The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations
Abstract
1. Introduction
2. Results
2.1. Effects of NA on Intracellular NAD+ in Hs68 Cells and C. elegans
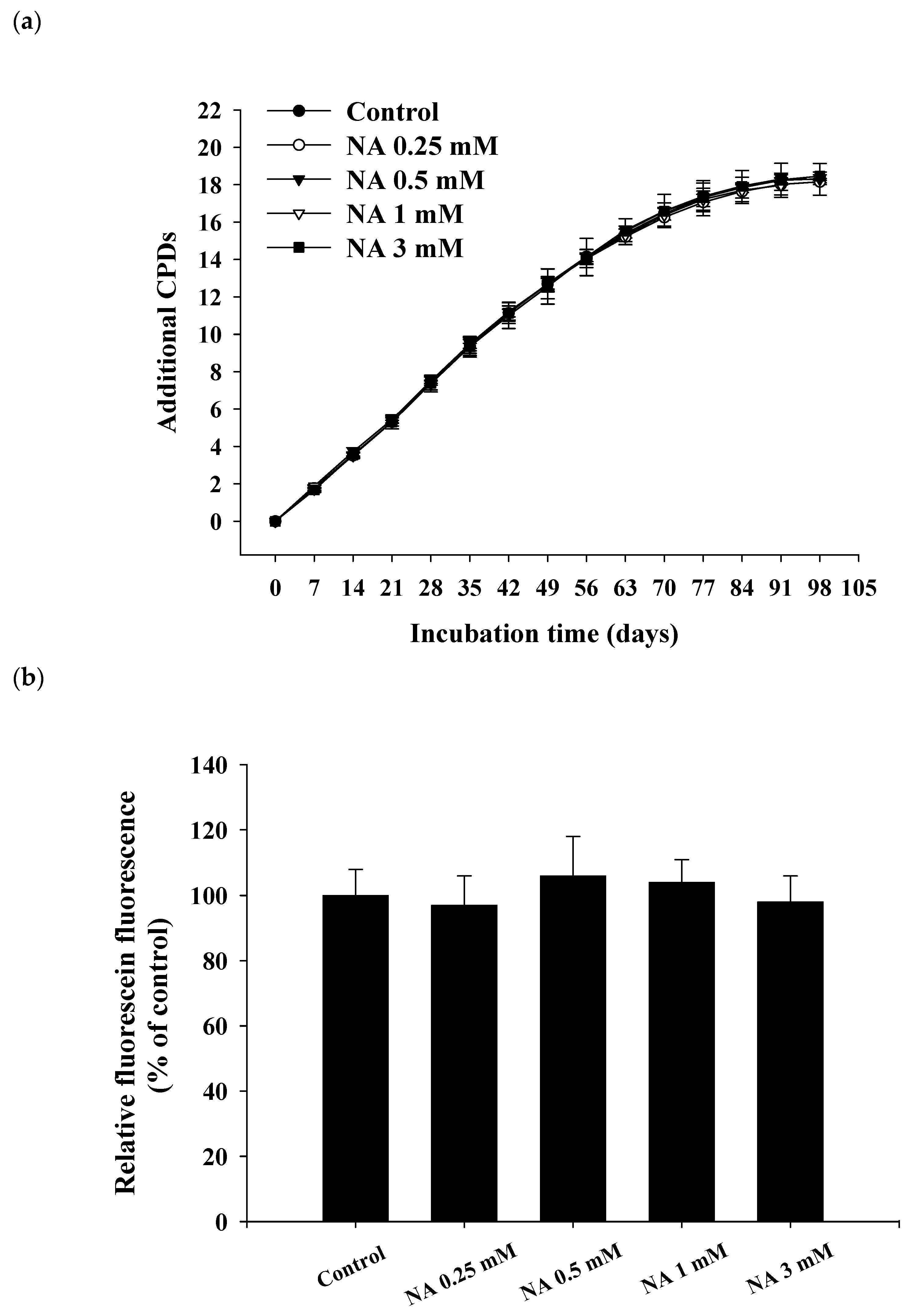
2.2. Effects of NA on the Replicative Lifespan and Senescence-Associated β-Galactosidase (SA-βG) Activity of Hs68 Cells
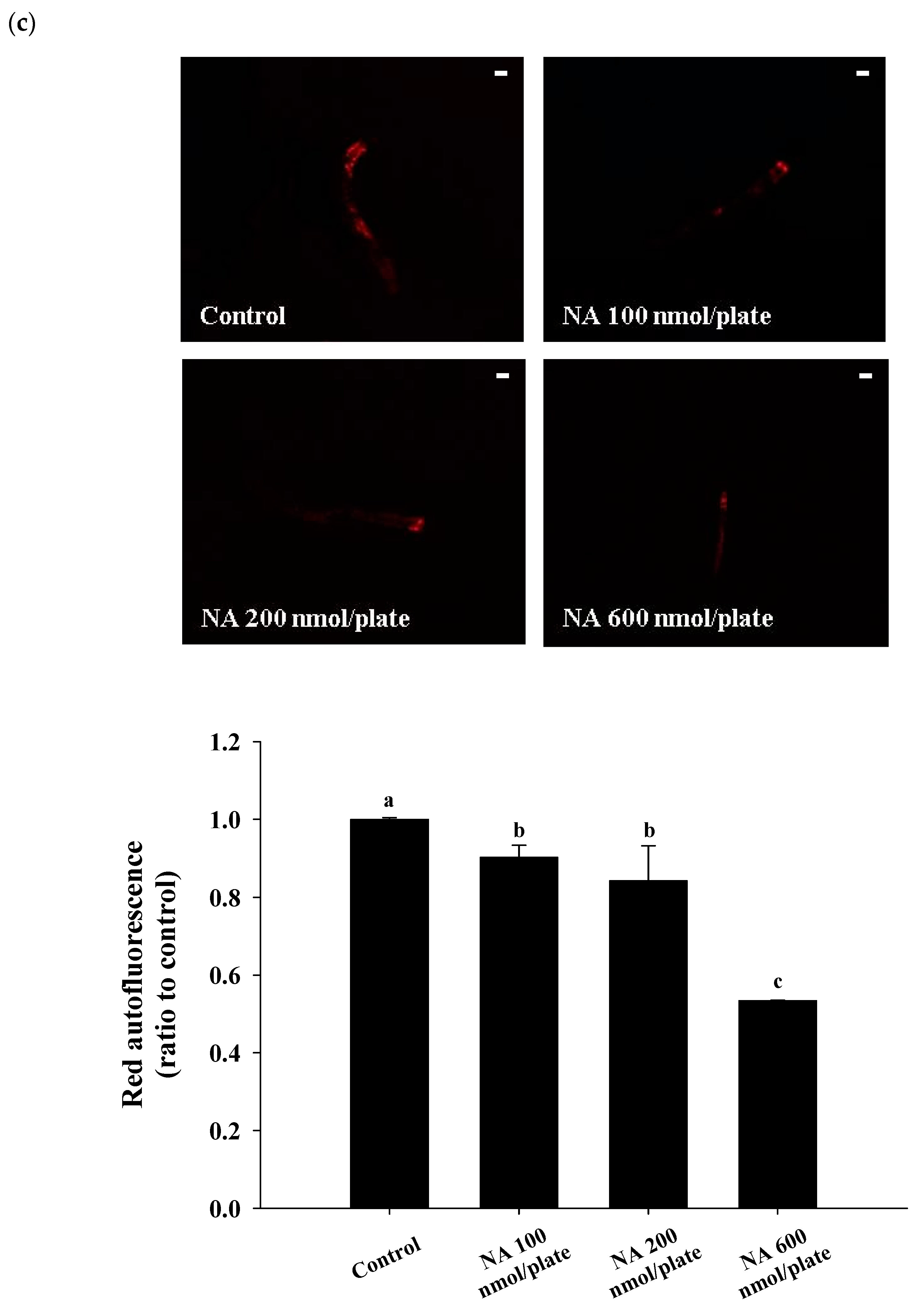
2.3. Effects of NA on the Lifespan and Physiological Indexes of C. elegans
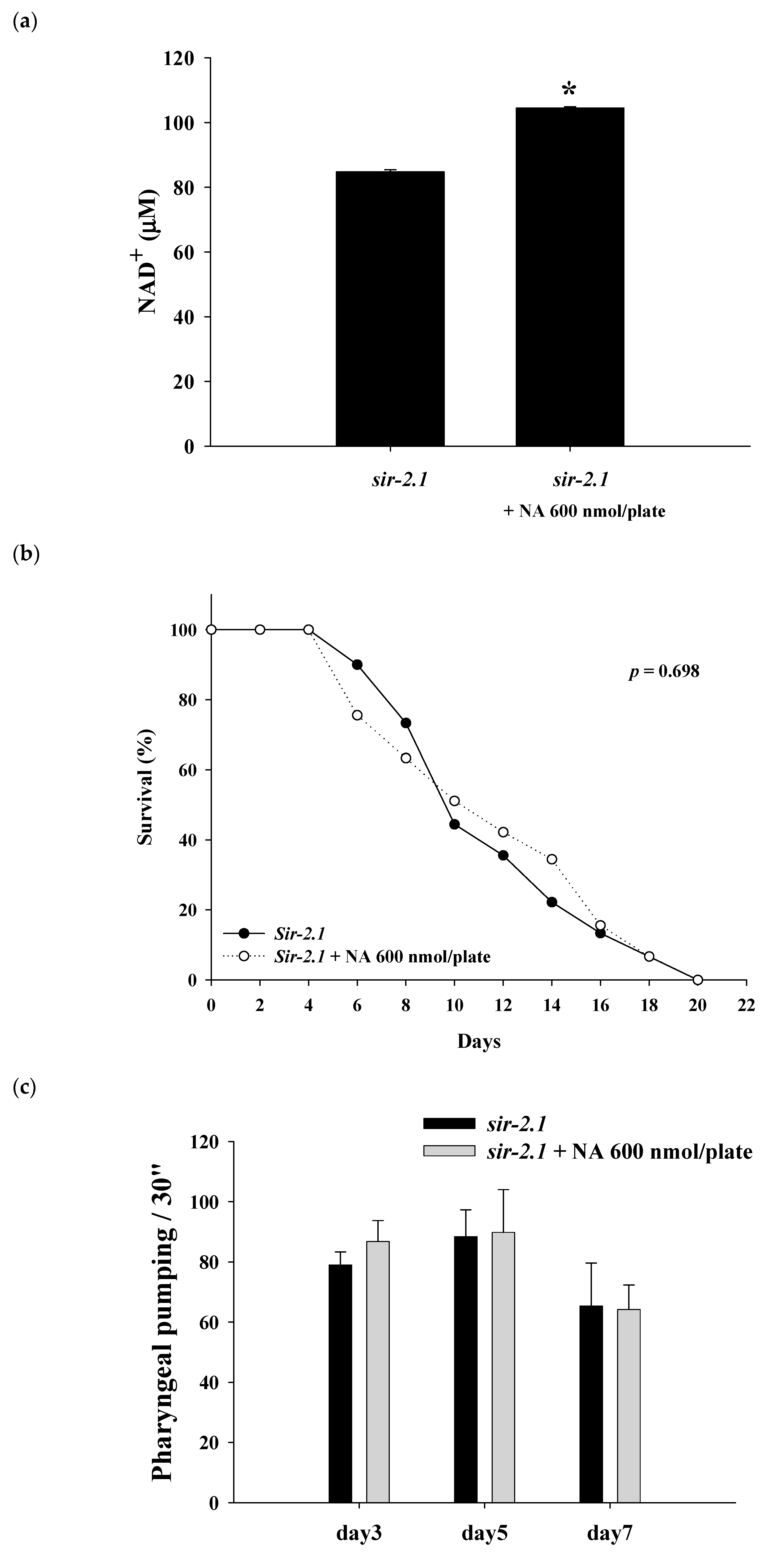
2.4. Effects of NA on the NAD+ Level, Lifespan, and Physiological Indexes of the sir-2.1 Mutant of C. elegans
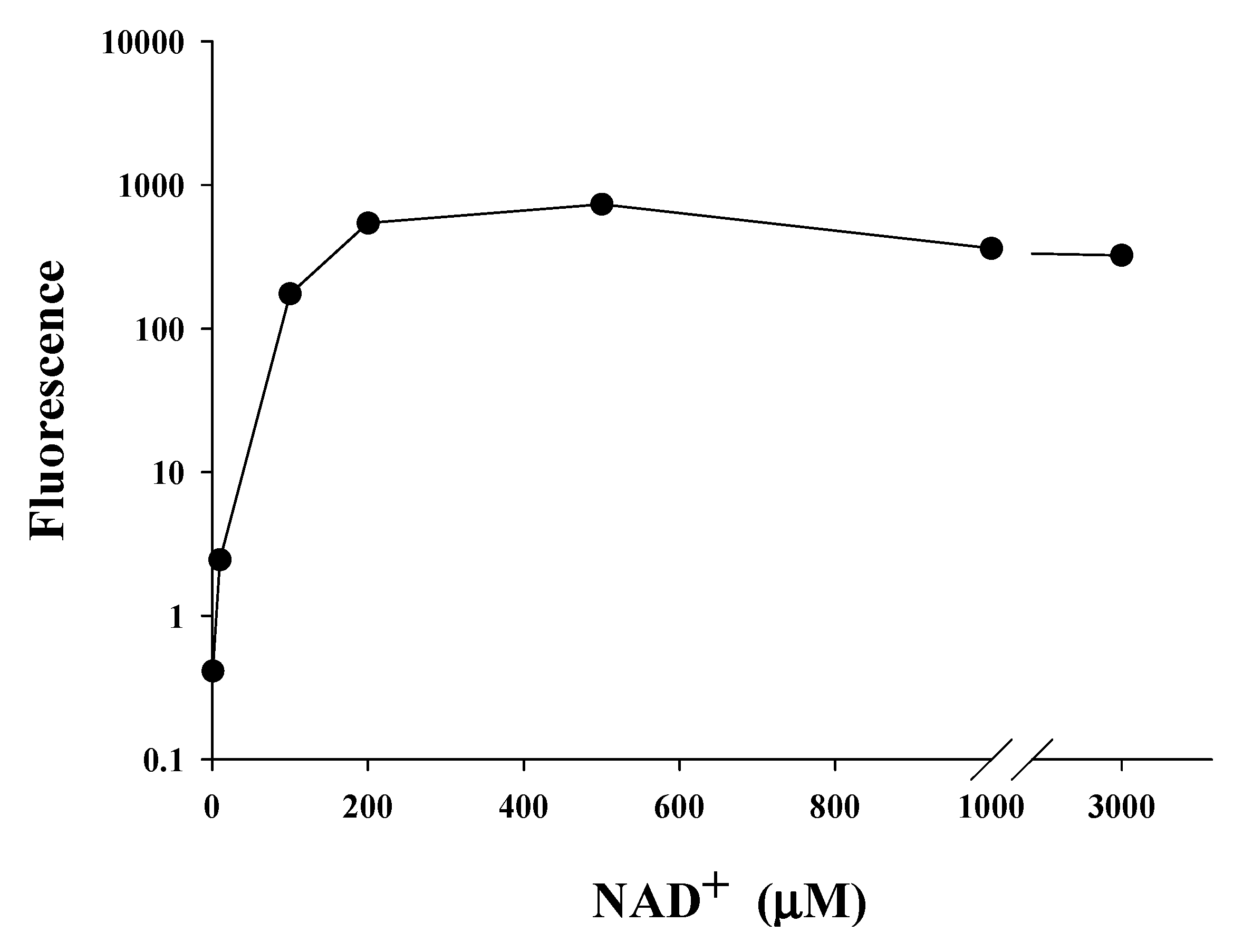
2.5. Saturating Concentration of NAD+ as a Cosubstrate for SIRT1
2.6. Effects of NA on the NAD+ Level, Lifespan, and Physiological Indexes of pme-1 Mutants of C. elegans
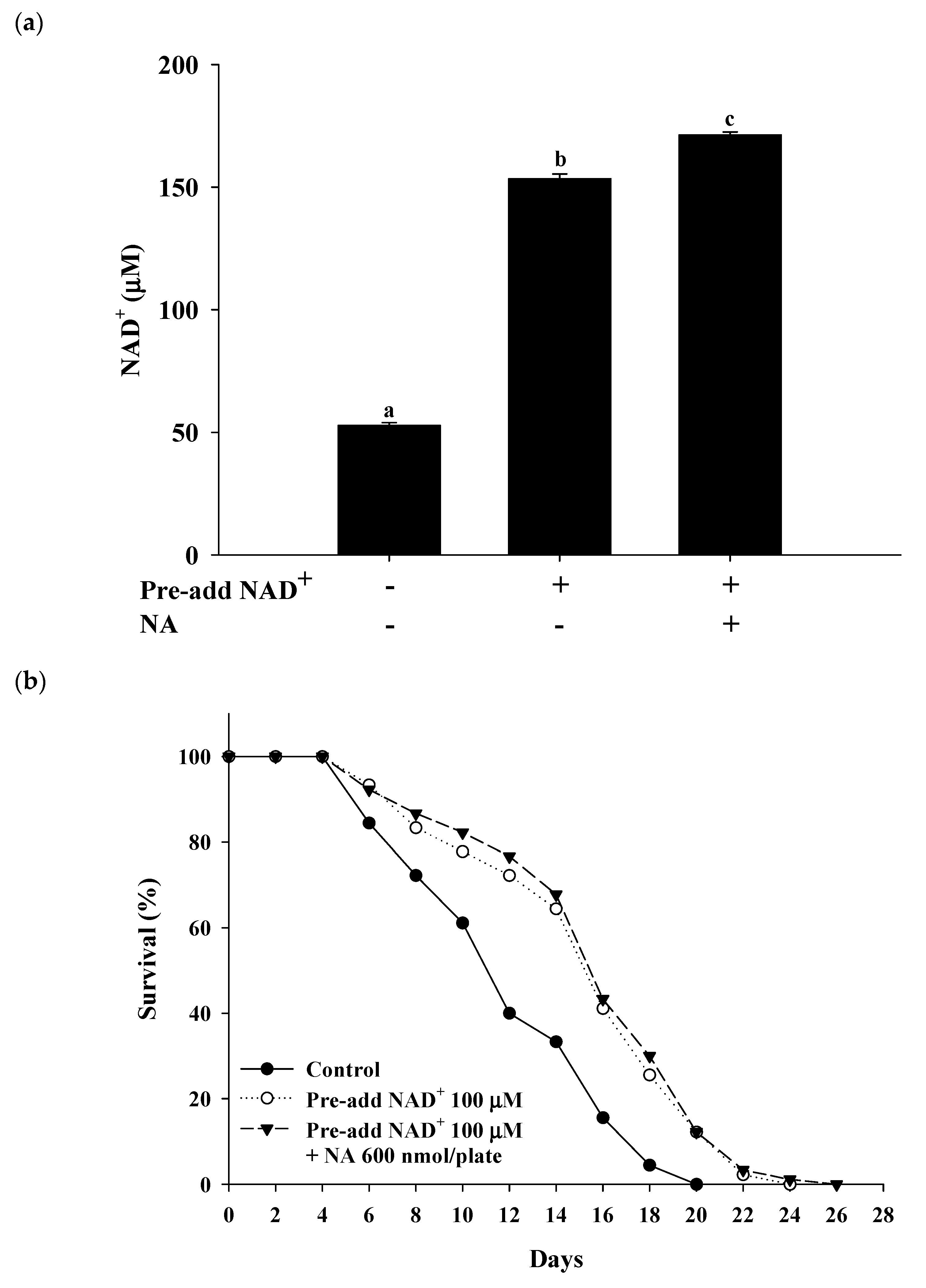
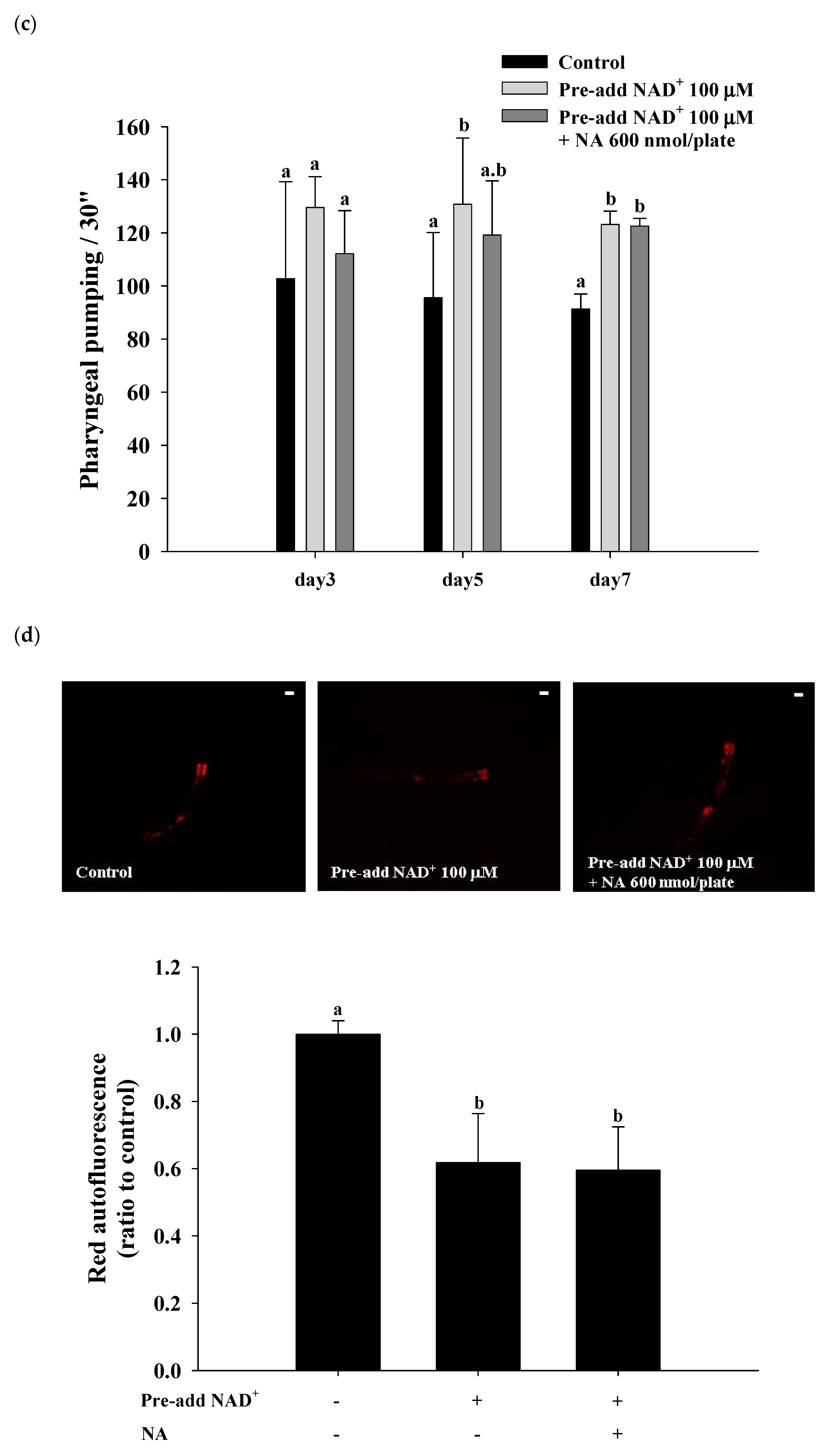
2.7. Effects of NA on the NAD+ Level, Lifespan, and Physiological Indexes in Normal C. elegans with an Increased Intracellular NAD+ Level
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Determination of the Intracellular NAD+ Level in Hs68 Cells
4.4. Assay of Replicative Lifespan of Hs68 Cells
4.5. Determination of the Senescence-Associated β-Galactosidase (SA-βG) Activity
4.6. Handling Procedures for C. elegans
4.7. Determination of the Intracellular NAD+ Level in C. elegans
4.8. Assay of the Lifespan of C. elegans
4.9. Assay of the Pharyngeal Pumping in C. elegans
4.10. Determination of the Autofluorescence in C. elegans
4.11. Assay of Body Bends of C. elegans
4.12. Determination of the SIRT1-Saturating Concentration of NAD+ In Vitro
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ingram, D.K.; Zhu, M.; Mamczarz, J.; Zou, S.; Lane, M.A.; Roth, G.S.; deCabo, R. Calorie restriction mimetics: An emerging research field. Aging Cell 2006, 5, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.C.; Song, T.Y.; Chang, Y.Z.; Chen, M.Y.; Hu, M.L. Up-regulation of nicotinamide phosphoribosyltransferase and increase of NAD+ levels by glucose restriction extend replicative lifespan of human fibroblast Hs68 cells. Biogerontology 2015, 16, 31–42. [Google Scholar] [CrossRef]
- Gille, A.; Bodor, E.T.; Ahmed, K.; Offermanns, S. Nicotinic acid: Pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 79–106. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.M.; Kim, L.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J. Nutr. Biochem. 2014, 25, 66–72. [Google Scholar] [CrossRef]
- Song, T.Y.; Yeh, S.L.; Hu, M.L.; Chen, M.Y.; Yang, N.C. A Nampt inhibitor FK866 mimics vitamin B3 deficiency by causing senescence of human fibroblastic Hs68 cells via attenuation of NAD+-SIRT1 signaling. Biogerontology 2015, 16, 789–800. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]
- Imai, S. A possibility of nutriceuticals as an anti-aging intervention: Activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol. Res. 2010, 6, 242–247. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Aon, M.A.; Cortassa, S.; Kim, E.Y.; Fang, E.F.; Palacios, H.H.; Ali, A.; Navas-Enamorado, I.; Di Francesco, A.; et al. Nicotinamide improves aspects of healthspan, but not lifespan, in mice. Cell Metab. 2018, 27, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Denu, J.M. Vitamin B3 and sirtuin function. Trends Biochem. Sci. 2005, 30, 479–483. [Google Scholar] [CrossRef] [PubMed]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly (ADP-ribosyl) ation reactions in the regulation of nuclear functions. Biochem. J. 1999, 342, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, K.; Mansfeld, J.; Kuhlow, D.; Weimer, S.; Priebe, S.; Heiland, I.; Birringer, M.; Groth, M.; Segref, A.; Kanfi, Y.; et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 2013, 9, 693–700. [Google Scholar] [CrossRef]
- Matuoka, K.; Chen, K.Y.; Takenawa, T. Rapid reversion of aging phenotypes by nicotinamide through possible modulation of histone acetylation. Cell Mol. Life Sci. 2001, 58, 2108–2116. [Google Scholar] [CrossRef]
- Yang, N.C.; Hu, M.L. The limitations and validities of senescence associated-beta-galactosidase activity as an aging marker for human foreskin fibroblast Hs68 cells. Exp. Gerontol. 2005, 40, 813–819. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, M.; Nomura, T.; Sakamoto, K. Nicotinamide adenine dinucleotide extends the lifespan of Caenorhabditis elegans mediated by sir-2.1 and daf-16. Biogerontology 2010, 11, 31–43. [Google Scholar] [CrossRef]
- Yu, J.; Haldar, M.; Mallik, S.; Srivastava, D.K. Role of the substrate specificity-defining residues of human SIRT5 in modulating the structural stability and inhibitory features of the enzyme. PLoS ONE 2016, 11, e0152467. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Dominy, J.E., Jr.; Blättler, S.M.; Jedrychowski, M.P.; Banks, A.S.; Lim, J.H.; Chim, H.; Gygi, S.P.; Puigserver, P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD+. Mol. Cell 2011, 44, 851–863. [Google Scholar] [CrossRef]
- Bakhtiari, N.; Mirzaie, S.; Hemmati, R.; Moslemee-Jalalvand, E.; Noori, A.R.; Kazemi, J. Mounting evidence validates Ursolic Acid directly activates SIRT1: A powerful STAC which mimic endogenous activator of SIRT1. Arch. Biochem. Biophys. 2018, 650, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rye, P.T.; Frick, L.E.; Ozbal, C.C.; Lamarr, W.A. Advances in label-free screening approaches for studying sirtuin-mediated deacetylation. J. Biomol. Screen 2011, 16, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Deota, S.; Chattopadhyay, T.; Ramachandran, D.; Armstrong, E.; Camacho, B.; Maniyadath, B.; Fulzele, A.; Gonzalez-de-Peredo, A.; Denu, J.M.; Kolthur-Seetharam, U. Identification of a tissue-restricted isoform of SIRT1 defines a regulatory domain that encodes specificity. Cell Rep. 2017, 18, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, Y.; Katayanagi, Y.; Tanaka, K.; Fukutomi, R.; Hiramoto, S.; Imai, S. Alkylresorcinols activate SIRT1 and delay ageing in Drosophila melanogaster. Sci. Rep. 2017, 7, 43679. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Yamada, K.; Shibata, T.; Osago, H.; Hashimoto, T.; Tsuchiya, M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J. Biol. Chem. 2007, 282, 24574–24582. [Google Scholar] [CrossRef]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Hong, J.; Kim, B.W.; Choo, H.J.; Park, J.J.; Yi, J.S.; Yu, D.M.; Lee, H.; Yoon, G.S.; Lee, J.S.; Ko, Y.G. Mitochondrial complex I deficiency enhances skeletal myogenesis but impairs insulin signaling through SIRT1 inactivation. J. Biol. Chem. 2014, 289, 20012–20025. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Cantó, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef]
- Schmidt, M.T.; Smith, B.C.; Jackson, M.D.; Denu, J.M. Coenzyme specificity of Sir2 protein deacetylases: Implications for physiological regulation. J. Biol. Chem. 2004, 279, 40122–40129. [Google Scholar] [CrossRef]
- Williams, G.T.; Lau, K.M.; Coote, J.M.; Johnstone, A.P. NAD metabolism and mitogen stimulation of human lymphocytes. Exp. Cell Res. 1985, 160, 419–426. [Google Scholar] [CrossRef]
- Zhu, X.H.; Lu, M.; Lee, B.Y.; Ugurbil, K.; Chen, W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Borra, M.T.; Langer, M.R.; Slama, J.T.; Denu, J.M. Substrate specificity and kinetic mechanism of the Sir2 family of NAD+-dependent histone/protein deacetylases. Biochemistry 2004, 43, 9877–9887. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D.; Shimazu, T.; Jing, E.; Grueter, C.A.; Collins, A.M.; Aouizerat, B.; Stančáková, A.; Goetzman, E.; Lam, M.M.; Schwer, B.; et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 2011, 44, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; German, N.J.; Saha, A.K.; de Boer, V.C.; Davies, M.; Koves, T.R.; Dephoure, N.; Fischer, F.; Boanca, G.; Vaitheesvaran, B.; et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 2013, 50, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Gertz, M.; Suenkel, B.; Lakshminarasimhan, M.; Schutkowski, M.; Steegborn, C. Sirt5 Deacylation Activities Show Differential Sensitivities to Nicotinamide Inhibition. PLoS ONE 2012, 7, e45098. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.W.; Feldman, J.L.; Devries, M.K.; Dong, A.; Edwards, A.M.; Denu, J.M. Structure and biochemical functions of SIRT6. J. Biol. Chem. 2011, 286, 14575–14587. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef]
- Yang, N.C.; Hu, M.L. A fluorimetric method using fluorescein di-beta-D-galactopyranoside for quantifying the senescence-associated beta-galactosidase activity in human foreskin fibroblast Hs68 cells. Anal. Biochem. 2004, 325, 337–343. [Google Scholar] [CrossRef]
- Sutphin, G.L.; Kaeberlein, M. Measuring Caenorhabditis elegans life span on solid media. JoVE J. Vis. Exp. 2009, 27, e1152. [Google Scholar] [CrossRef]
- Iwasa, H.; Yu, S.; Xue, J.; Driscoll, M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell 2010, 9, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gilliat, A.F.; Ziehm, M.; Turmaine, M.; Wang, H.; Ezcurra, M.; Yang, C.; Phillips, G.; McBay, D.; Zhang, W.B.; et al. Two forms of death in ageing Caenorhabditis elegans. Nat. Commun. 2017, 8, 15458. [Google Scholar] [CrossRef] [PubMed]
- Pincus, Z.; Mazer, T.C.; Slack, F.J. Autofluorescence as a measure of senescence in C. elegans: Look to red, not blue or green. Aging Albany N. Y. 2016, 8, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hwang, E.S. Fluorescence-based detection and quantification of features of cellular senescence. Methods Cell Biol. 2011, 103, 149–188. [Google Scholar]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.-C.; Cho, Y.-H.; Lee, I. The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations. Int. J. Mol. Sci. 2020, 21, 142. https://doi.org/10.3390/ijms21010142
Yang N-C, Cho Y-H, Lee I. The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations. International Journal of Molecular Sciences. 2020; 21(1):142. https://doi.org/10.3390/ijms21010142
Chicago/Turabian StyleYang, Nae-Cherng, Yu-Hung Cho, and Inn Lee. 2020. "The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations" International Journal of Molecular Sciences 21, no. 1: 142. https://doi.org/10.3390/ijms21010142
APA StyleYang, N.-C., Cho, Y.-H., & Lee, I. (2020). The Lifespan Extension Ability of Nicotinic Acid Depends on Whether the Intracellular NAD+ Level Is Lower than the Sirtuin-Saturating Concentrations. International Journal of Molecular Sciences, 21(1), 142. https://doi.org/10.3390/ijms21010142




