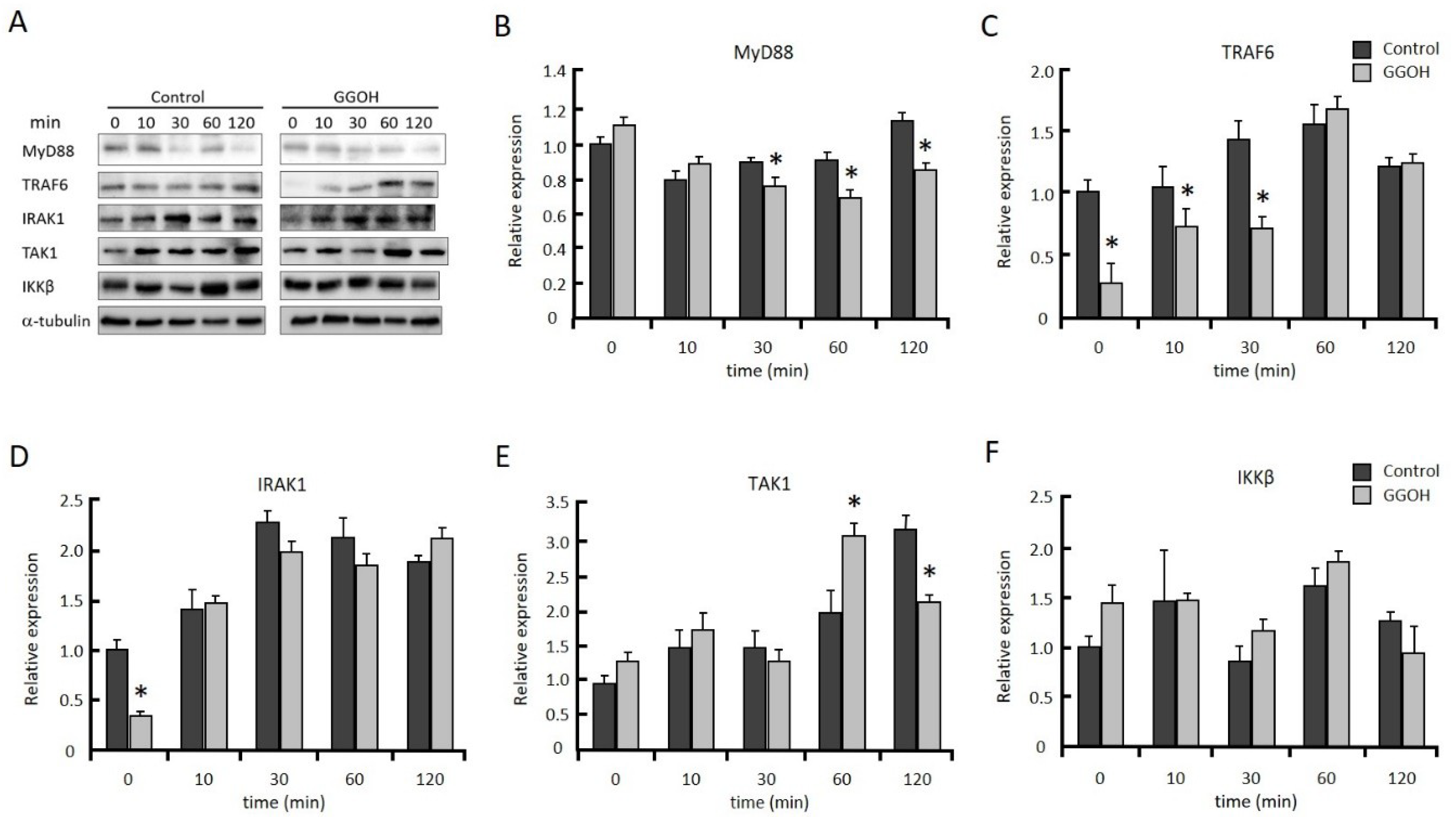
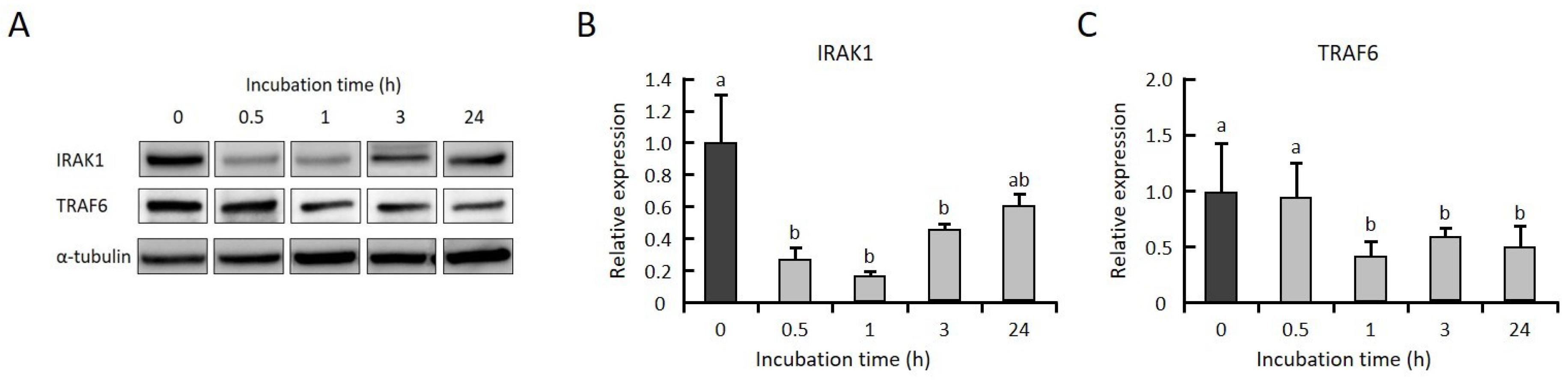
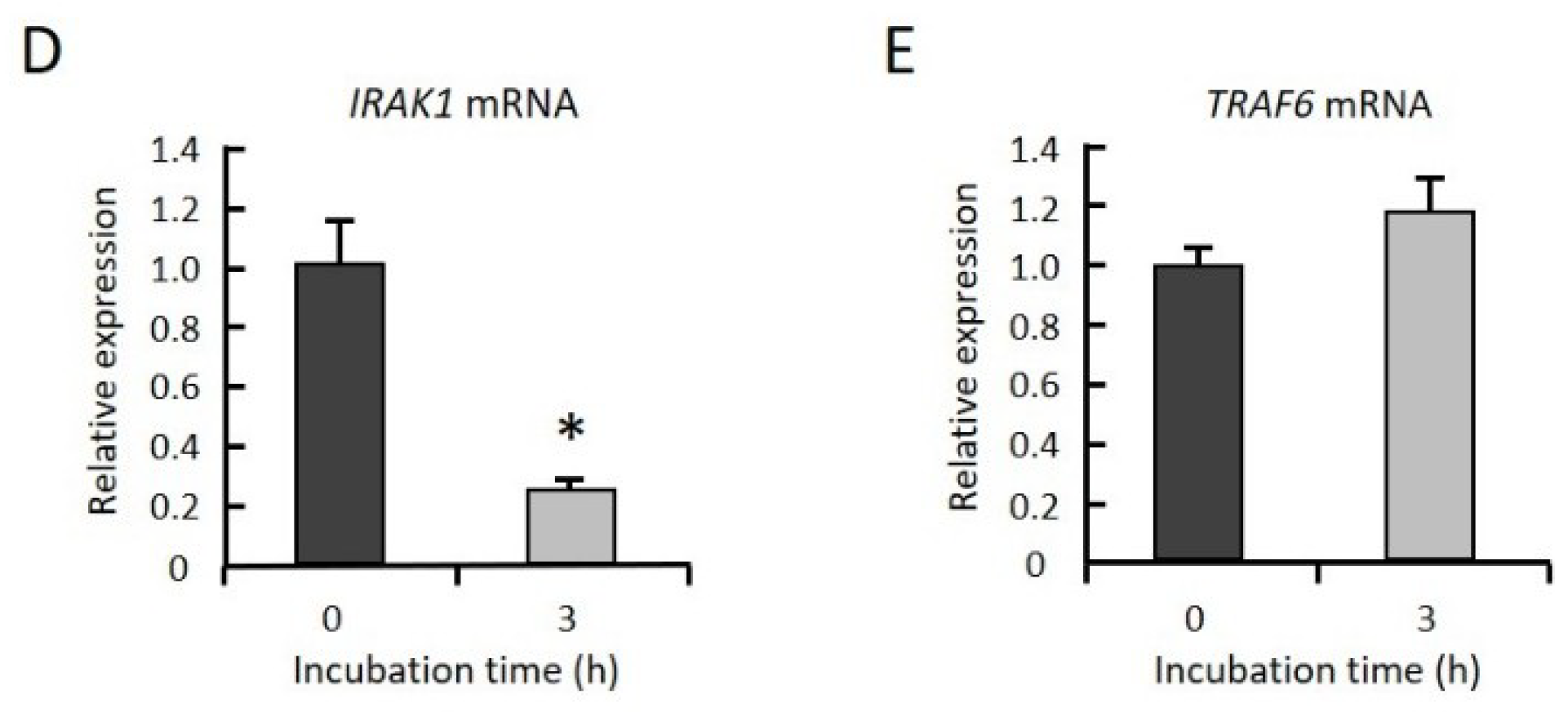
2.3. GGOH Suppresses IRAK1 and TRAF6 Expression and the Subsequent Phosphorylation of NFκB Signaling Molecules
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Growth Assays
4.4. RNA Preparation and Quantitative RT-PCR
4.5. Western Blot Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GGOH | Geranylgeraniol |
| LPS | Lipopolysaccharide |
| NFκB | Nuclear factor-kappa B |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
References
- Karin, M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect Biol. 2009, 1, a000141. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Staudt, L.M. Oncogenic Activation of NF-κB. Cold Spring Harb. Perspect Biol. 2010, 2, a000109. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Tilborghs, S.; Corthouts, J.; Verhoeven, Y.; Arias, D.; Rolfo, C.; Trinh, X.B.; van Dam, P.A. The role of Nuclear Factor-kappa B signaling in human cervical cancer. Crit. Rev. Oncol. Hematol. 2017, 120, 141–150. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: a central player in innate immune signaling. 1000prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef]
- Caplan, I.F.; Maguire-Zeiss, K.A. Toll-Like Receptor 2 Signaling and Current Approaches for Therapeutic Modulation in Synucleinopathies. Front. Pharm. 2018, 9, 417. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Dhingra, N.; Narsinghani, T.; Sharma, R. Insights into the mechanism of natural terpenoids as NF-κB inhibitors: an overview on their anticancer potential. Exp. Oncol. 2016, 38, 158–168. [Google Scholar] [CrossRef]
- Byun, E.B.; Choi, H.G.; Sung, N.Y.; Byun, E.H. Green tea polyphenolepigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa lamininreceptor on lipopolysaccharide-stimulated dendritic cells. Biochem. Biophys. Res. Commun. 2012, 426, 480–485. [Google Scholar] [CrossRef]
- Frenkel, J.; Rijkers, G.T.; Mandey, S.H.; Buurman, S.W.; Houten, S.M.; Wanders, R.J.; Waterham, H.R.; Kuis, W. Lack of isoprenoid products raises ex vivo interleukin-1β secretion in hyperimmunoglobulinemia D and periodic fever syndrome. Arthritis. Rheum. 2002, 46, 2794–2803. [Google Scholar] [CrossRef]
- Ruiz-Velascoa, N.; Dominguez, A.; Vega, M.A. Statins upregulate CD36 expression in human monocytes, an effect strengthened when combined with PPAR-γ ligands putative contribution of Rho GTPases in statin-induced CD36 expression. Biochem. Pharm. 2004, 67, 303–313. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Pontillo, A.; De Leo, L.; Tommasini, A.; Decorti, G.; Not, T.; Ventura, A. Natural isoprenoids are able to reduce inflammation in a mouse model of mevalonate kinase deficiency. Pediatr. Res. 2008, 64, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.N.; Ye, J.; Hao, R.; Debose-Boyd, R.; Ye, J. Sufficient production of geranylgeraniol is required to maintain endotoxin tolerance in macrophages. J. Lipid Res. 2013, 54, 3430–3437. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Kleiner, G.; Valencic, E.; Campisciano, G.; Girardelli, M.; Crovella, S.; Knowles, A.; Marcuzzi, A. Block of the mevalonate pathway triggers oxidative and inflammatory molecular mechanisms modulated by exogenous isoprenoid compounds. Int. J. Mol. Sci. 2014, 15, 6843–6856. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Tommasini, A.; Crovella, S.; Pontillo, A. Natural isoprenoids inhibit LPS-induced-production of cytokines and nitric oxide in aminobisphosphonate-treated monocytes. Int. Immunopharmacol 2010, 10, 639–642. [Google Scholar] [CrossRef] [PubMed]
- De Moura Espindola, R.; Mazzantini, R.P.; Ong, T.P.; de Conti, A.; Heidor, R.; Moreno, F.S. Geranylgeraniol and β-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-κB activation. Carcinogenesis 2005, 26, 1091–1099. [Google Scholar] [CrossRef]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-κB activation induced by carcinogenic agents through suppression of IκBα kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar] [PubMed]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Hata, S.; Kuriyama, H.; Sato, S.; Goto, T.; Komai, M. Dietary supplementation with geranylgeraniol suppresses lipopolysaccharide induced inflammation via inhibition of nuclear factor-κB activation in rats. Eur. J. Nutr. 2013, 52, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Zheng, M.H.; Feng, H.T.; Steer, J.H.; Joyce, D.A.; Xu, J. Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-κB activity. J. Bone Min. Res. 2004, 19, 1905–1916. [Google Scholar] [CrossRef]
- Lee, J.; Tae, N.; Lee, J.J.; Kim, T.; Lee, J.H. Eupatolide inhibits lipopolysaccharide-induced COX-2 and iNOS expression in RAW264.7 cells by inducing proteasomal degradation of TRAF6. Eur. J. Pharm. 2010, 636, 173–180. [Google Scholar] [CrossRef]
- Gottipati, S.; Rao, N.L.; Fung-Leung, W.P. IRAK1: a critical signaling mediator of innate immunity. Cell Signal. 2008, 20, 269–276. [Google Scholar] [CrossRef]
- Cui, W.; Xiao, N.; Xiao, H.; Zhou, H.; Yu, M.; Gu, J.; Li, X. β-TrCP-mediated IRAK1 degradation releases TAK1-TRAF6 from the membrane to the cytosol for TAK1-dependent NF-κB activation. Mol. Cell Biol. 2012, 32, 3990–4000. [Google Scholar] [CrossRef]
- Clark, K.; Nanda, S.; Cohen, P. Molecular control of the NEMO family of ubiquitin-binding proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 673–685. [Google Scholar] [CrossRef]
- Zotti, T.; Scudiero, I.; Settembre, P.; Ferravante, A.; Mazzone, P.; D’Andrea, L.; Reale, C.; Vito, P.; Stilo, R. TRAF6-mediated ubiquitination of NEMO requires p62/sequestosome-1. Mol. Immunol 2014, 58, 27–31. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. γ-Tocotrienol inhibits nuclear factor-κB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB–regulated gene products and TAK1-mediated NF-κB activation. Blood 2007, 109, 2727–2735. [Google Scholar]
- Ohsaki, Y.; Shirakawa, H.; Hiwatashi, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci. Biotechnol. Biochem. 2006, 70, 926–932. [Google Scholar] [CrossRef]
- Ozaki, I.; Zhang, H.; Mizuta, T.; Ide, Y.; Eguchi, Y.; Yasutake, T.; Sakamaki, T.; Pestell, R.G.; Yamamoto, K. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor κB activation. Clin. Cancer Res. 2007, 13, 2236–2245. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CCL2 | CAAGCAGAAGTGGGTTCAGGAT | AAGTCTTCGGAGTTTGGGTTTG |
| COX2 | TGAGCATCTACGGTTTGCTG | AACTGCTCATCACCCCATTC |
| EEF1a1 | GATGGCCCCAAATTCTTGAAG | GGACCATGTCAACAATTGCAG |
| IL-1β | CTGATGGCCCTAAACAGATGAAGT | GCCTGAAGCCCTTGCTGTAGT |
| IL-6 | ATGAGGAGACTTGCCTGGTGAA | ACTCTCAAATCTGTTCTGGAGGTACTC |
| IRAK1 | CCGGGCAATTCAGTTTCTAC | TCTCATCCAGAAGGACGTTG |
| TNF-α | TGTTGTAGCAAACCCTCAAGCTG | AGGACCTGGGAGTAGATGAGGTACA |
| TRAF6 | CTGCTTGATGGCATTACGAGAA | TGCAGGCTTTGCAGAACCTA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Sato, S.; Aoyama, Y.; Ho, H.-J.; Goto, T.; Komai, M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. Int. J. Mol. Sci. 2019, 20, 2320. https://doi.org/10.3390/ijms20092320
Giriwono PE, Shirakawa H, Ohsaki Y, Sato S, Aoyama Y, Ho H-J, Goto T, Komai M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. International Journal of Molecular Sciences. 2019; 20(9):2320. https://doi.org/10.3390/ijms20092320
Chicago/Turabian StyleGiriwono, Puspo E., Hitoshi Shirakawa, Yusuke Ohsaki, Shoko Sato, Yukihide Aoyama, Hsin-Jung Ho, Tomoko Goto, and Michio Komai. 2019. "Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells" International Journal of Molecular Sciences 20, no. 9: 2320. https://doi.org/10.3390/ijms20092320
APA StyleGiriwono, P. E., Shirakawa, H., Ohsaki, Y., Sato, S., Aoyama, Y., Ho, H.-J., Goto, T., & Komai, M. (2019). Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. International Journal of Molecular Sciences, 20(9), 2320. https://doi.org/10.3390/ijms20092320






