Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls
Abstract
:1. Introduction
2. Results
2.1. Foxp3 Findings
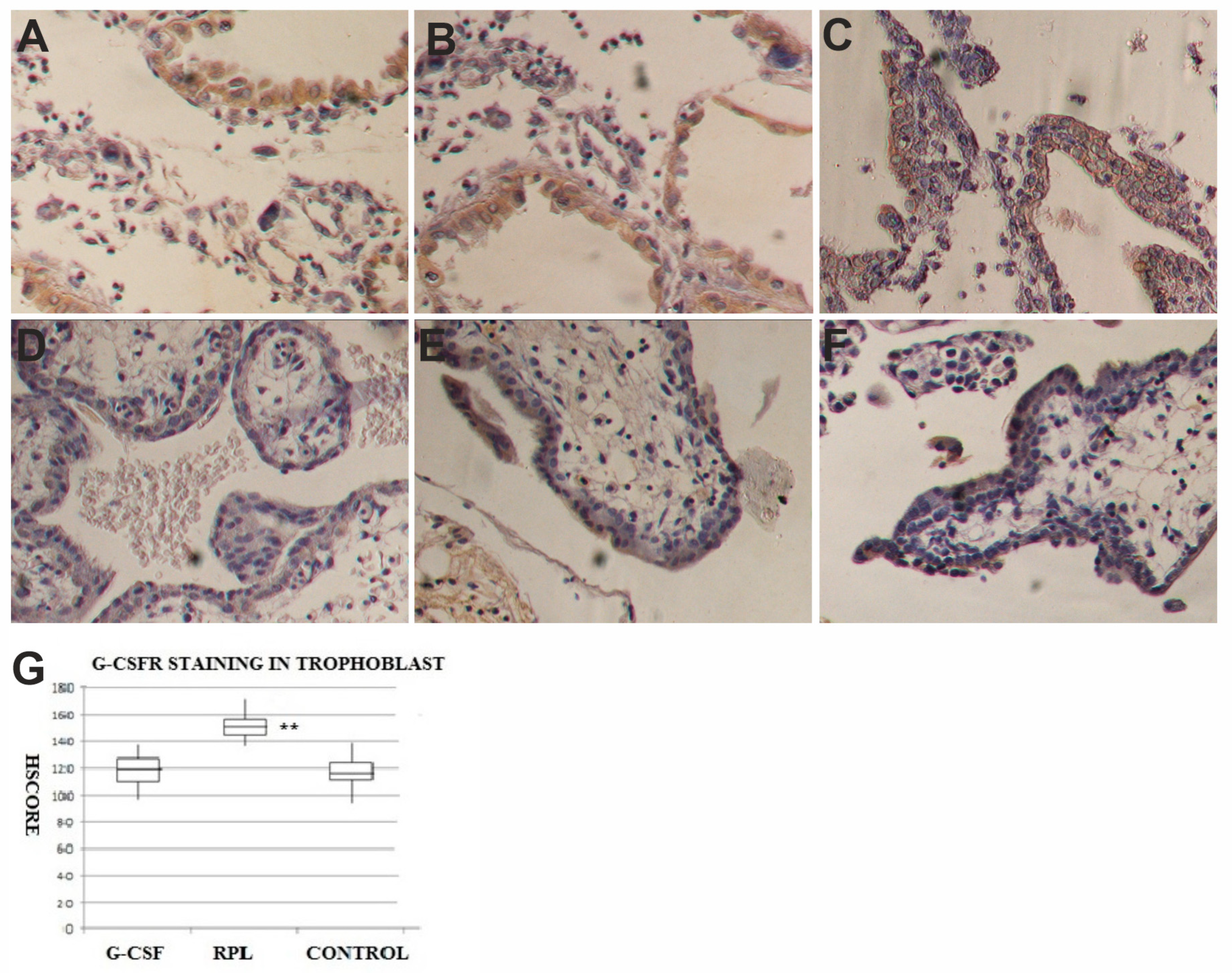
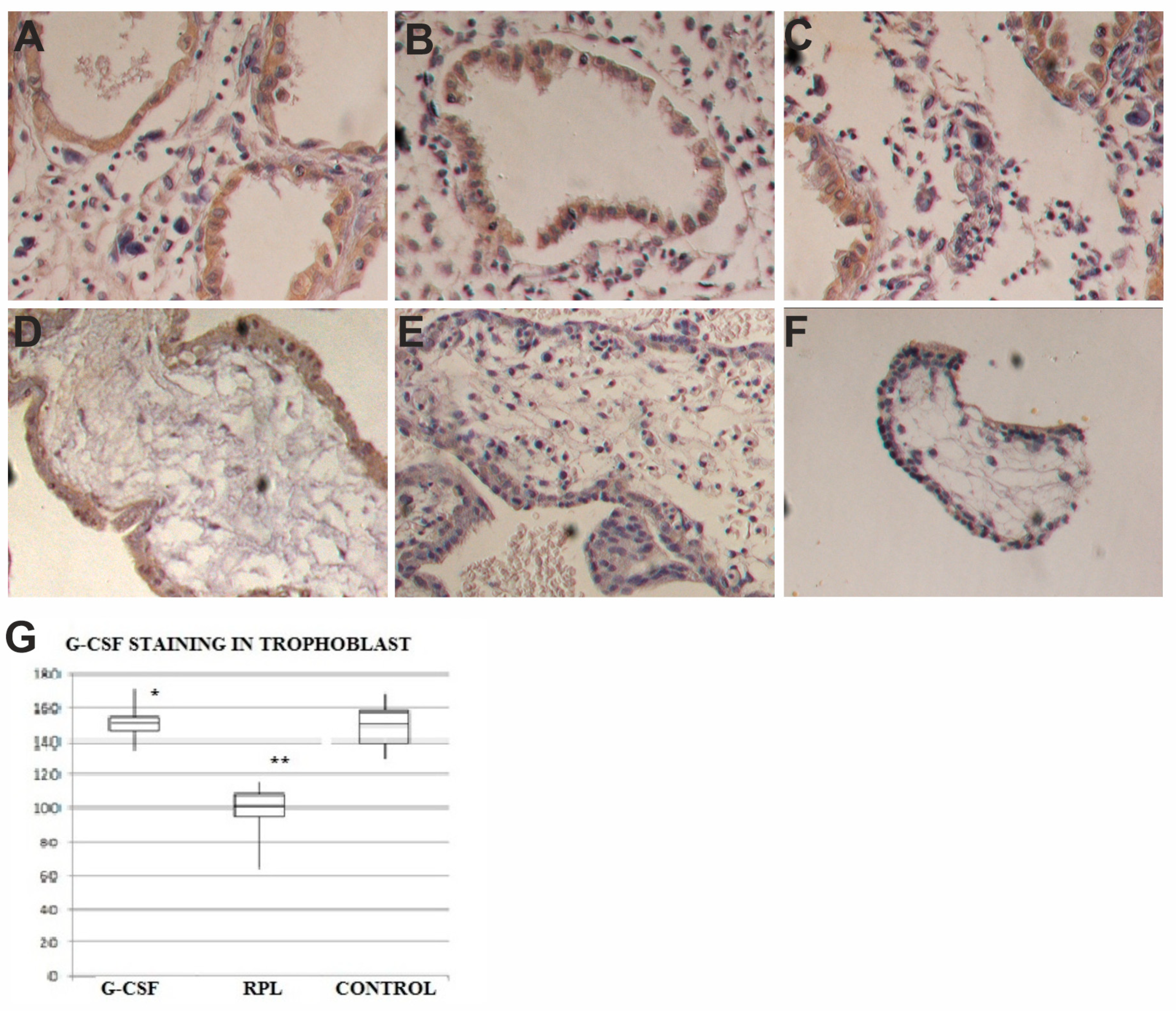
2.2. G-CSF and G-CSFR Findings
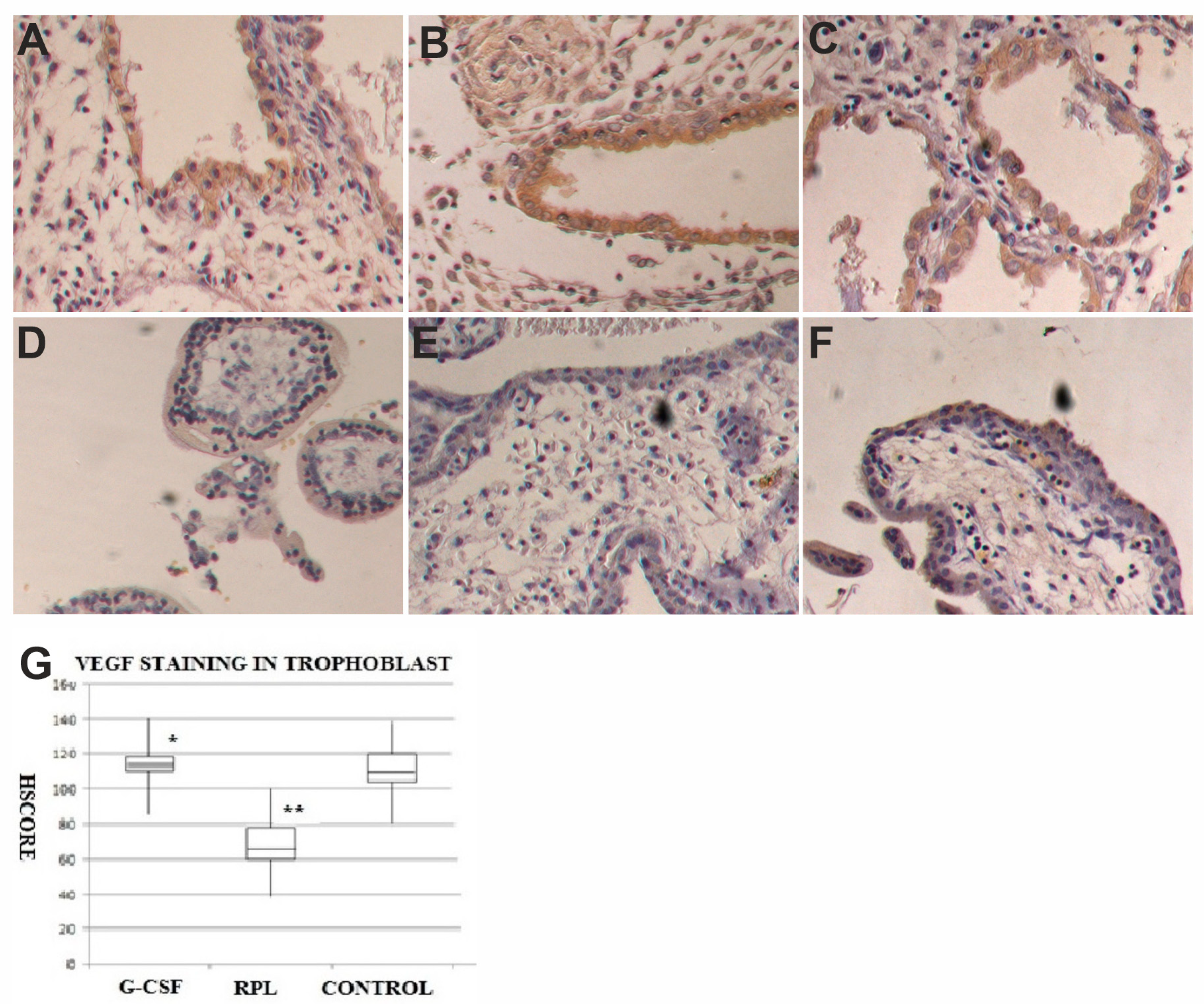
2.3. VEGF and VEGF-R1 Findings
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Conflicts of Interest
References
- The ESHRE Guideline Group on RPL; Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent preenter gnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef]
- Larsen, E.C.; Christiansen, O.B.; Kolte, A.M.; Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 2013, 11, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrington, B.; Sacks, G.; Regan, L. Recurrent miscarriage: Pathophysiology and outcome. Curr. Opin. Obstet. Gynecol. 2005, 17, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, F.; Sbracia, M. Use of granulocyte colony-stimulating factor for the treatment of unexplained recurrent miscarriage: A randomised controlled trial. Hum. Reprod. 2009, 24, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Tsuchiya, M.; Asano, S.; Kaziro, Y.; Yamazaki, T.; Yamamoto, O.; Hirata, Y.; Kubota, N.; Oheda, M.; Nomura, H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. Nature 1986, 319, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Uzumaki, H.; Okabe, T.; Sasaki, N.; Hagiwara, K.; Takaku, F.; Tobita, M.; Yasukawa, K.; Ito, S.; Umezawa, Y. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc. Natl. Acad. Sci. USA 1989, 86, 9323–9326. [Google Scholar] [CrossRef] [Green Version]
- Shorter, S.C.; Vince, G.S.; Starkey, P.M. Production of granulocyte colony-stimulating factor at the materno-foetal interface in human pregnancy. Immunology 1992, 75, 468–474. [Google Scholar]
- Saito, S.; Fukunaga, R.; Ichijo, M.; Nagata, S. Expression of granulocyte colony-stimulating factor and its receptor at the fetomaternal interface in murine and human pregnancy. Growth Factors 1994, 10, 135–143. [Google Scholar] [CrossRef]
- McCracken, S.; Layton, J.E.; Shorter, S.C.; Starkey, P.M.; Barlow, D.H.; Mardon, H.J. Expression of granulocyte-colony stimulating factor and its receptor is regulated during the development of the human placenta. J. Endocrinol. 1996, 149, 249–258. [Google Scholar] [CrossRef]
- Vandermolen, D.T.; Gu, Y. Human endometrial expression of granulocyte colony-stimulating factor (G-CSF) and its receptor, stimulation of endometrial G-CSF production by interleukin-1 beta, and G-CSF inhibition of choriocarcinoma cell proliferation. Am. J. Reprod. Immunol. 1996, 36, 278–284. [Google Scholar] [CrossRef]
- Miyama, M.; Umesaki, N.; Kawabata, M. Identification of the granulocyte colony-stimulating factor (G-CSF) producing cell population in human decidua and its biological action on trophoblast cells. Osaka City Med. J. 1998, 44, 85–96. [Google Scholar] [PubMed]
- McCracken, S.A.; Grant, K.E.; MacKenzie, I.Z.; Redman, C.W.; Mardon, H.J. Gestational regulation of granulocyte-colony stimulating factor receptor expression in the human placenta. Biol. Reprod. 1999, 60, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Litwin, S.; Lagadari, M.; Barrientos, G.; Roux, M.E.; Margni, R.; Miranda, S. Comparative immunohistochemical study of M-CSF and G-CSF in feto-maternal interface in a multiparity mouse model. Am. J. Reprod. Immunol. 2005, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Novales, J.S.; Salva, A.M.; Modanlou, H.D.; Kaplan, D.L.; del Castillo, J.; Andersen, J.; Medlock, E.S. Maternal administration of granulocyte colony-stimulating factor improves neonatal rat survival after a lethal group B streptococcal infection. Blood 1993, 81, 923–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, K.; Hayakawa, S.; Karasaki-Suzuki, M.; Hagiwara, H.; Chishima, F.; Aleemuzaman, S.; Li, J.A.; Nishinarita, S.; Yamamoto, T. Granulocyte colony stimulation factor (G-CSF) suppresses interleukin (IL)-12 and/or IL-2 induced interferon (IFN)-gamma production and cytotoxicity of decidual mononuclear cells. Am. J. Reprod. Immunol. 2003, 50, 83–89. [Google Scholar] [CrossRef]
- Svinarich, D.M.; Bitonti, O.M.; Araneda, H.; Romero, R.; Gonik, B. Induction and postranslational expression of G-CSF and RANTES in a first trimester trophoblast cell line by lipopolysaccharide. Am. J. Reprod. Immunol. 1996, 36, 256–259. [Google Scholar] [CrossRef]
- Marino, V.J.; Roguin, L.P. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J. Cell Biochem. 2008, 103, 1512–1523. [Google Scholar] [CrossRef]
- Connolly, D.T.; Olander, J.V.; Heuvelman, D.; Nelson, R.; Monsell, R.; Siegel, N.; Haymore, B.L.; Leimgruber, R.; Feder, J. Human vascular permeability factor. Isolation fromU937 cells. J. Biol. Chem. 1989, 264, 20017–20024. [Google Scholar]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef]
- Keck, P.J.; Hauser, S.D.; Krivi, G.; Sanzo, K.; Warren, T.; Feder, J.; Connolly, D.T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989, 246, 1309–1312. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [PubMed]
- Grimwood, J.; Bicknell, R.; Rees, M.C. The isolation, characterization and culture of human decidual endothelium. Hum. Reprod. 1995, 10, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Carney, E.W.; Lye, S.J.; Ritchie, J.W. Localization of two angiogenic growth factors (PDECGF and VEGF) in human placentae throughout gestation. Placenta 1994, 15, 341–353. [Google Scholar] [CrossRef]
- Cooper, J.C.; Sharkey, A.M.; McLaren, J.; Charnock-Jones, D.S.; Smith, S.K. Localization of vascular endothelial growth factor and its receptor, flt, in human placenta and decidua by immunohistochemistry. J. Reprod. Fertil. 1995, 105, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.E.; Smith, S.K.; Sharkey, A.M.; Charnock-Jones, D.S. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Hum. Reprod. 1996, 11, 1090–1098. [Google Scholar] [CrossRef] [Green Version]
- Vuorela, P.; Carpén, O.; Tulppala, M.; Halmesmäki, E. VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol. Hum. Reprod. 2000, 6, 276–282. [Google Scholar] [CrossRef]
- Von Wolff, M.; Thaler, C.J.; Strowitzki, T.; Broome, J.; Stolz, W.; Tabibzadeh, S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: Dysregulation in habitual abortion. Mol. Hum. Reprod. 2000, 6, 627–634. [Google Scholar] [CrossRef]
- Bilate, A.M.; Lafaille, J.J. Induced CD4 Foxp3 regulatory T Cells in immune tolerance. Annu. Rev. Immunol. 2012, 30, 733–758. [Google Scholar] [CrossRef] [Green Version]
- Somerset, D.A.; Zheng, Y.; Kilby, M.D.; Sansom, D.M.; Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25-CD4 regulatory T-cell subset. Immunology 2004, 112, 38–43. [Google Scholar] [CrossRef]
- Santner-Nanan, B.; Peek, M.J.; Khanam, R.; Richarts, L.; Zhu, E.; de St Groth, B.F.; Nanan, R. Systemic increase in the ratio between Foxp3 and IL-17-producing CD4 T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 2009, 183, 7023–7030. [Google Scholar] [CrossRef] [Green Version]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, J.Y.; Hur, S.E.; Kim, C.J.; Na, B.J.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3⁺ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.; Nanan, R.K. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front. Immunol. 2014, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Santner-Nanan, B.; Joung, S.; Peek, M.J.; Nanan, R. Expansion of CD4(+) HLA-G(+) T Cell in human pregnancy is impaired in pre-eclampsia. Am. J. Reprod. Immunol. 2014, 71, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Arruvito, L.; Sanz, M.; Banham, A.H.; Fainboim, L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: Implications for human reproduction. J. Immunol. 2007, 178, 2572–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441. [Google Scholar] [CrossRef] [Green Version]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Shima, T.; Inada, K.; Nakashima, A. Which types of regulatory T cells play important roles in implantation and pregnancy maintenance? Am. J. Reprod. Immunol. 2013, 69, 340–345. [Google Scholar] [CrossRef]
- Inada, K.; Shima, T.; Ito, M.; Ushijima, A.; Saito, S. Helios-positive functional regulatory T cells are decreased in decidua of miscarriage cases with normal fetal chromosomal content. J. Reprod. Immunol. 2015, 107, 10–19. [Google Scholar] [CrossRef]
- Akimova, T.; Beier, U.H.; Wang, L.; Levine, M.H.; Hancock, W.W. Helios expression is a marker of T cell activation and proliferation. PLoS ONE 2011, 6, e24226. [Google Scholar] [CrossRef] [Green Version]
- Mjösberg, J.; Berg, G.; Jenmalm, M.C.; Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 2010, 82, 698–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Darmochwal-Kolarz, D.; Suzuki, D.; Sakai, M.; Ito, M.; Shima, T.; Shiozaki, A.; Rolinski, J.; Saito, S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 2007, 149, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Santner-Nanan, B.; Dahlstrom, J.E.; Fadia, M.; Chandra, A.; Peek, M.; Nanan, R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am. J. Pathol. 2012, 181, 2149–2160. [Google Scholar] [PubMed]
- Quinn, K.H.; Lacoursiere, D.Y.; Cui, L.; Bui, J.; Parast, M.M. The unique pathophysiology of early-onset severe preeclampsia: Role of decidual T regulatory cells. J. Reprod. Immunol. 2011, 91, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Kludka-Sternik, M.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Schmitt, E.; Kisielewicz, A.; Rechenberg, S.; Seissler, N.; Mahnke, K.; Sohn, C. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin. Exp. Immunol. 2012, 167, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Jasper, M.J.; Tremellen, K.P.; Robertson, S.A. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 2006, 12, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Schlossberger, V.; Schober, L.; Rehnitz, J.; Schaier, M.; Zeier, M.; Meuer, S.; Steinborn, A. The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum. Reprod. 2013, 28, 3062–3073. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Barnett, B.; Safah, H.; Larussa, V.F.; Evdemon-Hogan, M.; Mottram, P.; Zou, W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004, 64, 8451–8455. [Google Scholar] [CrossRef] [Green Version]
- Adeegbe, D.; Serafini, P.; Bronte, V.; Zoso, A.; Ricordi, C.; Inverardi, L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011, 20, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Qiu, L.; Chen, G.; Ye, Z.; Lü, C.; Lin, Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil. Steril. 2008, 89, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.P.; Chen, Q.Y.; Zhang, T.; Guo, P.F.; Li, D.J. The CD4+CD25 bright regulatory Tcells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin. Immunol. 2009, 133, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Hao, C.F.; Yi, L.; Yin, G.J.; Bao, S.H.; Qiu, L.H.; Lin, Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sakai, M.; Miyazaki, S.; Higuma, S.; Shiozaki, A.; Saito, S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 2004, 10, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, K.; Shima, T.; Nakashima, A.; Aoki, K.; Ito, M.; Saito, S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J. Reprod. Immunol. 2013, 97, 104–111. [Google Scholar] [CrossRef]
- Nadkarni, S.; Smith, J.; Sferruzzi-Perri, A.N.; Ledwozyw, A.; Kishore, M.; Haas, R.; Perretti, M. Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc. Natl. Acad. Sci. USA 2016, 113, E8415–E8424. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, M.; Petitbarat, M.; Dubanchet, S.; Bensussan, A.; Chaouat, G.; Ledee, N. Granulocyte-Colony Stimulating Factor related pathways tested on an endometrial ex-vivo model. PLoS ONE 2014, 2, e102286. [Google Scholar] [CrossRef] [Green Version]


| G-CSF | RPL | Controls | |
|---|---|---|---|
| Number of patients | 8 | 15 | 15 |
| Age: when pregnancy started | 33.1 ± 3.0 | 31.6 ± 2.3 | 30.8 ± 2.2 |
| BMI: when pregnancy started | 27.7 + 2.1 | 27.4 ± 1.9 | 27.8 ± 1.8 |
| Smokers (more than 10 cigarette per day) | 0 | 1 | 2 |
| Number of previous abortions | 5.6 + 0.7 | 5.5 ± 0.4 | 0 |
| Gestational week of miscarriage (range) | 7.9 ± 1.3 (5–9) | 8.1 ± 1.2(5–9) | 9.4 ± 1.1(7–10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpellini, F.; Klinger, F.G.; Rossi, G.; Sbracia, M. Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls. Int. J. Mol. Sci. 2020, 21, 285. https://doi.org/10.3390/ijms21010285
Scarpellini F, Klinger FG, Rossi G, Sbracia M. Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls. International Journal of Molecular Sciences. 2020; 21(1):285. https://doi.org/10.3390/ijms21010285
Chicago/Turabian StyleScarpellini, Fabio, Francesca Gioia Klinger, Gabriele Rossi, and Marco Sbracia. 2020. "Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls" International Journal of Molecular Sciences 21, no. 1: 285. https://doi.org/10.3390/ijms21010285
APA StyleScarpellini, F., Klinger, F. G., Rossi, G., & Sbracia, M. (2020). Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls. International Journal of Molecular Sciences, 21(1), 285. https://doi.org/10.3390/ijms21010285





