Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth of Fibroblasts on Collagen Gel in Medium Containing FBS-1
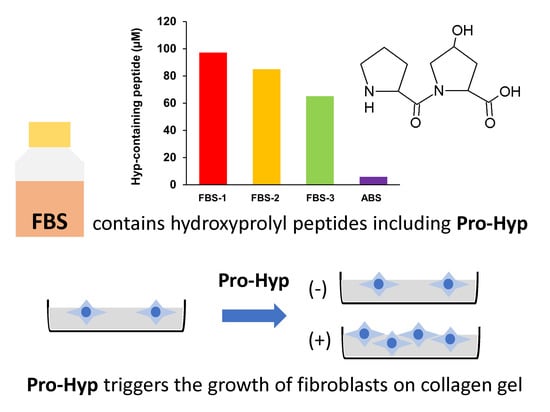
2.2. Presence of Hydroxyprolyl Peptides in FBS
2.3. Removal of Hydroxyprolyl Peptides from FBS-1
2.4. Effect of Hydroxyprolyl Peptides on Growth of Fibroblasts on Collagen Gel
2.5. Effect of Pro-Hyp on Number of Fibroblasts Migrated from Mouse Skin
3. Materials and Methods
3.1. Bovine Sera
3.2. Chemicals
3.3. Removal of Hydroxyprolyl Peptides from FBS-1
3.4. Animals
3.5. Estimation of the Number of Cells Migrated from Mouse Skin
3.6. Cell Proliferation Assay
3.7. Amino Acid Analysis
3.8. Determination of Pro-Hyp and Hyp-Gly
3.9. Statistical analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Pro-Hyp | prolyl-hydoxyproline |
| Hyp-Gly | hydroxyprolyl-glycine |
| Ala-Hyp | alanyl-hydroxyproline |
| Ile-Hyp | isoleucyl-hydroxyproline |
| Leu-Hyp | leucyl-hydroxyproline |
| Phe-Hyp | phenylalanyl-hydroxyproline |
| Glu-Hyp | glutamyl-hydroxyproline |
| Pro-Hyp-Gly | prolyl-hydroxyprolyl-glycine |
| Gly-Pro-Hyp | glycyl-prolyl-hydroxyproline |
| Ala-Hyp-Gly | alanyl-hydroxyprolyl-glycine |
| Ser-Hyp-Gly | serinyl-hydroxyprolyl-glycine |
| FBS | fetal bovine serum |
| LMW | low molecular weight |
| ABS | adult bovine serum |
| SEC | size-exclusion chromatography |
| HPLC | high performance liquid chromatography |
| EDTA | ethylenediaminetetraacetic acid |
| DMEM | Dulbecco’s Modified Eagle Medium |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| AccQ | 6-Aminoquinolyl-N-hydroxy succinimidyl carbamate |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| MRM | multiple reaction monitoring |
References
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol. 2014, 27, 113–119. [Google Scholar] [CrossRef]
- Proksch, E.; Segger, D.; Degwert, J.; Schunck, M.; Zague, V.; Oesser, S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol. Physiol. 2014, 27, 47–55. [Google Scholar] [CrossRef]
- Sugihara, F.; Inoue, N.; Wang, X. Clinical effects of ingesting collagen hydrolysate on facial skin properties: A randomized, placebo-controlled, double-blind trial. Jpn. Pharmacol. Ther. 2015, 43, 67–70. [Google Scholar]
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Benito-Ruiz, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-López, S.V.; Villacís-Tamayo, R.A.; Zurita-Gavilanes, L.A. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009, 60, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Lee, S.K.; Posthauer, M.E.; Dorner, B.; Redovian, V.; Maloney, M.J. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Adv. Skin Wound Care 2006, 19, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, H.; Okada, S.; Sanada, H. A multicenter, randomized, controlled study of the use of nutritional supplements containing collagen peptides to facilitate the healing of pressure ulcers. J. Nutr. Intermed. Metab. 2017, 8, 51–59. [Google Scholar] [CrossRef]
- Sugihara, F.; Inoue, N.; Venkateswarathirukumara, S. Ingestion of bioactive collagen hydrolysates enhanced pressure ulcer healing in a randomized double-blind placebo-controlled clinical study. Sci. Rep. 2018, 8, 11403. [Google Scholar] [CrossRef] [Green Version]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Shigemura, Y.; Akaba, S.; Kawashima, E.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate. Food Chem. 2011, 129, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Kubomura, D.; Sato, Y.; Sato, K. Dose-dependent changes in the levels of free and peptide forms of hydroxyproline in human plasma after collagen hydrolysate ingestion. Food Chem. 2014, 159, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Takahashi, A.; Ito, K.; Uetake, T.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Nakata, M.; Imanishi, Y.; Sato, K. Amount of collagen in the meat contained in Japanese daily dishes and the collagen peptide content in human blood after ingestion of cooked fish meat. J. Agric. Food Chem. 2019, 67, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Kusubata, M.; Koyama, Y.; Tometsuka, C.; Shigemura, Y.; Sato, K. Detection of endogenous and food-derived collagen dipeptide prolylhydroxyproline (Pro-Hyp) in allergic contact dermatitis-affected mouse ear. Biosci. Biotechnol. Biochem. 2015, 79, 1356–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimi, S.; Sato, K.; Kimura, M.; Suzumiya, J.; Hara, S.; De Francesco, F.; Ohjimi, H. G-CSF administration accelerates cutaneous wound healing accompanied with increased Pro-Hyp production in db/db mice. Clin. Res. Dermatol. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Tanii, T.; Furukawa, M.; Mizuno, N.; Kitajima, J.; Ishii, M.; Hamada, T.; Yoshizato, K. Cell cycle analysis of human dermal fibroblasts cultured on or in hydrated type I collagen lattices. Arch. Dermatol. Res. 1990, 282, 258–262. [Google Scholar] [CrossRef]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef]
- Price, P.J.; Gregory, E.A. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro 1982, 18, 576–584. [Google Scholar] [CrossRef]
- Bidlingmeyer, B.A.; Cohen, S.A.; Tarvin, T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 1984, 336, 93–104. [Google Scholar] [CrossRef]
- Kouguchi, T.; Ohmori, T.; Shimizu, M.; Takahata, Y.; Maeyama, Y.; Suzuki, T.; Morimatsu, F.; Tanabe, S. Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem. 2013, 77, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kouguchi, T.; Shimizu, K.; Sato, M.; Takahata, Y.; Morimatsu, F. Chicken collagen hydrolysate reduces proinflammatory cytokine production in C57BL/6.KOR-ApoEshl mice. J. Nutr. Sci. Vitaminol. 2010, 56, 208–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iba, Y.; Yokoi, K.; Eitoku, I.; Goto, M.; Koizumi, S.; Sugihara, F.; Oyama, H.; Yoshimoto, T. Oral administration of collagen hydrolysates improves glucose tolerance in normal mice through GLP-1-dependent and GLP-1-independent mechanisms. J. Med. Food 2016, 19, 836–843. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asai, T.T.; Oikawa, F.; Yoshikawa, K.; Inoue, N.; Sato, K. Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide. Int. J. Mol. Sci. 2020, 21, 229. https://doi.org/10.3390/ijms21010229
Asai TT, Oikawa F, Yoshikawa K, Inoue N, Sato K. Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide. International Journal of Molecular Sciences. 2020; 21(1):229. https://doi.org/10.3390/ijms21010229
Chicago/Turabian StyleAsai, Tomoko T., Fumi Oikawa, Kazunobu Yoshikawa, Naoki Inoue, and Kenji Sato. 2020. "Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide" International Journal of Molecular Sciences 21, no. 1: 229. https://doi.org/10.3390/ijms21010229
APA StyleAsai, T. T., Oikawa, F., Yoshikawa, K., Inoue, N., & Sato, K. (2020). Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide. International Journal of Molecular Sciences, 21(1), 229. https://doi.org/10.3390/ijms21010229