Genome-Wide Characterization and Gene Expression Analyses of GATA Transcription Factors in Moso Bamboo (Phyllostachys edulis)
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Characterization of GATA Factors in Moso Bamboo
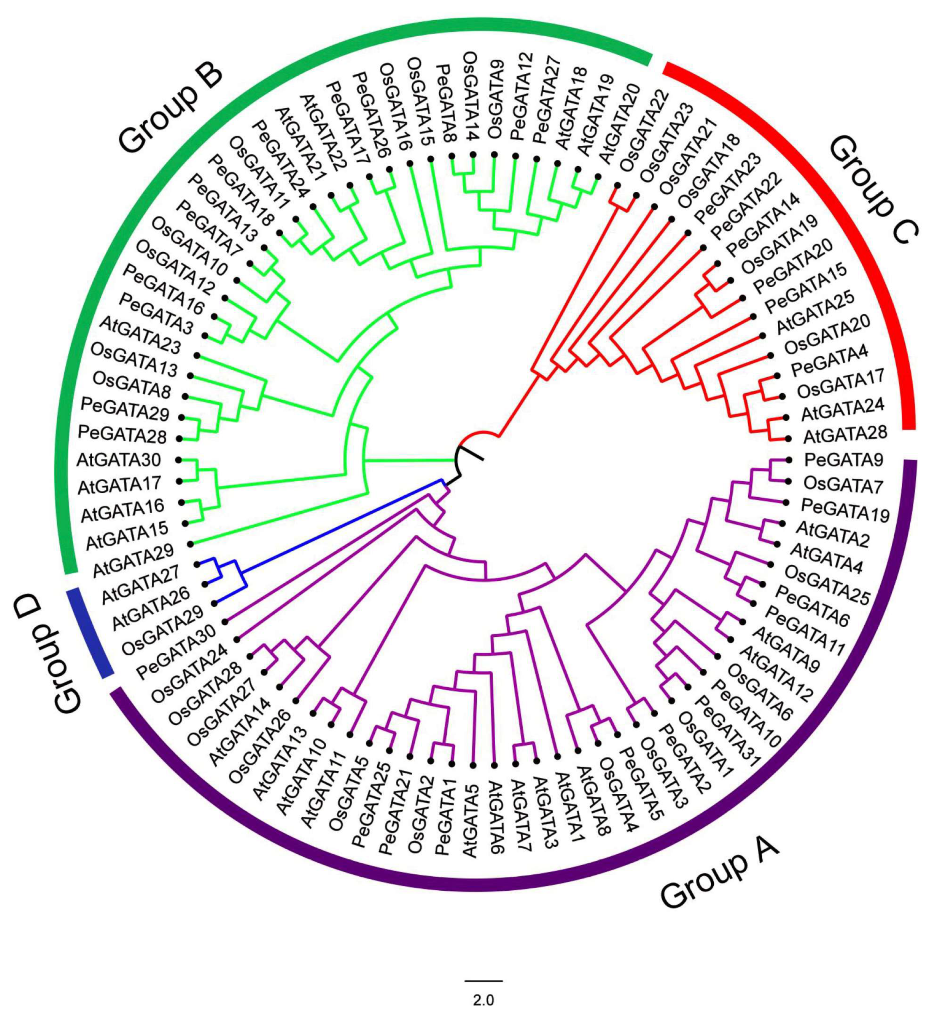
2.2. Phylogenetic Analysis of Bamboo GATA Factors
2.3. The Conservation of GATA Motifs in Bamboo GATA Factors
2.4. Gene Structure of Bamboo GATA Genes
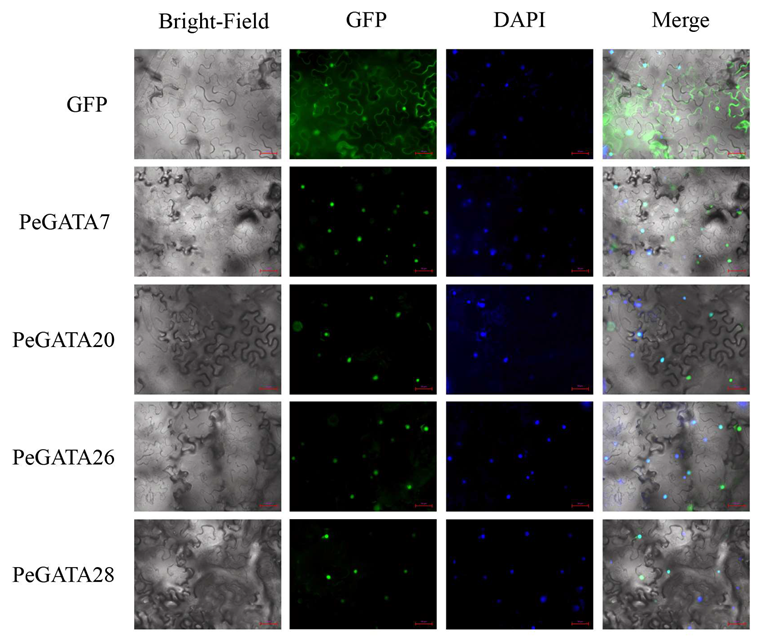
2.5. Subcellular Localization of Bamboo GATA Factors
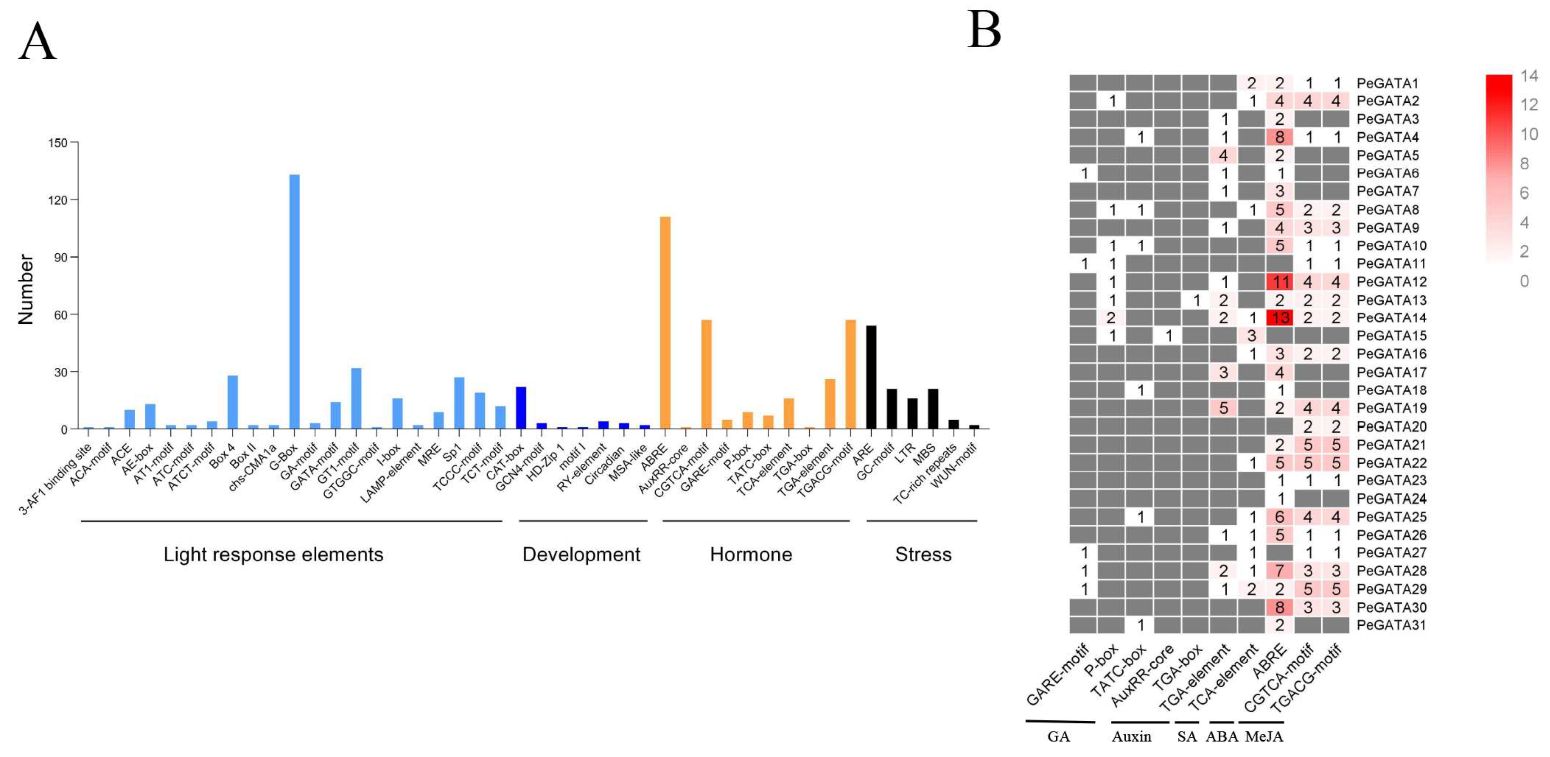
2.6. The Regulatory Cis-Elements in the Promoters of Bamboo GATA Genes
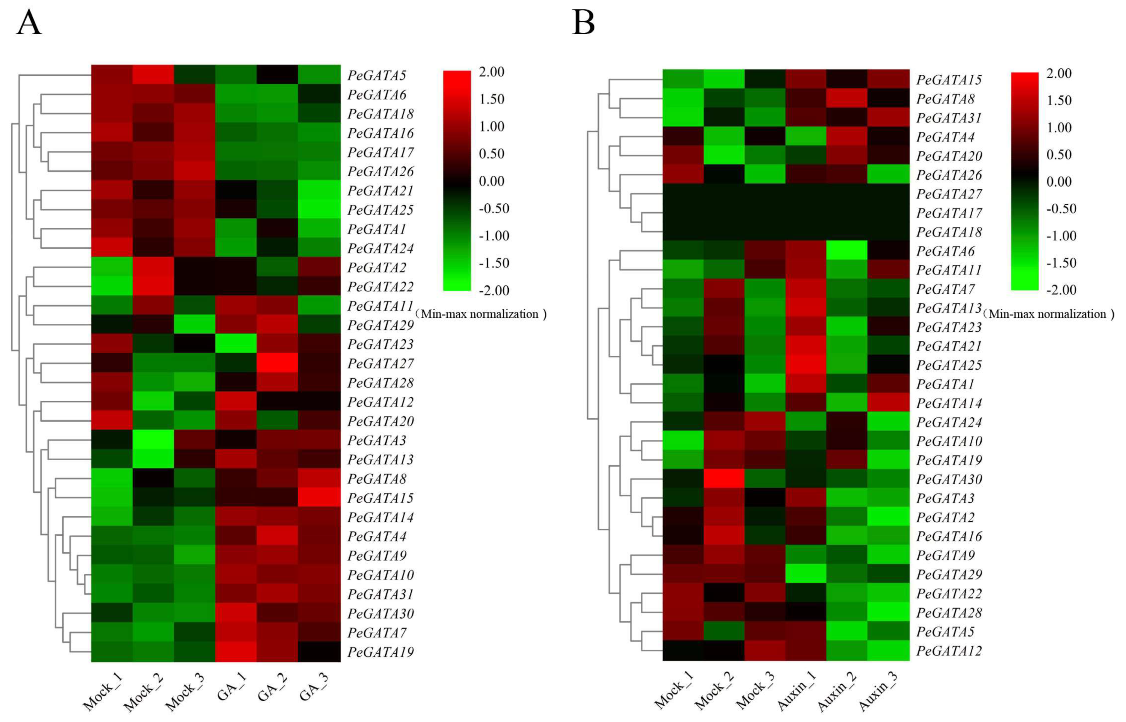
2.7. Gene Expression Analysis of Bamboo GATAs under Exogenous Hormone Treatment
2.8. Gene Expression Analysis of Bamboo GATA Genes during the Rapid-Growth of Bamboo Shoots
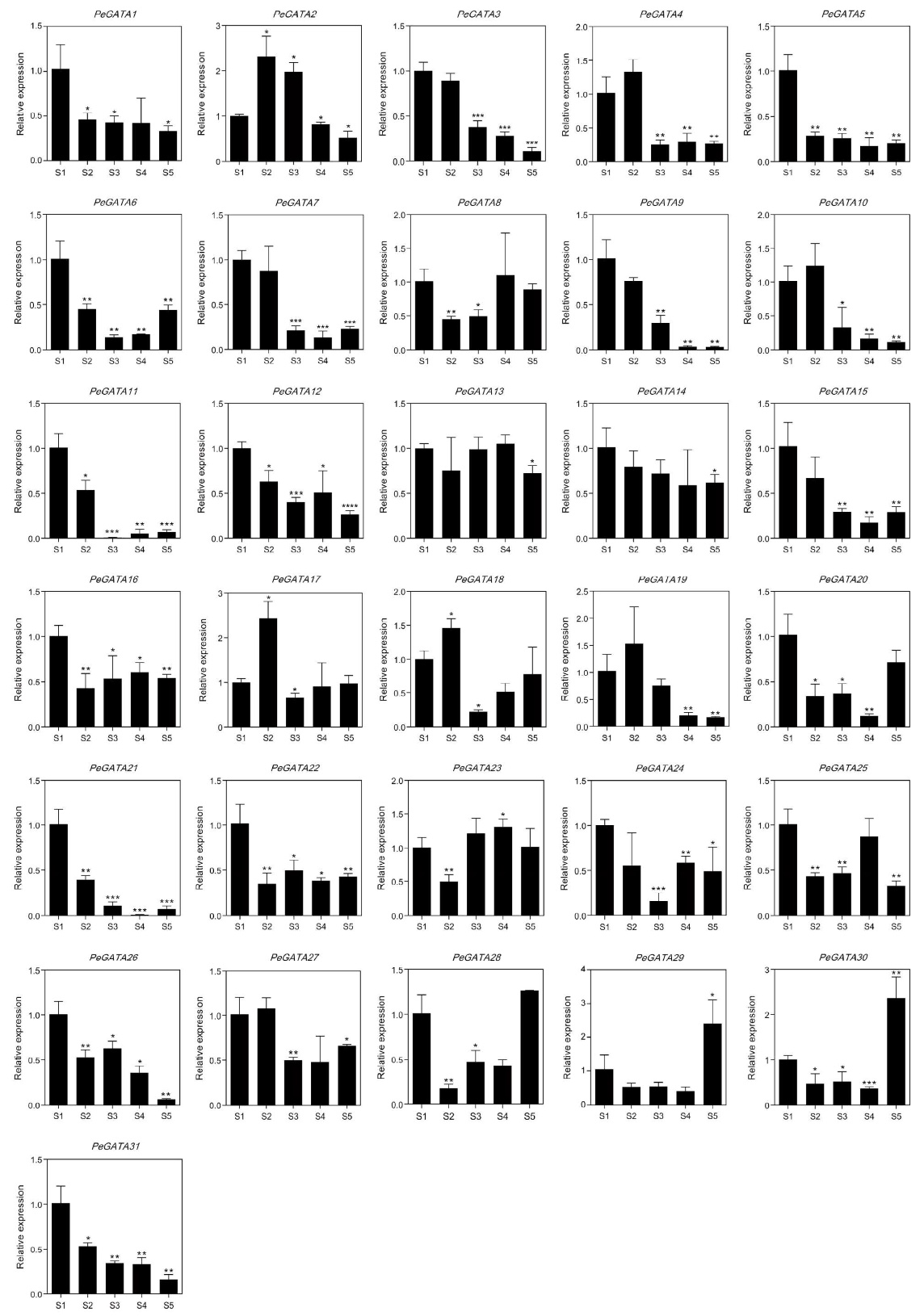
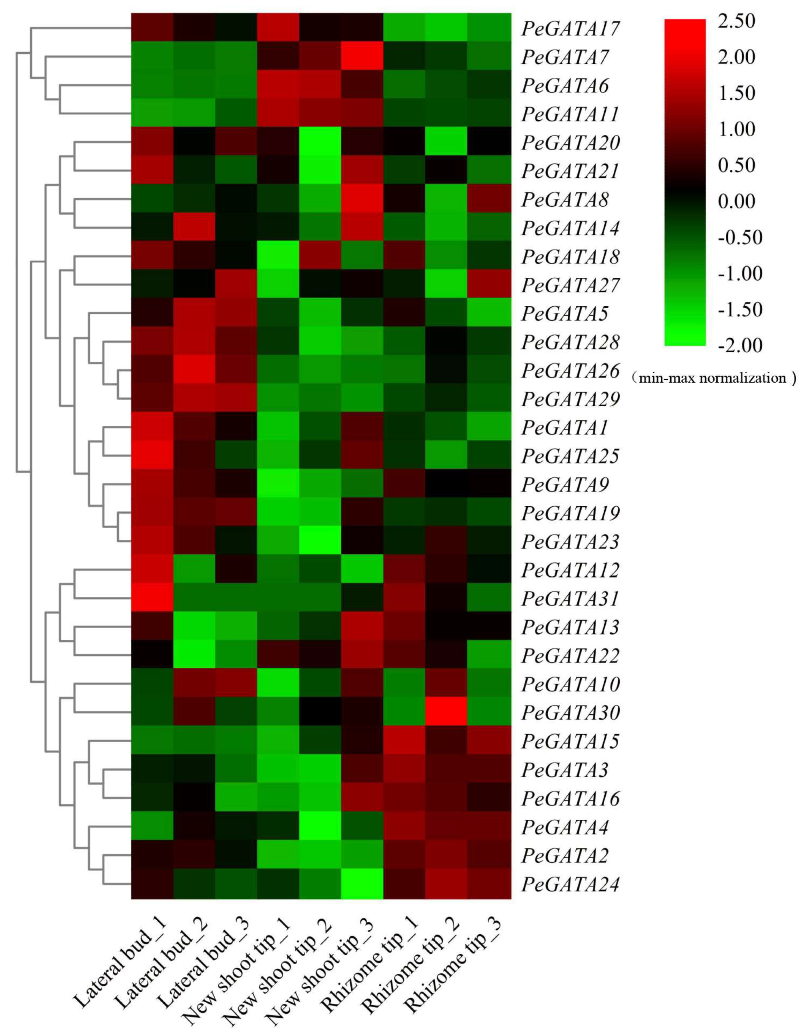
2.9. Gene Expression Analysis of Bamboo GATAs during Rhizome Development
2.10. PeGATA26 May Negatively Regulate Plant Height in Arabidopsis
3. Discussion
4. Material and Methods
4.1. Plant Materials and ABA Treatment
4.2. Identification of GATA Factors in Moso Bamboo
4.3. Phylogenetic Tree, Conserved Domain, Motif Recognition and Cis-Elements Analyses
4.4. Subcellular Localization Analysis
4.5. Gene Expression Analysis
4.6. Ectopic Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Li, Q.; Lou, S.; Yang, Y.; Peng, L.; Lin, Z.; Hu, Q.; Ma, L. GSK3/shaggy-like kinase 1 ubiquitously regulates cell growth from Arabidopsis to Moso bamboo (Phyllostachys edulis). Plant Sci. 2019, 283, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, H.; Cai, D.; Gao, Y.; Zhang, H.; Wang, Y.; Lin, C.; Ma, L.; Gu, L. Comprehensive profiling of rhizome-associated alternative splicing and alternative polyadenylation in moso bamboo (Phyllostachys edulis). Plant J. 2017, 91, 684–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, J.A.; Atchley, W.R. Molecular evolution of the GATA family of transcription factors: Conservation within the DNA-binding domain. J. Mol. Evol. 2000, 50, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, C. The fungal GATA factors. Curr. Opin. Microbiol. 2000, 3, 126–131. [Google Scholar] [CrossRef]
- Katsumura, K.R.; Bresnick, E.H.; Group, G.F.M. The GATA factor revolution in hematology. Blood 2017, 129, 2092–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patient, R.K.; McGhee, J.D. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 2002, 12, 416–422. [Google Scholar] [CrossRef]
- Crispino, J.D.; Horwitz, M.S. GATA factor mutations in hematologic disease. Blood 2017, 129, 2103–2110. [Google Scholar] [CrossRef]
- Teakle, G.R.; Gilmartin, P.M. Two forms of type IV zinc-finger motif and their kingdom-specific distribution between the flora, fauna and fungi. Trends Biochem. Sci. 1998, 23, 100–102. [Google Scholar] [CrossRef]
- Reyes, J.C.; Muro-Pastor, M.I.; Florencio, F.J. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004, 134, 1718–1732. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hou, Y.; Hao, Q.; Chen, H.; Chen, L.; Yuan, S.; Shan, Z.; Zhang, X.; Yang, Z.; Qiu, D.; et al. Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS ONE 2015, 10, e0125174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ren, C.; Zou, L.; Wang, Y.; Li, S.; Liang, Z. Characterization of the GATA gene family in Vitis vinifera: Genome-wide analysis, expression profiles, and involvement in light and phytohormone response. Genome 2018, 61, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, M.; Sanchez-Ferras, O.; Bouchard, M. GATA transcription factors in development and disease. Development 2018, 145, dev164384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzaghi, W.B.; Cashmore, A.R. Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 445–474. [Google Scholar] [CrossRef]
- Daniel-Vedele, F.; Caboche, M. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. Mol. Gen. Genet. 1993, 240, 365–373. [Google Scholar] [PubMed]
- Chen, H.; Shao, H.; Li, K.; Zhang, D.; Fan, S.; Li, Y.; Han, M. Genome-wide identification, evolution, and expression analysis of GATA transcription factors in apple (Malus×domestica Borkh.). Gene 2017, 627, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.; Guevara, D.R.; Hand, A.J.; Xu, Z.; Hao, L.; Chen, X.; Zhu, T.; Bi, Y.M.; Rothstein, S.J. Rice cytokinin GATA transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiol. 2013, 162, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.; Bastakis, E.; Schwechheimer, C. Cross-repressive interactions between SOC1 and the GATAs GNC and GNL/CGA1 in the control of greening, cold tolerance, and flowering time in Arabidopsis. Plant Physiol. 2013, 162, 1992–2004. [Google Scholar] [CrossRef] [Green Version]
- Shikata, M.; Matsuda, Y.; Ando, K.; Nishii, A.; Takemura, M.; Yokota, A.; Kohchi, T. Characterization of Arabidopsis ZIM, a member of a novel plant-specific GATA factor gene family. J. Exp. Bot. 2004, 55, 631–639. [Google Scholar] [CrossRef]
- Liu, P.-P.; Koizuka, N.; Martin, R.C.; Nonogaki, H. The BME3 (Blue Micropylar End 3) GATA zinc finger transcription factor is a positive regulator of Arabidopsis seed germination. Plant J. 2005, 44, 960–971. [Google Scholar] [CrossRef]
- Fu, Y.H.; Marzluf, G.A. nit-2, the major nitrogen regulatory gene of Neurospora crassa, encodes a protein with a putative zinc finger DNA-binding domain. Mol. Cell Biol. 1990, 10, 1056–1065. [Google Scholar] [CrossRef]
- Richter, R.; Behringer, C.; Müller, I.K.; Schwechheimer, C. The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes. Dev. 2010, 24, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.-M.; Lin, W.H.; Zhu, S.; Zhu, J.Y.; Sun, Y.; Fan, X.Y.; Cheng, M.; Hao, Y.; Oh, E.; Tian, M.; et al. Integration of light-and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev. Cell 2010, 19, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, R.; Behringer, C.; Zourelidou, M.; Schwechheimer, C. Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2013, 110, 13192–13197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzby, J.S.; Yamada, T.; Tobin, E.M. A light-regulated DNA-binding activity interacts with a conserved region of a Lemna gibba rbcS promoter. Plant Cell 1990, 2, 805–814. [Google Scholar]
- Carre, I.A.; Kay, S.A. Multiple DNA-Protein Complexes at a Circadian-Regulated Promoter Element. Plant Cell 1995, 7, 2039–2051. [Google Scholar] [CrossRef] [Green Version]
- Naito, T.; Kiba, T.; Koizumi, N.; Yamashino, T.; Mizuno, T. Characterization of a unique GATA family gene that responds to both light and cytokinin in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2007, 71, 1557–1560. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Cheng, Z.; Ma, Y.; Bai, Q.; Li, X.; Cao, Z.; Wu, Z.; Gao, J. The association of hormone signalling genes, transcription and changes in shoot anatomy during moso bamboo growth. Plant Biotechnol. J. 2018, 16, 72–85. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, Q.; Gao, Y.; Wang, H.; Zhao, L.; Wang, Y.; Xi, F.; Wang, W.; Yang, Y.; et al. Transcriptome characterization of moso bamboo (Phyllostachys edulis) seedlings in response to exogenous gibberellin applications. BMC Plant Biol. 2018, 18, 125. [Google Scholar] [CrossRef]
- Zhao, H.; Lou, Y.; Sun, H.; Li, L.; Wang, L.; Dong, L.; Gao, Z. Transcriptome and comparative gene expression analysis of Phyllostachys edulis in response to high light. BMC Plant Biol. 2016, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-L.; Wu, M.; Li, F.; Gao, Y.-M.; Chen, F.; Xiang, Y. TCP Transcription Factors in Moso Bamboo (Phyllostachys edulis): Genome-Wide Identification and Expression Analysis. Front. Plant Sci. 2018, 9, 1263. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Xiong, R.; Yan, H.; Gao, Y.; Liu, H.; Wu, M.; Xiang, Y. The trihelix family of transcription factors: Functional and evolutionary analysis in Moso bamboo (Phyllostachys edulis). BMC Plant Biol. 2019, 19, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, D.; Cheng, Z.; Xie, L.; Li, X.; Li, J.; Mu, S.; Gao, J. The R2R3MYB Gene Family in Phyllostachys edulis: Genome-Wide Analysis and Identification of Stress or Development-Related R2R3MYBs. Front. Plant Sci. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, K.; Obayashi, T.; Masuda, T. Role of the G-box element in regulation of chlorophyll biosynthesis in Arabidopsis roots. Plant Signal Behav. 2012, 7, 922–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Gu, L.; Ye, S.; Zhang, H.; Cai, C.; Xiang, M.; Gao, Y.; Wang, Q.; Lin, C.; Zhu, Q. Genome-wide analysis and transcriptomic profiling of the auxin biosynthesis, transport and signaling family genes in moso bamboo (Phyllostachys heterocycla). BMC Genomics 2017, 18, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Peng, C.; Zhou, G.; Gu, H.; Li, Q.; Zhang, C. Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2016, 6, 25908. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; Li, Y.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2013, 45, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Wang, S.; Lou, Y.; Zhu, C.; Zhao, H.; Li, Y.; Li, X.; Gao, Z. Whole-Genome and Expression Analyses of Bamboo Aquaporin Genes Reveal Their Functions Involved in Maintaining Diurnal Water Balance in Bamboo Shoots. Cells 2018, 7, 195. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.J.; Shih, M.C. Interaction of a GATA factor with cis-acting elements involved in light regulation of nuclear genes encoding chloroplast glyceraldehyde-3-phosphate dehydrogenase in Arabidopsis. Biochem. Biophys. Res. Commun. 2003, 300, 555–562. [Google Scholar] [CrossRef]
- Jiang, K.; Yung, V.; Chiba, T.; Feldman, L.J. Longitudinal patterning in roots: A GATA2-auxin interaction underlies and maintains the root transition domain. Planta 2018, 247, 831–843. [Google Scholar] [CrossRef]
- Kollmer, I.; Werner, T.; Schmulling, T. Ectopic expression of different cytokinin-regulated transcription factor genes of Arabidopsis thaliana alters plant growth and development. J. Plant Physiol. 2011, 168, 1320–1327. [Google Scholar] [CrossRef]
- Ravindran, P.; Verma, V.; Stamm, P.; Kumar, P.P. A Novel RGL2-DOF6 Complex Contributes to Primary Seed Dormancy in Arabidopsis thaliana by Regulating a GATA Transcription Factor. Mol. Plant 2017, 10, 1307–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.Y.; Cui, K.; Zhang, J.G.; Duan, A.G.; Zeng, Y.F. Next-generation sequencing-based mRNA and microRNA expression profiling analysis revealed pathways involved in the rapid growth of developing culms in Moso bamboo. BMC Plant Biol. 2013, 13, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, Y.; Zhang, H.; Wang, H.; Liu, X.; Xu, X.; Zhang, Z.; Kohnen, M.V.; Hu, K.; Wang, H.; et al. Genome-Wide Profiling of Circular RNAs in the Rapidly Growing Shoots of Moso Bamboo (Phyllostachys edulis). Plant Cell Physiol. 2019, 60, 1354–1373. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.-R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zahur, M.; Maqbool, A.; Irfan, M.; Barozai, M.Y.; Qaiser, U.; Rashid, B.; Husnain, T.; Riazuddin, S. Functional analysis of cotton small heat shock protein promoter region in response to abiotic stresses in tobacco using Agrobacterium-mediated transient assay. Mol. Biol. Rep. 2009, 36, 1915–1921. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.; Pimentel, H.; Salzberg, S.; Rinn, J.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Name | Gene ID | Location | ORF Length (bp) | Size (aa) | MW (kDa) | PI | Group |
|---|---|---|---|---|---|---|---|
| PeGATA1 | PH01000001G0820 | 603239–605821 (− strand) | 762 | 253 | 27.2 | 7.11 | A |
| PeGATA2 | PH01000036G1110 | 651571–655167 (+ strand) | 1212 | 403 | 42.4 | 5.2 | A |
| PeGATA3 | PH01000040G1560 | 1013911–1015300 (− strand) | 801 | 266 | 28.7 | 9.77 | B |
| PeGATA4 | PH01000114G0660 | 460195–464981 (+ strand) | 912 | 303 | 32.2 | 5.96 | C |
| PeGATA5 | PH01000157G0800 | 521887–523791 (− strand) | 654 | 217 | 23.2 | 6.15 | A |
| PeGATA6 | PH01000162G1360 | 945504–954318 (+ strand) | 1500 | 499 | 56.4 | 9.4 | A |
| PeGATA7 | PH01000232G0180 | 85809–87165 (− strand) | 420 | 139 | 15.6 | 9.23 | B |
| PeGATA8 | PH01000242G0460 | 296415–297844 (− strand) | 648 | 215 | 22.6 | 8.2 | B |
| PeGATA9 | PH01000263G0760 | 473691–475362 (− strand) | 1095 | 364 | 37.5 | 7.72 | A |
| PeGATA10 | PH01000284G0590 | 365850–367843 (− strand) | 1131 | 376 | 39.3 | 5.89 | A |
| PeGATA11 | PH01000417G1130 | 669097–674119 (− strand) | 948 | 315 | 35.8 | 8.87 | A |
| PeGATA12 | PH01000468G1050 | 681872–683301 (+ strand) | 663 | 220 | 22.7 | 5.78 | A |
| PeGATA13 | PH01000604G0620 | 351426–352613 (+ strand) | 399 | 132 | 14.6 | 9.36 | A |
| PeGATA14 | PH01000750G0690 | 435897–442169 (+ strand) | 777 | 258 | 28.1 | 8.18 | C |
| PeGATA15 | PH01000836G0660 | 444348–452795 (− strand) | 1413 | 470 | 51.6 | 8.57 | C |
| PeGATA16 | PH01000985G0260 | 141878–143100 (+ strand) | 402 | 133 | 14.8 | 9.87 | B |
| PeGATA17 | PH01001002G0190 | 175975–177694 (+ strand) | 831 | 276 | 29.4 | 8.98 | B |
| PeGATA18 | PH01001129G0380 | 296297–297943 (+ strand) | 699 | 232 | 26 | 9.16 | B |
| PeGATA19 | PH01001155G0480 | 343290–344746 (− strand) | 741 | 246 | 25.3 | 9.66 | A |
| PeGATA20 | PH01001253G0390 | 263024–267886 (− strand) | 516 | 171 | 18.9 | 9.95 | C |
| PeGATA21 | PH01001451G0450 | 270274–272851 (− strand) | 930 | 309 | 32.6 | 8.68 | A |
| PeGATA22 | PH01001557G0370 | 244289–248001 (+ strand) | 594 | 197 | 20.7 | 9.14 | C |
| PeGATA23 | PH01001584G0350 | 297120–303918 (− strand) | 1035 | 344 | 37.8 | 4.75 | C |
| PeGATA24 | PH01001907G0160 | 109783–111580 (+ strand) | 1017 | 338 | 36.2 | 9.26 | B |
| PeGATA25 | PH01002105G0190 | 151795–153666 (+ strand) | 1248 | 415 | 44 | 8.6 | A |
| PeGATA26 | PH01002473G0050 | 19960–21655 (− strand) | 1011 | 336 | 36.1 | 9.64 | B |
| PeGATA27 | PH01002681G0110 | 59436–60698 (− strand) | 690 | 229 | 24 | 8.49 | B |
| PeGATA28 | PH01002830G0260 | 174984–177126 (− strand) | 381 | 126 | 13.8 | 9.69 | B |
| PeGATA29 | PH01003365G0100 | 53196–55342 (+ strand) | 369 | 122 | 13.3 | 9.4 | B |
| PeGATA30 | PH01003433G0110 | 88789–92096 (− strand) | 1167 | 388 | 39.8 | 9.33 | A |
| PeGATA31 | PH01004789G0060 | 58264–61570 (− strand) | 993 | 330 | 35 | 6.06 | A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Yang, Y.; Lou, S.; Wei, W.; Zhao, Z.; Ren, Y.; Lin, C.; Ma, L. Genome-Wide Characterization and Gene Expression Analyses of GATA Transcription Factors in Moso Bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2020, 21, 14. https://doi.org/10.3390/ijms21010014
Wang T, Yang Y, Lou S, Wei W, Zhao Z, Ren Y, Lin C, Ma L. Genome-Wide Characterization and Gene Expression Analyses of GATA Transcription Factors in Moso Bamboo (Phyllostachys edulis). International Journal of Molecular Sciences. 2020; 21(1):14. https://doi.org/10.3390/ijms21010014
Chicago/Turabian StyleWang, Taotao, Yong Yang, Shuaitong Lou, Wei Wei, Zhixin Zhao, Yujun Ren, Chentao Lin, and Liuyin Ma. 2020. "Genome-Wide Characterization and Gene Expression Analyses of GATA Transcription Factors in Moso Bamboo (Phyllostachys edulis)" International Journal of Molecular Sciences 21, no. 1: 14. https://doi.org/10.3390/ijms21010014





