Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases
Abstract
1. Introduction
2. Historical Thinking of Trophoblast Invasion
3. Looking into Invaded Uterine Structures from the Embryo’s Nutritional Point of View
4. New Routes of Trophoblast Invasion
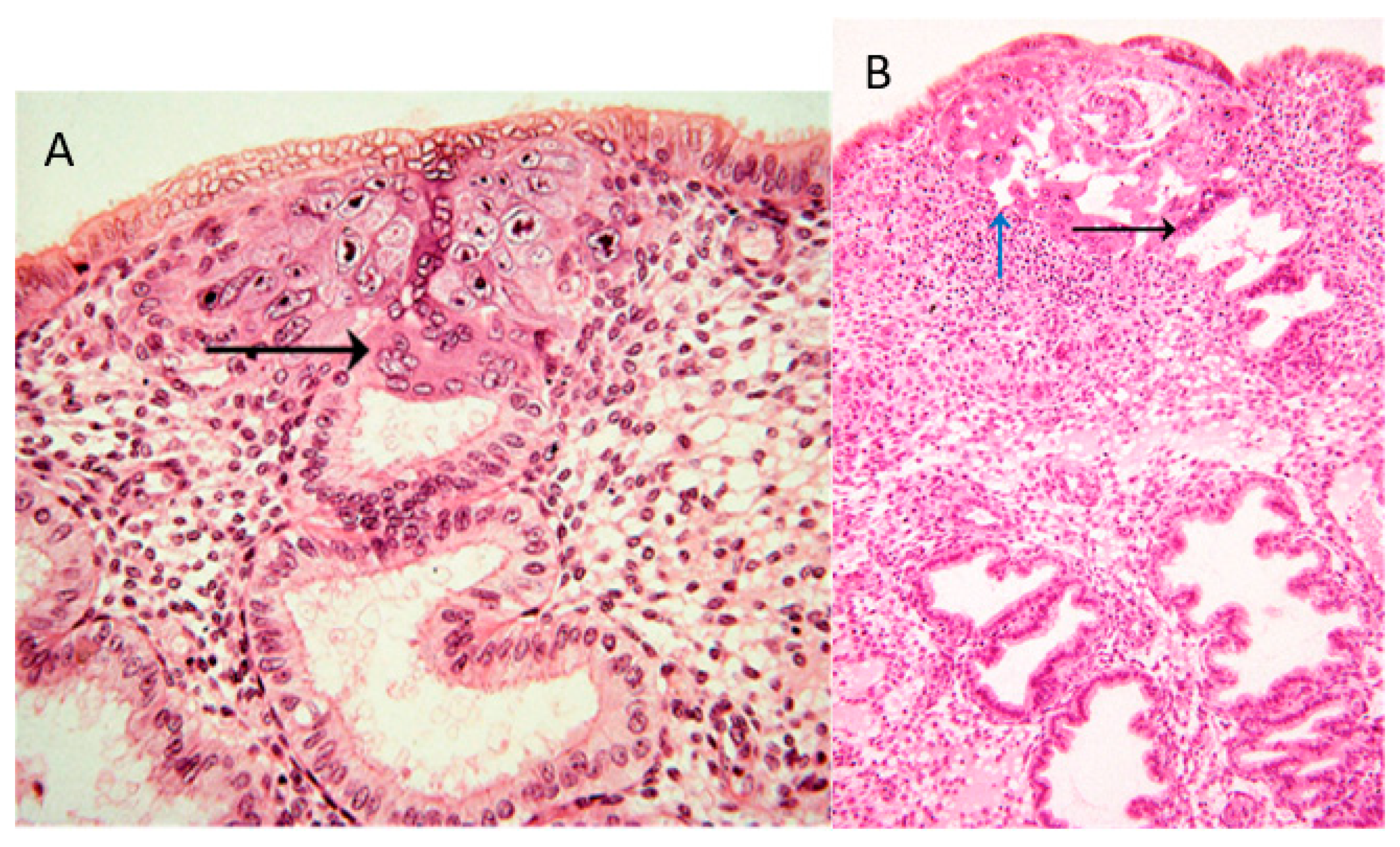
4.1. Endoglandular Trophoblast
4.2. Endovenous Trophoblast
4.3. Endoarterial Trophoblast
4.4. Endolymphatic Trophoblast
5. Alterations of Trophoblast Invasion and the Putative Effects on Pregnancy Outcome
5.1. One Example of Non-Arterial Changes of Trophoblast Invasion in a Pregnancy Pathology
5.2. General Considerations of Changes of Trophoblast Invasion and Their Effects on Pregnancy Outcome
6. New Omics Technologies and Morphological Assessment of Tissues
7. Conclusions
Funding
Conflicts of Interest
References
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Nonvillous Parts and Trophoblast Invasion. In Pathology of the Human Placenta, 6th ed.; Springer: New York, NY, USA, 2012; pp. 157–240. [Google Scholar]
- Kadyrov, M.; Schmitz, C.; Black, S.; Kaufmann, P.; Huppertz, B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta 2003, 24, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Goffin, F.; Munaut, C.; Malassiné, A.; Evain-Brion, D.; Frankenne, F.; Fridman, V.; Dubois, M.; Uzan, S.; Merviel, P.; Foidart, J.M. Evidence of a limited contribution of feto-maternal interactions to trophoblast differentiation along the invasive pathway. Tissue Antigens 2003, 62, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Lian, I.A.; Toft, J.H.; Olsen, G.D.; Langaas, M.; Bjørge, L.; Eide, I.P.; Børdahl, P.E.; Austgulen, R. Matrix metalloproteinase 1 in pre-eclampsia and fetal growth restriction: Reduced gene expression in decidual tissue and protein expression in extravillous trophoblasts. Placenta 2010, 31, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Gauster, M.; Orendi, K.; Glasner, A.; Theuerkauf, R.; Huppertz, B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum. Reprod. 2010, 25, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Orendi, K.; Gauster, M.; Siwetz, M.; Helige, C.; Huppertz, B. The art of identification of extravillous trophoblast. Placenta 2011, 32, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Gardner, L.; Moffett, A. A critical look at HLA-G. Trends Immunol. 2008, 29, 313–321. [Google Scholar] [CrossRef]
- McMaster, M.T.; Librach, C.L.; Zhou, Y.; Lim, K.H.; Janatpour, M.J.; DeMars, R.; Kovats, S.; Damsky, C.; Fisher, S.J. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 1995, 154, 3771–3778. [Google Scholar]
- Weetman, A.P. The immunology of pregnancy. Thyroid 1999, 9, 643–646. [Google Scholar] [CrossRef]
- James, J.L.; Chamley, L.W. A caution on the use of HLA-G isoforms as markers of extravillous trophoblasts. Placenta 2008, 29, 305–306. [Google Scholar] [CrossRef]
- Huppertz, B. The critical role of abnormal trophoblast development in the etiology of preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 771–780. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. An updated view on the origin and use of angiogenic biomarkers for preeclampsia. Expert Rev. Mol. Diagn. 2018, 18, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W. Anatomia uteri humani gravidi tabulis illustrata [The Anatomy of the Human Gravid Uterus Exhibited in Figures]; John Baskerville: Birmingham, UK, 1774. [Google Scholar]
- Grosser, O. Frühentwicklung, Eihautbildung und Placentation des Menschen und der Säugetiere; J.F. Bergmann: München, Germany, 1927; p. 454. [Google Scholar]
- Ramsey, E.M.; Harris, J.W.S. Comparison of uteroplacental vasculature and circulation in the rhesus monkey and man. Contrib. Embryol. Carnegie Inst. Wash. 1966, 38, 59e70. [Google Scholar]
- Harris, J.W.S.; Ramsey, E.M. The morphology of human uteroplacental vasculature. Contrib. Embryol. Carnegie Inst. Wash. 1966, 38, 43e58. [Google Scholar]
- Boyd, J.D.; Hamilton, W.J. Cells in the spiral arteries of the pregnant uterus. J. Anat. 1956, 90, 595. [Google Scholar]
- Hamilton, W.J.; Boyd, J.D. Trophoblast in human utero-placental arteries. Nature 1966, 212, 906–908. [Google Scholar] [CrossRef]
- Robertson, W.B.; Brosens, I.; Dixon, H.G. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J. Pathol. 1967, 93, 581–592. [Google Scholar] [CrossRef]
- Moser, G.; Huppertz, B. Implantation and extravillous trophoblast invasion: From rare archival specimens to modern biobanking. Placenta 2017, 56, 19–26. [Google Scholar] [CrossRef]
- Enders, A. Available online: https://www.trophoblast.cam.ac.uk/Resources/enders (accessed on 25 October 2019).
- Burton, G.J.; Watson, A.L.; Hempstock, J.; Skepper, J.N.; Jauniaux, E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 2954–2959. [Google Scholar] [CrossRef]
- He, N.; van Iperen, L.; de Jong, D.; Szuhai, K.; Helmerhorst, F.M.; van der Westerlaken, L.A.J.; Chuva de Sousa Lopes, S.M. Human extravillous trophoblasts penetrate decidual veins and lymphatics before remodeling spiral arteries during early pregnancy. PLoS ONE 2017, 12, e0169849. [Google Scholar] [CrossRef]
- Moser, G.; Weiss, G.; Sundl, M.; Gauster, M.; Siwetz, M.; Lang-Olip, I.; Huppertz, B. Extravillous trophoblasts invade more than uterine arteries: Evidence for the invasion of uterine veins. Histochem. Cell Biol. 2017, 147, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Windsperger, K.; Dekan, S.; Pils, S.; Golletz, C.; Kunihs, V.; Fiala, C.; Kristiansen, G.; Knöfler, M.; Pollheimer, J. Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. 2017, 32, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Sundl, M.; Glasner, A.; Huppertz, B.; Moser, G. The trophoblast plug during early pregnancy: A deeper insight. Histochem. Cell Biol. 2016, 146, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Moser, G.; Windsperger, K.; Pollheimer, J.; de Sousa Lopes, S.C.; Huppertz, B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Biol. 2018, 150, 361–370. [Google Scholar] [CrossRef]
- Vogel, P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta 2005, 26, 591–596. [Google Scholar] [CrossRef]
- Moser, G.; Weiss, G.; Gauster, M.; Sundl, M.; Huppertz, B. Evidence from the very beginning: Endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro. Hum. Reprod. 2015, 30, 2747–2757. [Google Scholar] [CrossRef]
- Moser, G.; Drewlo, S.; Huppertz, B.; Armant, D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 2018, 24, 484–496. [Google Scholar] [CrossRef]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C.P. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Ball, E.; Robson, S.C.; Ayis, S.; Lyall, F.; Bulmer, J.N. Early embryonic demise: No evidence of abnormal spiral artery transformation or trophoblast invasion. J. Pathol. 2006, 208, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.Z.; Khong, T.Y.; Clark, D.A.; Beard, R.W. A morphological and immunological study of human placental bed biopsies in miscarriage. BJOG Int. J. Obstet. Gynaecol. 1990, 97, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Fox, H.; Backos, M.; Rai, R.; Paterson, C.; Regan, L. Defective endovascular trophoblast invasion in primary antiphospholipid antibody syndrome-associated early pregnancy failure. Hum. Reprod. 2002, 17, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Nik, H.; Innes, B.; Lash, G.; Turner, M.; Drury, J.; Bulmer, J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. 2009, 24, 45–54. [Google Scholar] [CrossRef]
- Habib, N.; Avraham-Davidi, I.; Basu, A.; Burks, T.; Shekhar, K.; Hofree, M.; Choudhury, S.R.; Aguet, F.; Gelfand, E.; Ardlie, K.; et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 2017, 14, 955–958. [Google Scholar] [CrossRef]
- Cao, J.; Packer, J.S.; Ramani, V.; Cusanovich, D.A.; Huynh, C.; Daza, R.; Qiu, X.; Lee, C.; Furlan, S.N.; Steemers, F.J.; et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 2017, 357, 661–667. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Wang, R.; Lu, X.; Dang, Y.L.; Wang, H.; Lin, H.Y.; Zhu, C.; Ge, H.; Cross, J.C.; et al. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res. 2018, 28, 819–832. [Google Scholar] [CrossRef]
- Suryawanshi, H.; Morozov, P.; Straus, A.; Sahasrabudhe, N.; Max, K.E.A.; Garzia, A.; Kustagi, M.; Tuschl, T.; Williams, Z. A single-cell survey of the human first-trimester placenta and decidua. Sci. Adv. 2018, 4, eaau4788. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Mezger, A.; Öhrmalm, C.; Herthnek, D.; Blomberg, J.; Nilsson, M. Detection of rotavirus using padlock probes and rolling circle amplification. PLoS ONE 2014, 9, e111874. [Google Scholar] [CrossRef]
- El-Heliebi, A.; Kashofer, K.; Fuchs, J.; Jahn, S.W.; Viertler, C.; Matak, A.; Sedlmayr, P.; Hoefler, G. Visualization of tumor heterogeneity by in situ padlock probe technology in colorectal cancer. Histochem. Cell Biol. 2017, 148, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Siwetz, M.; Blaschitz, A.; El-Heliebi, A.; Hiden, U.; Desoye, G.; Huppertz, B.; Gauster, M. TNF-α alters the inflammatory secretion profile of human first trimester placenta. Lab. Investig. 2016, 96, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo, S.; Lewis, R.M.; Sengers, B.G. Modelling the effect of intervillous flow on solute transfer based on 3D imaging of the human placental microstructure. Placenta 2017, 60, 21–27. [Google Scholar] [CrossRef] [PubMed]

| Extravillous Trophoblast Subtype | Invaded Structure | Putative Alteration | Putative Effect | Possibly Involved Pathologies |
|---|---|---|---|---|
| Interstitial trophoblast | Uterine tissues (decidua & myometrium) | Reduced | Less cells invading the uterus in general | IUGR w and w/o preeclampsia |
| Enhanced | Deeper invasion than normal | Placenta accreta/increta/percreta OR Maternal anemia, pregnancy at high altitude | ||
| Endoarterial trophoblast | Uterine spiral arteries | Reduced | Faster blood flow into the placenta | IUGR w and w/o preeclampsia |
| Enhanced | Further widening of the arteries | Maternal anemia, pregnancy at high altitude | ||
| Endovenous trophoblast | Uterine veins | Reduced | Decreased backflow of maternal blood into the maternal system | Early pregnancy loss, IUGR, spontaneous abortion, stillbirth |
| Enhanced | Increased backflow of blood into the maternal system | Mild IUGR | ||
| Endoglandular trophoblast | Uterine glands | Reduced | Decreased nutrition of the embryo | Early pregnancy loss, spontaneous abortion |
| Enhanced | Increased nutrition of the embryo | LGA | ||
| Endolymphatic trophoblast | Uterine lymph vessels | Reduced | Decreased regulation of placental fluid pressure | Spontaneous abortion |
| Enhanced | ? | ? |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2020, 21, 289. https://doi.org/10.3390/ijms21010289
Huppertz B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. International Journal of Molecular Sciences. 2020; 21(1):289. https://doi.org/10.3390/ijms21010289
Chicago/Turabian StyleHuppertz, Berthold. 2020. "Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases" International Journal of Molecular Sciences 21, no. 1: 289. https://doi.org/10.3390/ijms21010289
APA StyleHuppertz, B. (2020). Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. International Journal of Molecular Sciences, 21(1), 289. https://doi.org/10.3390/ijms21010289





