“Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era
Abstract
:1. Introduction
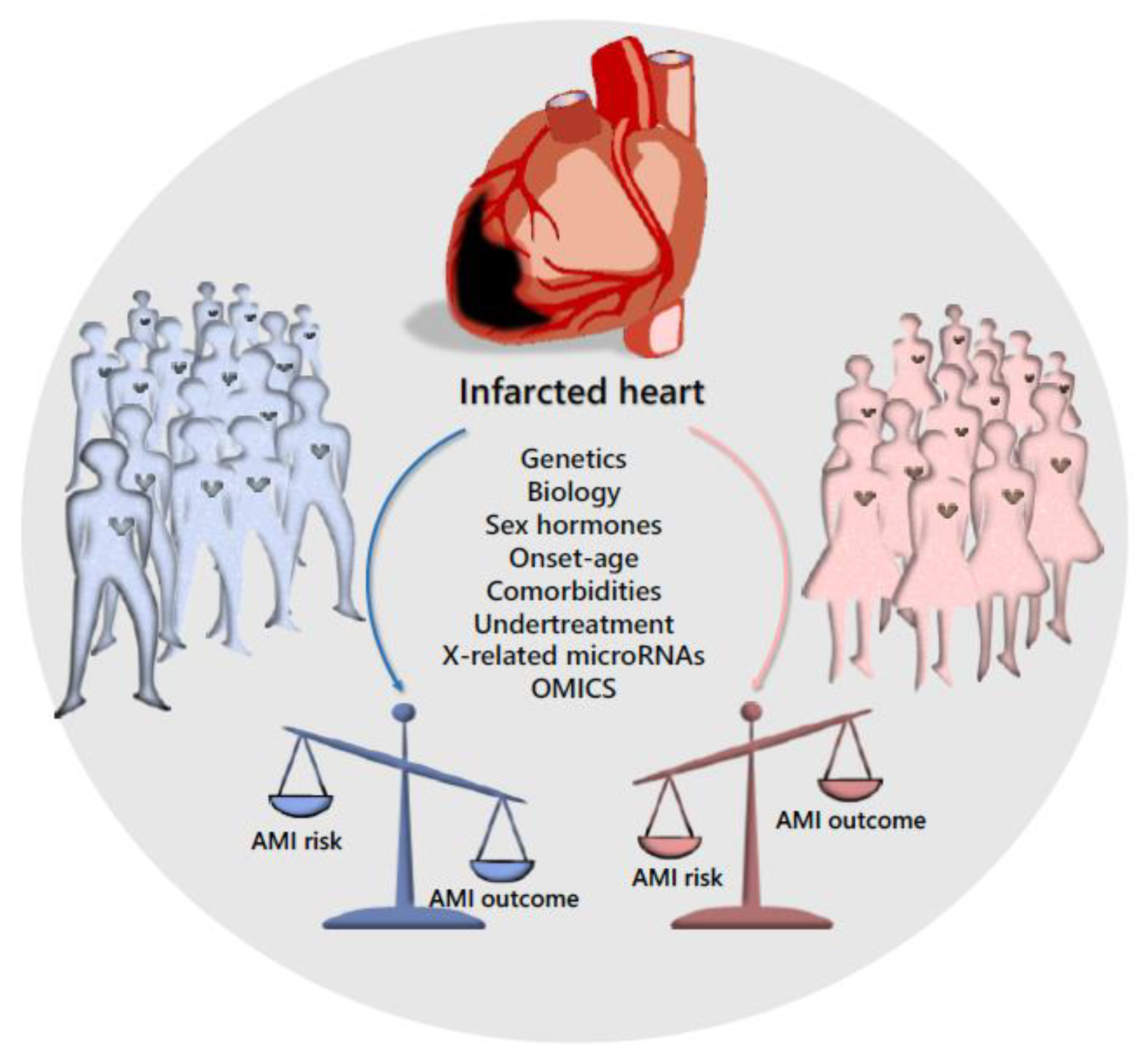
2. Sex Disparity in Cardiovascular Disease
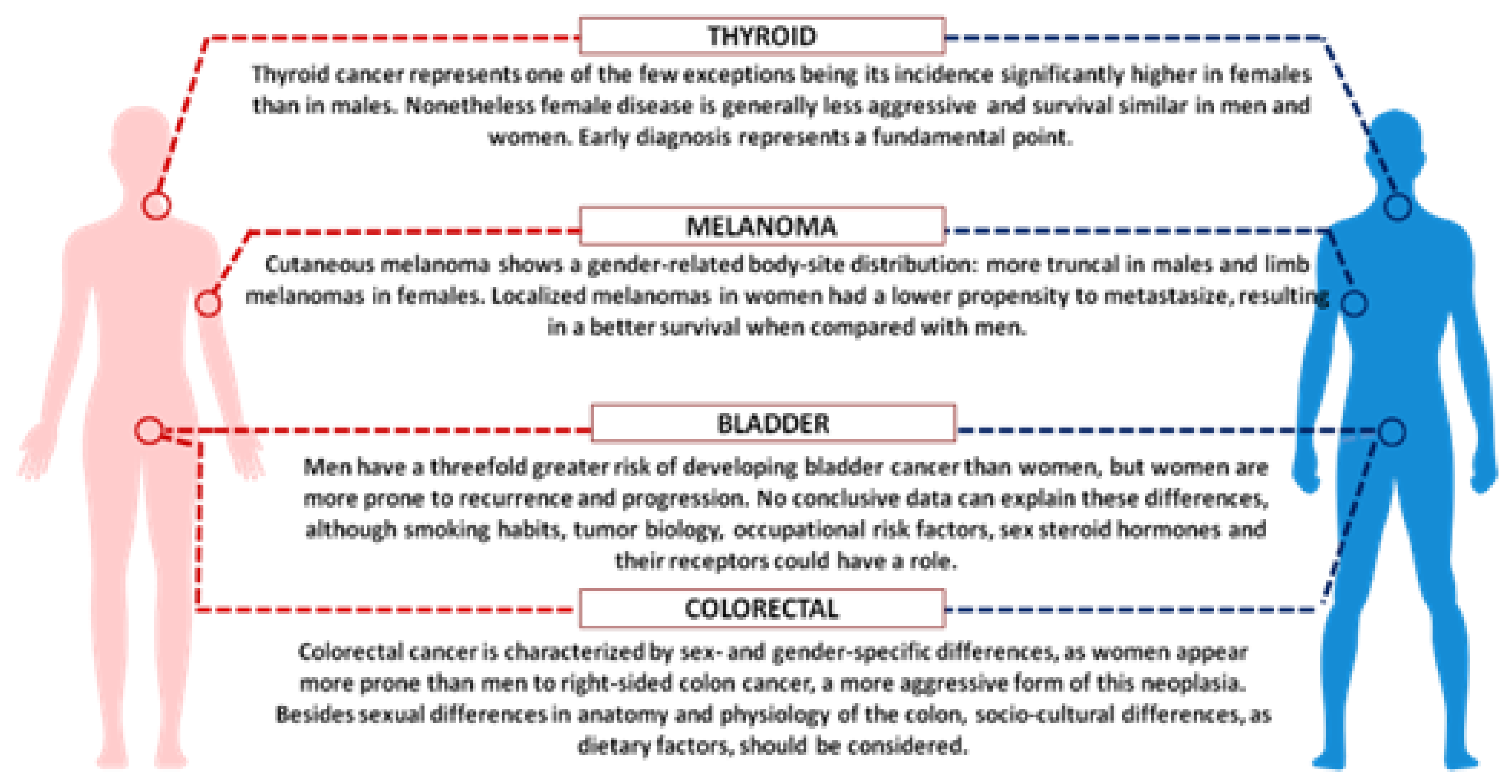
3. Sex Disparity in Cancer
4. Sex Disparity in Neurodegenerative Disorders
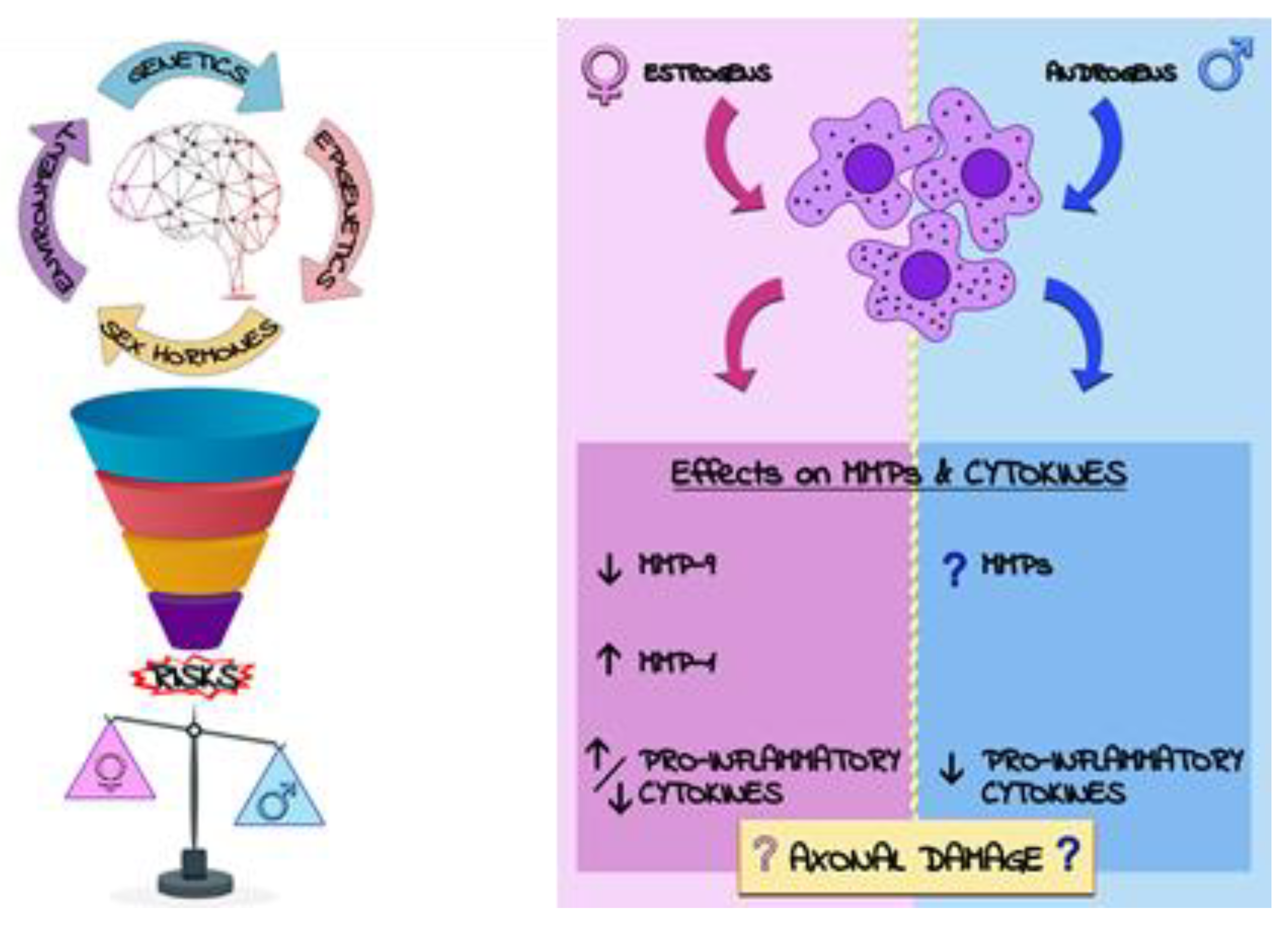
4.1. Sex Disparity in Multiple Sclerosis
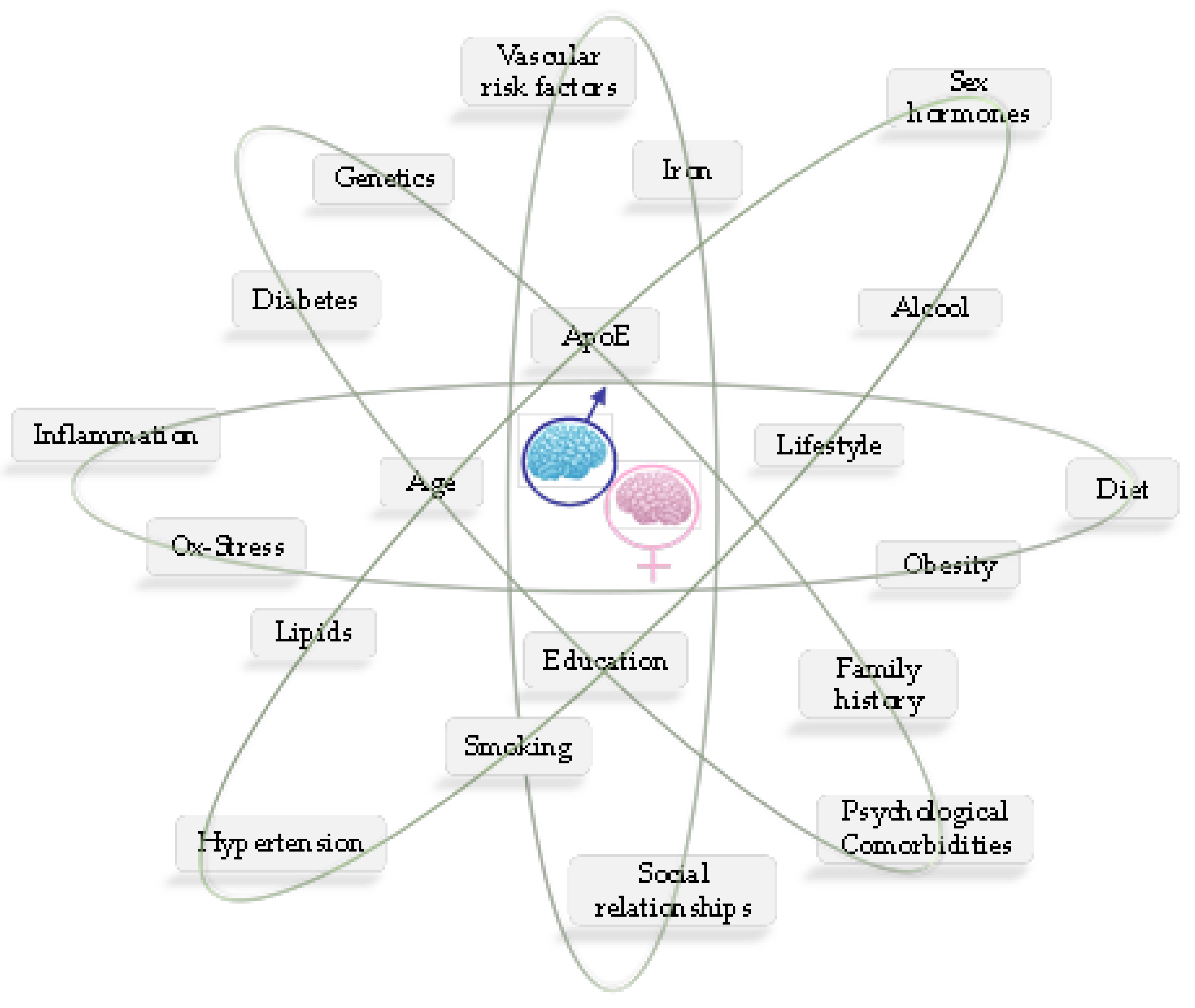
4.2. Sex Disparity in Alzheimer’s Disease
5. Sex Disparity in Bone Homeostasis Diseases
- the heterogeneity of the cell population: osteoclasts, osteoblasts, and osteocytes are the primary cells responsible for bone remodelling, yet other cells play a significant role, included their progeny, hematopoietic stem cells (HSC), cells of the immune system, mesenchymal stromal cells (MSC), adipocytes, endothelial cells and cells of the perivascular niche [178,184];
- the cellular adaption to mechanical signals [190].
6. Sex Disparity in Osteoporosis Risk and Prevention
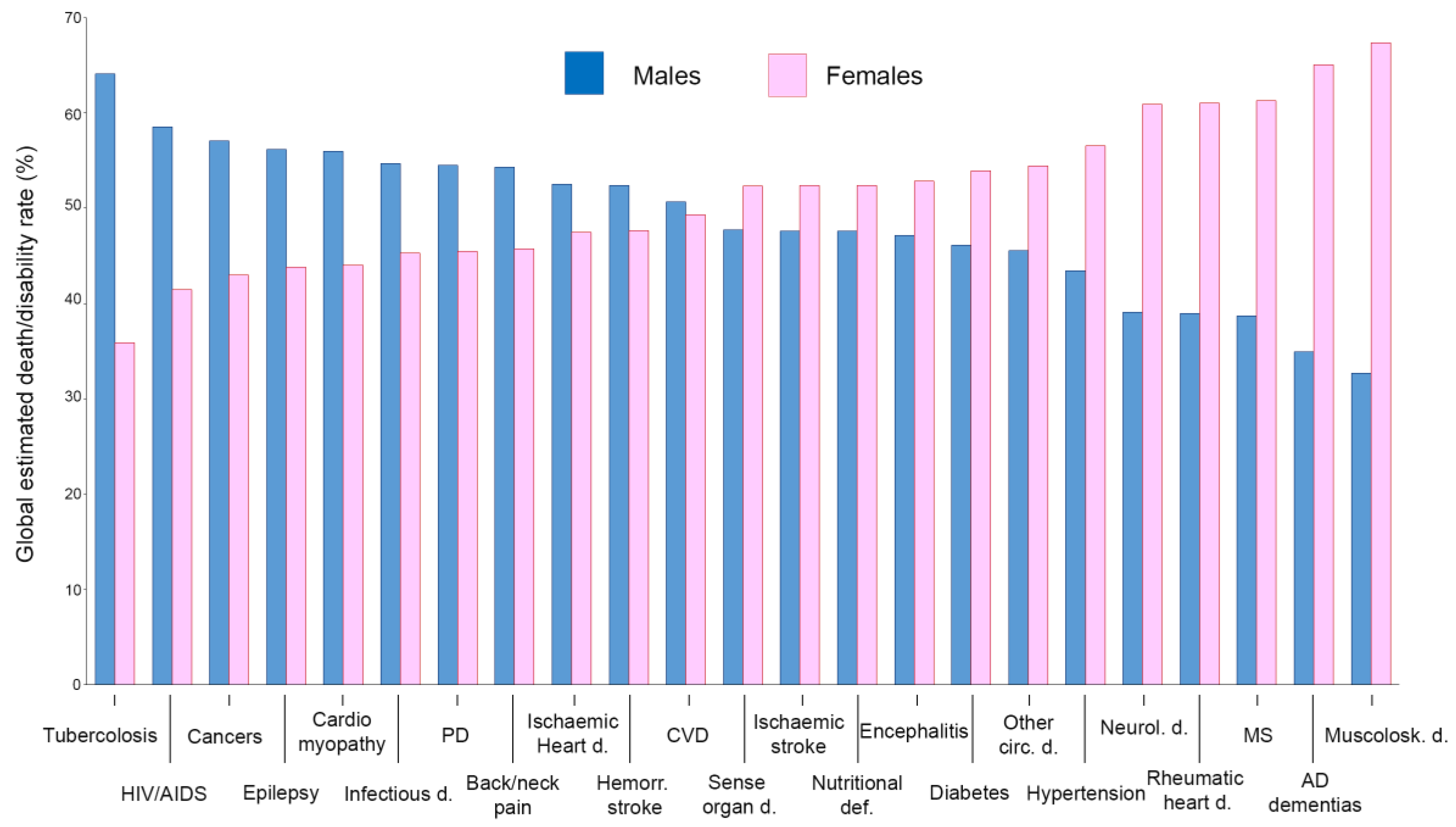
7. Sex Disparity in Morbidity/Mortality of Infectious Diseases
8. Sex Disparity in Pain Threshold and Feelings
9. Sex/Gender Disparity in the OMICs Era: Sex-Omics and Gender-Omics
10. Conclusions
“It is also important to note that the study of sex/gender differences benefits men as much as it benefits women. Therefore, when we fail to routinely consider the impact of sex/gender in research, we are leaving everyone’s health to chance”.[298]
Acknowledgments
Conflicts of Interest
References
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Vlassoff, C. Gender differences in determinants and consequences of health and illness. J. Health Popul. Nutr. 2007, 25, 47–61. [Google Scholar] [PubMed]
- Kimmel, M. The Gendered Society, 6th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Lips, H.M. The Gender Gap in Possible Selves: Divergence of Academic Self-Views Among High School and University Students. Sex Roles 2004, 50, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Prakash, V.S.; Mansukhani, N.A.; Helenowski, I.B.; Woodruff, T.K.; Kibbe, M.R. Sex Bias in Interventional Clinical Trials. J. Womens Health 2018, 27, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.; Tingen, C.M.; Woodruff, T.K. Sex bias in trials and treatment must end. Nature 2010, 465, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Douglas, P.S. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N. Engl. J. Med. 2000, 343, 475–480. [Google Scholar] [CrossRef]
- Kim, E.S.; Carrigan, T.P.; Menon, V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J. Am. Coll. Cardiol. 2008, 52, 672–673. [Google Scholar] [CrossRef] [Green Version]
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef]
- Mostertz, W.; Stevenson, M.; Acharya, C.; Chan, I.; Walters, K.; Lamlertthon, W.; Barry, W.; Crawford, J.; Nevins, J.; Potti, A. Age- and sex-specific genomic profiles in non-small cell lung cancer. JAMA 2010, 303, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Franconi, F.; Rosano, G.; Campesi, I. Need for gender-specific pre-analytical testing: The dark side of the moon in laboratory testing. Int. J. Cardiol. 2015, 179, 514–535. [Google Scholar] [CrossRef]
- McCullough, L.D.; de Vries, G.J.; Miller, V.M.; Becker, J.B.; Sandberg, K.; McCarthy, M.M. NIH initiative to balance sex of animals in preclinical studies: Generative questions to guide policy, implementation, and metrics. Biol. Sex Differ. 2014, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef] [PubMed]
- Veliskova, J. Estrogens and epilepsy: Why are we so excited? Neuroscientist 2007, 13, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Voskuhl, R.R. Estrogen treatment in multiple sclerosis. J. Neurol. Sci. 2009, 286, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Rosvall, K.A.; Bentz, A.B.; George, E.M. How research on female vertebrates contributes to an expanded challenge hypothesis. Horm. Behav. 2019, 104565. [Google Scholar] [CrossRef]
- Legato, M.J.; Johnson, P.A.; Manson, J.E. Consideration of Sex Differences in Medicine to Improve Health Care and Patient Outcomes. JAMA 2016, 316, 1865–1866. [Google Scholar] [CrossRef]
- Putting gender on the agenda. Nature 2010, 465, 665. [CrossRef]
- Blaustein, J.D. Animals have a sex, and so should titles and methods sections of articles in Endocrinology. Endocrinology 2012, 153, 2539–2540. [Google Scholar] [CrossRef]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Oertelt-Prigione, S.; Dalibert, L.; Verdonk, P.; Stutz, E.Z.; Klinge, I. Implementation Strategies for Gender-Sensitive Public Health Practice: A European Workshop. J. Womens Health 2017, 26, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Kaminsky, Z.; Wang, S.C.; Petronis, A. Complex disease, gender and epigenetics. Ann. Med. 2006, 38, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Sioud, M.; Melien, O. Treatment options and individualized medicine. Methods Mol. Biol. 2007, 361, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Raparelli, V.; Regitz-Zagrosek, V. Sex and gender landscape in pharmacology. Pharmacol. Res. 2017, 123, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, M.; Aquino, R.P.; D’Ursi, A.M. Is it time to integrate sex and gender into drug design and development? Future Med. Chem. 2015, 7, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Campesi, I. Sex and gender influences on pharmacological response: An overview. Expert Rev. Clin. Pharmacol. 2014, 7, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Basili, S.; Raparelli, V.; Proietti, M.; Tanzilli, G.; Franconi, F. Impact of sex and gender on the efficacy of antiplatelet therapy: The female perspective. J. Atheroscler. Thromb. 2015, 22, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Di Giosia, P.; Passacquale, G.; Petrarca, M.; Giorgini, P.; Marra, A.M.; Ferro, A. Gender differences in cardiovascular prophylaxis: Focus on antiplatelet treatment. Pharmacol. Res. 2017, 119, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Campesi, I.; Franconi, F.; Seghieri, G.; Meloni, M. Sex-gender-related therapeutic approaches for cardiovascular complications associated with diabetes. Pharmacol. Res. 2017, 119, 195–207. [Google Scholar] [CrossRef]
- Franconi, F.; Campesi, I.; Colombo, D.; Antonini, P. Sex-Gender Variable: Methodological Recommendations for Increasing Scientific Value of Clinical Studies. Cells 2019, 8, 476. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Snyder, M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baetta, R.; Pontremoli, M.; Fernandez, A.M.; Spickett, C.M.; Banfi, C. Reprint of: Proteomics in cardiovascular diseases: Unveiling sex and gender differences in the era of precision medicine. J. Proteom. 2018, 178, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Legato, M.J. Gender-specific medicine in the genomic era. Clin. Sci. 2016, 130, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.M. Why are sex and gender important to basic physiology and translational and individualized medicine? Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H781–H788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legato, M.J. The “Biological Sex”. Available online: http://www.genderbasic.nl/ (accessed on 13 December 2019).
- Legato, M.J. The “ biological sex or gender?” debate: “Everything flows, nothing stands still. Nothing endures but change”. Gend. Med. 2011, 8, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Klinge, I. Gender perspectives in European research. Pharmacol. Res. 2008, 58, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://cordis.europa.eu/project/id/18833 (accessed on 13 December 2019).
- Available online: https://eupha.org/gencad (accessed on 13 December 2019).
- Available online: http://www.eugenmed.eu/index.php/eugennet (accessed on 13 December 2019).
- Cardiovascular Clinical Study Group; Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Clapier, R.; Dworatzek, E.; Seeland, U.; Kararigas, G.; Arnal, J.F.; Brunelleschi, S.; Carpenter, T.C.; Erdmann, J.; Franconi, F.; Giannetta, E.; et al. Sex in basic research: Concepts in the cardiovascular field. Cardiovasc. Res. 2017, 113, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://eige.europa.eu/about (accessed on 13 December 2019).
- Available online: http://www.worldheartfailure.org/WHFS (accessed on 24 October 2019).
- Available online: http://www.medicographia.com/2012/02/the-heart-failure-epidemic (accessed on 24 October 2019).
- Davis, E.; Gorog, D.A.; Rihal, C.; Prasad, A.; Srinivasan, M. “Mind the gap” acute coronary syndrome in women: A contemporary review of current clinical evidence. Int. J. Cardiol. 2017, 227, 840–849. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S. Gender and cardiovascular disease in the workplace—It’s not just about pay gaps. Int. J. Cardiol. 2018, 262, 108–109. [Google Scholar] [CrossRef]
- Myocardial Infarction Genetics Consortium; Kathiresan, S.; Voight, B.F.; Purcell, S.; Musunuru, K.; Ardissino, D.; Mannucci, P.M.; Anand, S.; Engert, J.C.; Samani, N.J.; et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009, 41, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatochi, M.; Ichihara, S.; Yamamoto, K.; Naruse, K.; Yokota, S.; Asano, H.; Matsubara, T.; Yokota, M. Epigenome-wide association of myocardial infarction with DNA methylation sites at loci related to cardiovascular disease. Clin. Epigenet. 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, T.R.; Erdmann, J.; Stirrups, K.E.; Stitziel, N.O.; Masca, N.G.; Jansen, H.; Kanoni, S.; Nelson, C.P.; Ferrario, P.G.; Konig, I.R.; et al. Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Sukhi, A.; Chaudhary, R.; Jindal, M.; Vyas, A.; Rout, A.; Bliden, K.; Tantry, U.; Gurbel, P. Gender differences in thrombogenicity among patients with angina and non-obstructive coronary artery disease. J. Thromb. Thrombolysis 2019. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Tragante, V.; Schmidt, A.F.; McCubrey, R.O.; Holmes, M.V.; Howe, L.J.; Direk, K.; Akerblom, A.; Leander, K.; Virani, S.S.; et al. Subsequent Event Risk in Individuals with Established Coronary Heart Disease: Design and Rationale of the GENIUS-CHD Consortium. Circ. Genom. Precis. Med. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmati, D.; Serino, M.L.; Trivellato, C.; Fiorini, S.; Scapoli, G.L. C677T substitution in the methylenetetrahydrofolate reductase gene as a risk factor for venous thrombosis and arterial disease in selected patients. Haematologica 1999, 84, 824–828. [Google Scholar]
- Gemmati, D.; Serino, M.L.; Ongaro, A.; Tognazzo, S.; Moratelli, S.; Resca, R.; Moretti, M.; Scapoli, G.L. A common mutation in the gene for coagulation factor XIII-A (VAL34Leu): A risk factor for primary intracerebral hemorrhage is protective against atherothrombotic diseases. Am. J. Hematol. 2001, 67, 183–188. [Google Scholar] [CrossRef]
- Campo, G.; Valgimigli, M.; Ferraresi, P.; Malagutti, P.; Baroni, M.; Arcozzi, C.; Gemmati, D.; Percoco, G.; Parrinello, G.; Ferrari, R.; et al. Tissue factor and coagulation factor VII levels during acute myocardial infarction: Association with genotype and adverse events. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2800–2806. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Federici, F.; Campo, G.; Tognazzo, S.; Serino, M.L.; De Mattei, M.; Valgimigli, M.; Malagutti, P.; Guardigli, G.; Ferraresi, P.; et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: Effects on survival in myocardial infarction patients. Mol. Med. 2007, 13, 112–120. [Google Scholar] [CrossRef]
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation Factor XIIIA (F13A1): Novel Perspectives in Treatment and Pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, L.; Marini, M.; Gonzini, L.; Boccanelli, A.; Casella, G.; Chiarella, F.; De Servi, S.; Di Chiara, A.; Di Pasquale, G.; Olivari, Z.; et al. Contemporary Trends and Age-Specific Sex Differences in Management and Outcome for Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, R.P.; Ranasinghe, I.; Wang, Y.; Dharmarajan, K.; Murugiah, K.; Nuti, S.V.; Hsieh, A.F.; Spertus, J.A.; Krumholz, H.M. Sex Differences in the Rate, Timing, and Principal Diagnoses of 30-Day Readmissions in Younger Patients with Acute Myocardial Infarction. Circulation 2015, 132, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyer, R.P.; Dharmarajan, K.; Kennedy, K.F.; Jones, P.G.; Vaccarino, V.; Murugiah, K.; Nuti, S.V.; Smolderen, K.G.; Buchanan, D.M.; Spertus, J.A.; et al. Sex Differences in 1-Year All-Cause Rehospitalization in Patients After Acute Myocardial Infarction: A Prospective Observational Study. Circulation 2017, 135, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [CrossRef]
- Wells, G.L. Cardiovascular Risk Factors: Does Sex Matter? Curr. Vasc. Pharmacol. 2016, 14, 452–457. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L. Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr. Heart Fail. Rep. 2012, 9, 267–276. [Google Scholar] [CrossRef]
- Seeland, U.; Regitz-Zagrosek, V. Sex and gender differences in cardiovascular drug therapy. Handb. Exp. Pharmacol. 2012, 211–236. [Google Scholar] [CrossRef]
- Ranasinghe, I.; Wang, Y.; Dharmarajan, K.; Hsieh, A.F.; Bernheim, S.M.; Krumholz, H.M. Readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia among young and middle-aged adults: A retrospective observational cohort study. PLoS Med. 2014, 11, e1001737. [Google Scholar] [CrossRef] [Green Version]
- Bauters, C.; Dubois, E.; Porouchani, S.; Saloux, E.; Fertin, M.; de Groote, P.; Lamblin, N.; Pinet, F. Long-term prognostic impact of left ventricular remodeling after a first myocardial infarction in modern clinical practice. PLoS ONE 2017, 12, e0188884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanya, V.; Zhao, D.; Ouyang, P.; Lima, J.A.; Vaidya, D.; Ndumele, C.E.; Bluemke, D.A.; Shah, S.J.; Guallar, E.; Nwabuo, C.C.; et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018, 108, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, E.N.; Hakimzadeh, N.; van der Laan, A.M.; Krijnen, P.A.; Niessen, H.W.; Piek, J.J. Modulators of Macrophage Polarization Influence Healing of the Infarcted Myocardium. Int. J. Mol. Sci. 2015, 16, 29583–29591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. Int. J. Mol. Sci. 2016, 17, 63. [Google Scholar] [CrossRef]
- Musial-Wysocka, A.; Kot, M.; Sulkowski, M.; Majka, M. Regenerative Potential of the Product “CardioCell” Derived from the Wharton’s Jelly Mesenchymal Stem Cells for Treating Hindlimb Ischemia. Int. J. Mol. Sci. 2019, 20, 4632. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Dong, Y.H.; Du, W.; Shi, C.Y.; Wang, K.; Tariq, M.A.; Wang, J.X.; Li, P.F. The Role of MicroRNAs in Myocardial Infarction: From Molecular Mechanism to Clinical Application. Int. J. Mol. Sci. 2017, 18, 745. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Subhashree, L.; Milani, P.; Gemmati, D.; Zamboni, P. Interplay of iron metallobiology, metalloproteinases, and FXIII, and role of their gene variants in venous leg ulcer. Int. J. Low Extrem. Wounds 2010, 9, 166–179. [Google Scholar] [CrossRef]
- Zamboni, P.; Gemmati, D. Clinical implications of gene polymorphisms in venous leg ulcer: A model in tissue injury and reparative process. Thromb. Haemost. 2007, 98, 131–137. [Google Scholar]
- Zamboni, P.; De Mattei, M.; Ongaro, A.; Fogato, L.; Carandina, S.; De Palma, M.; Tognazzo, S.; Scapoli, G.L.; Serino, M.L.; Caruso, A.; et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc. Endovasc. Surg. 2004, 38, 431–438. [Google Scholar] [CrossRef]
- Tognazzo, S.; Gemmati, D.; Palazzo, A.; Catozzi, L.; Carandina, S.; Legnaro, A.; Tacconi, G.; Scapoli, G.L.; Zamboni, P. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J. Vasc. Surg. 2006, 44, 815–819. [Google Scholar] [CrossRef] [Green Version]
- Gemmati, D.; Tognazzo, S.; Catozzi, L.; Federici, F.; De Palma, M.; Gianesini, S.; Scapoli, G.L.; De Mattei, M.; Liboni, A.; Zamboni, P. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J. Vasc. Surg. 2006, 44, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmati, D.; Tognazzo, S.; Serino, M.L.; Fogato, L.; Carandina, S.; De Palma, M.; Izzo, M.; De Mattei, M.; Ongaro, A.; Scapoli, G.L.; et al. Factor XIII V34L polymorphism modulates the risk of chronic venous leg ulcer progression and extension. Wound Repair Regen. 2004, 12, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Occhionorelli, S.; Tisato, V.; Vigliano, M.; Longo, G.; Gonelli, A.; Sibilla, M.G.; Serino, M.L.; Zamboni, P. Inherited genetic predispositions in F13A1 and F13B genes predict abdominal adhesion formation: Identification of gender prognostic indicators. Sci. Rep. 2018, 8, 16916. [Google Scholar] [CrossRef] [PubMed]
- Greiten, L.E.; Holditch, S.J.; Arunachalam, S.P.; Miller, V.M. Should there be sex-specific criteria for the diagnosis and treatment of heart failure? J. Cardiovasc. Transl. Res. 2014, 7, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: www.istat.it/it/archivio/230897 (accessed on 24 October 2019).
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straface, E.; Gambardella, L.; Brandani, M.; Malorni, W. Sex differences at cellular level: “Cells have a sex”. Handb. Exp. Pharmacol. 2012, 49–65. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signalling, thereby contributing to the enhanced immune response of females. Bioessays 2011, 33, 791–802. [Google Scholar] [CrossRef]
- Capone, I.; Marchetti, P.; Ascierto, P.A.; Malorni, W.; Gabriele, L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Front. Immunol. 2018, 9, 552. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, L.; Buoncervello, M.; Ascione, B.; Bellenghi, M.; Matarrese, P.; Care, A. The gender perspective in cancer research and therapy: Novel insights and on-going hypotheses. Ann. Ist. Super. Sanita 2016, 52, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Paik, H.Y.; Yoon, H.; Lee, J.E.; Kim, N.; Sung, M.K. Sex- and gender-specific disparities in colorectal cancer risk. World J. Gastroenterol. 2015, 21, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef] [Green Version]
- Marks, P.; Soave, A.; Shariat, S.F.; Fajkovic, H.; Fisch, M.; Rink, M. Female with bladder cancer: What and why is there a difference? Transl. Androl. Urol. 2016, 5, 668–682. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Roh, M.R.; Eliades, P.; Gupta, S.; Grant-Kels, J.M.; Tsao, H. Cutaneous melanoma in women. Int. J. Womens Dermatol. 2017, 3, S11–S15. [Google Scholar] [CrossRef]
- Faramarzi, S.; Ghafouri-Fard, S. Melanoma: A prototype of cancer-testis antigen-expressing malignancies. Immunotherapy 2017, 9, 1103–1113. [Google Scholar] [CrossRef]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336–99346. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Sacca, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Care, A.; Bellenghi, M.; Matarrese, P.; Gabriele, L.; Salvioli, S.; Malorni, W. Sex disparity in cancer: Roles of microRNAs and related functional players. Cell Death Differ. 2018, 25, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, Y.M.; Peng, M.; Hu, M.X.; Yao, Y.; Song, Q.B. Clinical and molecular characteristics associated with the efficacy of PD-1/PD-L1 inhibitors for solid tumors: A meta-analysis. OncoTargets Ther. 2018, 11, 7529–7542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abu-Duhier, F.M.; Barreto, G.E.; Ashraf, G.M. Impact of sex differences and gender specificity on behavioral characteristics and pathophysiology of neurodegenerative disorders. Neurosci. Biobehav. Rev. 2019, 102, 95–105. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintore, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Karussis, D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: A critical review. J. Autoimmun. 2014, 48-49, 134–142. [Google Scholar] [CrossRef]
- Airas, L.; Kaaja, R. Pregnancy and multiple sclerosis. Obstet. Med. 2012, 5, 94–97. [Google Scholar] [CrossRef]
- Fainardi, E.; Castellazzi, M.; Tamborino, C.; Trentini, A.; Manfrinato, M.C.; Baldi, E.; Tola, M.R.; Dallocchio, F.; Granieri, E.; Bellini, T. Potential relevance of cerebrospinal fluid and serum levels and intrathecal synthesis of active matrix metalloproteinase-2 (MMP-2) as markers of disease remission in patients with multiple sclerosis. Mult. Scler. 2009, 15, 547–554. [Google Scholar] [CrossRef]
- Trentini, A.; Manfrinato, M.C.; Castellazzi, M.; Tamborino, C.; Roversi, G.; Volta, C.A.; Baldi, E.; Tola, M.R.; Granieri, E.; Dallocchio, F.; et al. TIMP-1 resistant matrix metalloproteinase-9 is the predominant serum active isoform associated with MRI activity in patients with multiple sclerosis. Mult. Scler. 2015, 21, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Castellazzi, M.; Cervellati, C.; Manfrinato, M.C.; Tamborino, C.; Hanau, S.; Volta, C.A.; Baldi, E.; Kostic, V.; Drulovic, J.; et al. Interplay between Matrix Metalloproteinase-9, Matrix Metalloproteinase-2, and Interleukins in Multiple Sclerosis Patients. Dis. Markers 2016, 2016, 3672353. [Google Scholar] [CrossRef] [PubMed]
- Kallaur, A.P.; Oliveira, S.R.; Colado Simao, A.N.; Delicato de Almeida, E.R.; Kaminami Morimoto, H.; Lopes, J.; de Carvalho Jennings Pereira, W.L.; Marques Andrade, R.; Muliterno Pelegrino, L.; Donizete Borelli, S.; et al. Cytokine profile in relapsingremitting multiple sclerosis patients and the association between progression and activity of the disease. Mol. Med. Rep. 2013, 7, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Khaibullin, T.; Ivanova, V.; Martynova, E.; Cherepnev, G.; Khabirov, F.; Granatov, E.; Rizvanov, A.; Khaiboullina, S. Elevated Levels of Proinflammatory Cytokines in Cerebrospinal Fluid of Multiple Sclerosis Patients. Front. Immunol. 2017, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Comabella, M.; Tintore, M.; Koel-Simmelink, M.J.; Killestein, J.; Roos, B.; Rovira, A.; Korth, C.; Ottis, P.; Blankenstein, M.A.; et al. N-acetylaspartate and neurofilaments as biomarkers of axonal damage in patients with progressive forms of multiple sclerosis. J. Neurol. 2014, 261, 2338–2343. [Google Scholar] [CrossRef]
- Khalil, M.; Enzinger, C.; Langkammer, C.; Ropele, S.; Mader, A.; Trentini, A.; Vane, M.L.; Wallner-Blazek, M.; Bachmaier, G.; Archelos, J.J.; et al. CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult. Scler. 2013, 19, 436–442. [Google Scholar] [CrossRef]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; Alvarez-Cermeno, J.C.; Andreasson, U.; Axelsson, M.; Backstrom, D.C.; Bartos, A.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019. [Google Scholar] [CrossRef]
- Hamedani, S.Y.; Taheri, M.; Sajjadi, E.; Omrani, M.D.; Mazdeh, M.; Arsang-Jang, S.; Panah, A.S.; Sayad, A. Up regulation of MMP9 gene expression in female patients with multiple sclerosis. Hum. Antibodies 2016, 24, 59–64. [Google Scholar] [CrossRef]
- Gold, S.M.; Sasidhar, M.V.; Morales, L.B.; Du, S.; Sicotte, N.L.; Tiwari-Woodruff, S.K.; Voskuhl, R.R. Estrogen treatment decreases matrix metalloproteinase (MMP)-9 in autoimmune demyelinating disease through estrogen receptor alpha (ERalpha). Lab. Invest. 2009, 89, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Castellazzi, M.; Ligi, D.; Contaldi, E.; Quartana, D.; Fonderico, M.; Borgatti, L.; Bellini, T.; Trentini, A.; Granieri, E.; Fainardi, E.; et al. Multiplex Matrix Metalloproteinases Analysis in the Cerebrospinal Fluid Reveals Potential Specific Patterns in Multiple Sclerosis Patients. Front. Neurol. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Powell, B.S.; Dhaher, Y.Y.; Szleifer, I.G. Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation. Crit. Rev. Biomed. Eng. 2015, 43, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Ramien, C.; Taenzer, A.; Lupu, A.; Heckmann, N.; Engler, J.B.; Patas, K.; Friese, M.A.; Gold, S.M. Sex effects on inflammatory and neurodegenerative processes in multiple sclerosis. Neurosci. Biobehav. Rev. 2016, 67, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signalling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldan, S.S.; Alvarez Retuerto, A.I.; Sicotte, N.L.; Voskuhl, R.R. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 2003, 171, 6267–6274. [Google Scholar] [CrossRef] [PubMed]
- Pelfrey, C.M.; Cotleur, A.C.; Lee, J.C.; Rudick, R.A. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J. Neuroimmunol. 2002, 130, 211–223. [Google Scholar] [CrossRef]
- Eikelenboom, M.J.; Killestein, J.; Uitdehaag, B.M.; Polman, C.H. Sex differences in proinflammatory cytokine profiles of progressive patients in multiple sclerosis. Mult. Scler. 2005, 11, 520–523. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Ramanathan, M.; Weinstock-Guttman, B.; Baier, M.; Brownscheidle, C.; Jacobs, L.D. Sex differences in in vitro pro-inflammatory cytokine production from peripheral blood of multiple sclerosis patients. J. Neurol. Sci. 2003, 209, 93–99. [Google Scholar] [CrossRef]
- Eikelenboom, M.J.; Killestein, J.; Kragt, J.J.; Uitdehaag, B.M.; Polman, C.H. Gender differences in multiple sclerosis: Cytokines and vitamin D. J. Neurol. Sci. 2009, 286, 40–42. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lee, W.J.; Wang, S.J.; Fuh, J.L. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci. Rep. 2018, 8, 17368. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Schreiber, S.; Bittner, D.; Kreipe, J.; Leypoldt, F.; Rupprecht, K.; Carare, R.O.; Meuth, S.G.; Vielhaber, S.; Kortvelyessy, P. CSF Neurofilament Light Chain Levels in Primary Progressive MS: Signs of Axonal Neurodegeneration. Front. Neurol. 2018, 9, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92, e1007–e1015. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Dring, A.; Zetterberg, H.; Blennow, K.; Norgren, N.; Gilthorpe, J.; Bergenheim, T.; Svenningsson, A. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmati, D.; Zeri, G.; Orioli, E.; De Gaetano, F.E.; Salvi, F.; Bartolomei, I.; D’Alfonso, S.; Dall’osso, C.; Leone, M.A.; Singh, A.V.; et al. Polymorphisms in the genes coding for iron binding and transporting proteins are associated with disability, severity, and early progression in multiple sclerosis. BMC Med. Genet. 2012, 13, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef]
- Ziliotto, N.; Marchetti, G.; Scapoli, C.; Bovolenta, M.; Meneghetti, S.; Benazzo, A.; Lunghi, B.; Balestra, D.; Laino, L.A.; Bozzini, N.; et al. C6orf10 Low-Frequency and Rare Variants in Italian Multiple Sclerosis Patients. Front. Genet. 2019, 10, 573. [Google Scholar] [CrossRef] [Green Version]
- Ferlini, A.; Bovolenta, M.; Neri, M.; Gualandi, F.; Balboni, A.; Yuryev, A.; Salvi, F.; Gemmati, D.; Liboni, A.; Zamboni, P. Custom CGH array profiling of copy number variations (CNVs) on chromosome 6p21.32 (HLA locus) in patients with venous malformations associated with multiple sclerosis. BMC Med. Genet. 2010, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Paraboschi, E.M.; Solda, G.; Gemmati, D.; Orioli, E.; Zeri, G.; Benedetti, M.D.; Salviati, A.; Barizzone, N.; Leone, M.; Duga, S.; et al. Genetic association and altered gene expression of mir-155 in multiple sclerosis patients. Int. J. Mol. Sci. 2011, 12, 8695–8712. [Google Scholar] [CrossRef] [Green Version]
- Paraboschi, E.M.; Rimoldi, V.; Solda, G.; Tabaglio, T.; Dall’Osso, C.; Saba, E.; Vigliano, M.; Salviati, A.; Leone, M.; Benedetti, M.D.; et al. Functional variations modulating PRKCA expression and alternative splicing predispose to multiple sclerosis. Hum. Mol. Genet. 2014, 23, 6746–6761. [Google Scholar] [CrossRef] [Green Version]
- Paraboschi, E.M.; Cardamone, G.; Rimoldi, V.; Gemmati, D.; Spreafico, M.; Duga, S.; Solda, G.; Asselta, R. Meta-Analysis of Multiple Sclerosis Microarray Data Reveals Dysregulation in RNA Splicing Regulatory Genes. Int. J. Mol. Sci. 2015, 16, 23463–23481. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/mental_health/neurology/dementia/en/ (accessed on 8 December 2019).
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Du, Y.; Li, J.; Qiu, C. Lifespan Intellectual Factors, Genetic Susceptibility, and Cognitive Phenotypes in Aging: Implications for Interventions. Front. Aging Neurosci. 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [PubMed]
- Mielke, M.M. Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in Alzheimer’s disease. Curr. Opin. Psychiatry 2018, 31, 133–139. [Google Scholar] [CrossRef]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019, 1710, 43–60. [Google Scholar] [CrossRef]
- Eid, A.; Mhatre, I.; Richardson, J.R. Gene-environment interactions in Alzheimer’s disease: A potential path to precision medicine. Pharmacol. Ther. 2019, 199, 173–187. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.L.; Tybjaerg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. Absolute 10-year risk of dementia by age, sex and APOE genotype: A population-based cohort study. CMAJ 2018, 190, E1033–E1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.; Ling, H.; Lashley, T.; Holton, J.L.; Warner, T.T. Review: Clinical, neuropathological and genetic features of Lewy body dementias. Neuropathol. Appl. Neurobiol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, S.; Li, J.; Zhang, Y.; Chen, J. Meta-Analysis of the Relationship between the APOE Gene and the Onset of Parkinson’s Disease Dementia. Parkinsons Dis. 2018, 2018, 9497147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinvang, I.; Espeseth, T.; Westlye, L.T. APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2013, 37, 1322–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Boueiz, A.; Abougergi, M.S.; Kitner-Triolo, M.H.; Beydoun, H.A.; Resnick, S.M.; O’Brien, R.; Zonderman, A.B. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol. Aging 2012, 33, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Janicki, S.C.; Park, N.; Cheng, R.; Clark, L.N.; Lee, J.H.; Schupf, N. Estrogen receptor alpha variants affect age at onset of Alzheimer’s disease in a multiethnic female cohort. Dement. Geriatr. Cogn. Disord. 2014, 38, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Tisato, V.; Zuliani, G.; Vigliano, M.; Longo, G.; Franchini, E.; Secchiero, P.; Zauli, G.; Paraboschi, E.M.; Vikram Singh, A.; Serino, M.L.; et al. Gene-gene interactions among coding genes of iron-homeostasis proteins and APOE-alleles in cognitive impairment diseases. PLoS ONE 2018, 13, e0193867. [Google Scholar] [CrossRef] [Green Version]
- Peters, D.G.; Connor, J.R.; Meadowcroft, M.D. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: Two sides of the same coin. Neurobiol. Dis. 2015, 81, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell 2005, 120, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali-Rahmani, F.; Schengrund, C.L.; Connor, J.R. HFE gene variants, iron, and lipids: A novel connection in Alzheimer’s disease. Front. Pharmacol. 2014, 5, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Li, R.; Cui, J.; Shen, Y. Brain sex matters: Estrogen in cognition and Alzheimer’s disease. Mol. Cell. Endocrinol. 2014, 389, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Toro, C.A.; Zhang, L.; Cao, J.; Cai, D. Sex differences in Alzheimer’s disease: Understanding the molecular impact. Brain Res. 2019, 1719, 194–207. [Google Scholar] [CrossRef]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Cervellati, C.; Wood, P.L.; Romani, A.; Valacchi, G.; Squerzanti, M.; Sanz, J.M.; Ortolani, B.; Zuliani, G. Oxidative challenge in Alzheimer’s disease: State of knowledge and future needs. J. Investig. Med. 2016, 64, 21–32. [Google Scholar] [CrossRef]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef]
- Brombo, G.; Bonetti, F.; Ortolani, B.; Morieri, M.L.; Bosi, C.; Passaro, A.; Vigna, G.B.; Borgna, C.; Arcidicono, M.V.; Tisato, V.; et al. Lower Plasma Klotho Concentrations Are Associated with Vascular Dementia but Not Late-Onset Alzheimer’s Disease. Gerontology 2018, 64, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Rimondi, E.; Brombo, G.; Volpato, S.; Zurlo, A.; Zauli, G.; Secchiero, P.; Zuliani, G. Serum Soluble Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Levels in Older Subjects with Dementia and Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2016, 41, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.L.; Vied, C.; Badger, C.; Nowakowski, R.S. Sex-biased hippocampal pathology in the 5XFAD mouse model of Alzheimer’s disease: A multi-omic analysis. J. Comp. Neurol. 2019, 527, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Strafella, C.; Caputo, V.; Galota, M.R.; Zampatti, S.; Marella, G.; Mauriello, S.; Cascella, R.; Giardina, E. Application of Precision Medicine in Neurodegenerative Diseases. Front. Neurol. 2018, 9, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosp, F.; Mann, M. A Primer on Concepts and Applications of Proteomics in Neuroscience. Neuron 2017, 96, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Avramouli, A.; Vlamos, P.M. Integrating Omic Technologies in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2017, 987, 177–184. [Google Scholar] [CrossRef]
- Bundy, J.L.; Vied, C.; Nowakowski, R.S. Sex differences in the molecular signature of the developing mouse hippocampus. BMC Genom. 2017, 18, 237. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, M.; Yuen, T.; Sun, L.; Rosen, C.J. Regulation of Skeletal Homeostasis. Endocr. Rev. 2018, 39, 701–718. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, M.; Sawamoto, K.; Almeciga-Diaz, C.J.; Mackenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, J.; Duda, G.N.; Sass, F.A.; Dienelt, A. The Metabolic Microenvironment Steers Bone Tissue Regeneration. Trends Endocrinol. Metab. 2018, 29, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.; Bianco, P. Skeletal stem cells in space and time. Cell 2015, 160, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.L.; Li, W.J. Identification of Bone Marrow-Derived Soluble Factors Regulating Human Mesenchymal Stem Cells for Bone Regeneration. Stem Cell Rep. 2017, 8, 387–400. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Szulc, P. Bone turnover: Biology and assessment tools. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Yellowley, C.E.; Genetos, D.C. Hypoxia Signalling in the Skeleton: Implications for Bone Health. Curr. Osteoporos. Rep. 2019, 17, 26–35. [Google Scholar] [CrossRef]
- Spyropoulou, A.; Karamesinis, K.; Basdra, E.K. Mechanotransduction pathways in bone pathobiology. Biochim. Biophys. Acta 2015, 1852, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Curtis, E.M.; Ward, K.A.; Harvey, N.C.; Dennison, E.M.; Cooper, C. Fracture prediction, imaging and screening in osteoporosis. Nat. Rev. Endocrinol. 2019, 15, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Zheng, H.F.; Spector, T.D. Genetics of osteoporosis from genome-wide association studies: Advances and challenges. Nat. Rev. Genet. 2012, 13, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Schulz, A.; Coxon, F.P.; Villa, A.; Helfrich, M.H. Osteopetrosis: Genetics, treatment and new insights into osteoclast function. Nat. Rev. Endocrinol. 2013, 9, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.; Calero-Paniagua, I.; Del Pino-Montes, J. Clinical and Genetic Advances in Paget’s Disease of Bone: A Review. Clin. Rev. Bone Miner. Metab. 2017, 15, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Raterman, H.G.; Bultink, I.E.M.; Lems, W.F. Current Treatments and New Developments in the Management of Glucocorticoid-induced Osteoporosis. Drugs 2019, 79, 1065–1087. [Google Scholar] [CrossRef]
- Adler, R.A. Update on osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 759–772. [Google Scholar] [CrossRef]
- Zuo, H.; Wan, Y. Nuclear Receptors in Skeletal Homeostasis. Curr. Top. Dev. Biol. 2017, 125, 71–107. [Google Scholar] [CrossRef]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Yakar, S.; Werner, H.; Rosen, C.J. Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Caenegem, E.; Taes, Y.; Wierckx, K.; Vandewalle, S.; Toye, K.; Kaufman, J.M.; Schreiner, T.; Haraldsen, I.; T’Sjoen, G. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone 2013, 54, 92–97. [Google Scholar] [CrossRef]
- Van Caenegem, E.; Wierckx, K.; Taes, Y.; Dedecker, D.; Van de Peer, F.; Toye, K.; Kaufman, J.M.; T’Sjoen, G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J. Clin. Endocrinol. Metab. 2012, 97, 2503–2511. [Google Scholar] [CrossRef] [Green Version]
- Reppe, S.; Datta, H.K.; Gautvik, K.M. Omics analysis of human bone to identify genes and molecular networks regulating skeletal remodeling in health and disease. Bone 2017, 101, 88–95. [Google Scholar] [CrossRef]
- Lv, H.; Jiang, F.; Guan, D.; Lu, C.; Guo, B.; Chan, C.; Peng, S.; Liu, B.; Guo, W.; Zhu, H.; et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int. J. Mol. Sci. 2016, 17, 2018. [Google Scholar] [CrossRef] [Green Version]
- Bjornerem, A.; Wang, X.; Bui, M.; Ghasem-Zadeh, A.; Hopper, J.L.; Zebaze, R.; Seeman, E. Menopause-Related Appendicular Bone Loss is Mainly Cortical and Results in Increased Cortical Porosity. J. Bone Miner. Res. 2018, 33, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Vignozzi, L.; Malavolta, N.; Villa, P.; Mangili, G.; Migliaccio, S.; Lello, S. Consensus statement on the use of HRT in postmenopausal women in the management of osteoporosis by SIE, SIOMMMS and SIGO. J. Endocrinol. Invest. 2019, 42, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Borjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Hormone Therapy for Primary Prevention of Chronic Conditions in Postmenopausal Women. JAMA 2017, 318, 2265. [Google Scholar] [CrossRef] [Green Version]
- Ramchand, S.K.; Seeman, E. Advances and Unmet Needs in the Therapeutics of Bone Fragility. Front. Endocrinol. 2018, 9, 505. [Google Scholar] [CrossRef] [Green Version]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef]
- Mikkola, T.S.; Tuomikoski, P.; Lyytinen, H.; Korhonen, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Estradiol-based postmenopausal hormone therapy and risk of cardiovascular and all-cause mortality. Menopause 2015, 22, 976–983. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Hormones and heart disease in women: The timing hypothesis. Am. J. Epidemiol. 2007, 166, 506–510. [Google Scholar] [CrossRef] [Green Version]
- Gambacciani, M.; Cagnacci, A.; Lello, S. Hormone replacement therapy and prevention of chronic conditions. Climacteric 2019, 22, 303–306. [Google Scholar] [CrossRef]
- Davis, S.R.; Baber, R.; Panay, N.; Bitzer, J.; Perez, S.C.; Islam, R.M.; Kaunitz, A.M.; Kingsberg, S.A.; Lambrinoudaki, I.; Liu, J.; et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. J. Clin. Endocrinol. Metab. 2019, 104, 4660–4666. [Google Scholar] [CrossRef] [Green Version]
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Baber, R.J.; Panay, N.; Fenton, A.; A. Fenton the IMS Writing Group. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Jared, D. Guns, Germs, and Steel: The Fates of Human Societies; W. W. Norton & Company: New York, NY, USA, 1997; Volume 14. [Google Scholar]
- Kay, G.L.; Sergeant, M.J.; Giuffra, V.; Bandiera, P.; Milanese, M.; Bramanti, B.; Bianucci, R.; Pallen, M.J. Recovery of a medieval Brucella melitensis genome using shotgun metagenomics. MBio 2014, 5, e01337-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guellil, M.; Kersten, O.; Namouchi, A.; Bauer, E.L.; Derrick, M.; Jensen, A.Ø.; Stenseth, N.C.; Bramanti, B. Genomic blueprint of a relapsing fever pathogen in 15th century Scandinavia. Proc. Natl. Acad. Sci. USA 2018, 115, 10422–10427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascovan, N.; Sjögren, K.-G.; Kristiansen, K.; Nielsen, R.; Willerslev, E.; Desnues, C.; Rasmussen, S. Emergence and spread of basal lineages of Yersinia pestis during the Neolithic decline. Cell 2019, 176, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.D.; Mideo, N. Linking sex differences to the evolution of infectious disease life-histories. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramanti, B.; Hummel, S.; Chiarelli, A.B.; Herrmann, B. Ancient DNA analysis of the delta F508 mutation. Hum. Biol. 2003, 75, 105–115. [Google Scholar] [CrossRef]
- Hughes, J.F.; Page, D.C. The biology and evolution of mammalian Y chromosomes. Annu. Rev. Genet. 2015, 49, 507–527. [Google Scholar] [CrossRef] [Green Version]
- Washburn, T.C.; Medearis, D.N.; Childs, B. Sex differences in susceptibility to infections. Pediatrics 1965, 35, 57–64. [Google Scholar]
- World Health Organization. Addressing Sex and Gender in Epidemic-Prone Infectious Diseases; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Bramanti, B.; Dean, K.R.; Walløe, L.; Chr. Stenseth, N. The Third Plague Pandemic in Europe. Proc. R. Soc. B 2019, 286, 20182429. [Google Scholar] [CrossRef]
- Pandey, A.; Sengupta, P.G.; Mondal, S.K.; Gupta, D.N.; Manna, B.; Ghosh, S.; Sur, D.; Bhattacharya, S.K. Gender differences in healthcare-seeking during common illnesses in a rural community of West Bengal, India. J. Health Popul. Nutr. 2002, 20, 306–311. [Google Scholar]
- Mitra, A.K.; Rahman, M.M.; Fuchs, G.J. Risk factors and gender differentials for death among children hospitalized with diarrhoea in Bangladesh. J. Health Popul. Nutr. 2000, 18, 151–156. [Google Scholar] [PubMed]
- Markle, J.G.; Fish, E.N. SeXX matters in immunity. Trends Immunol. 2014, 35, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. Infections reveal inequality between the sexes. Nat. News 2016, 534, 447. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Vom Steeg, L.G.; Klein, S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays 2012, 34, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Úbeda, F.; Jansen, V.A.A. The evolution of sex-specific virulence in infectious diseases. Nat. Commun. 2016, 7, 13849. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Marriott, I.; Huet-Hudson, Y.M. Sexual dimorphism in innate immune responses to infectious organisms. Immunol. Res. 2006, 34, 177–192. [Google Scholar] [CrossRef]
- Schurz, H.; Salie, M.; Tromp, G.; Hoal, E.G.; Kinnear, C.J.; Möller, M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum. Genom. 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Silveira, F.; Abad-Franch, F. Sex bias in infectious disease epidemiology: Patterns and processes. PLoS ONE 2013, 8, e62390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef] [Green Version]
- Borgdorff, M.W.; Veen, J.; Kalisvaart, N.A.; Nagelkerke, N. Mortality among tuberculosis patients in The Netherlands in the period 1993–1995. Eur. Respir. J. 1998, 11, 816–820. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Corona, M.-E.; Garcia-Garcia, L.; DeRiemer, K.; Ferreyra-Reyes, L.; Bobadilla-del-Valle, M.; Cano-Arellano, B.; Canizales-Quintero, S.; Martinez-Gamboa, A.; Small, P.M.; Sifuentes-Osornio, J. Gender differentials of pulmonary tuberculosis transmission and reactivation in an endemic area. Thorax 2006, 61, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Zumla, A.; George, A.; Sharma, V.; Herbert, R.H.N.; Oxley, A.; Oliver, M. The WHO 2014 global tuberculosis report—Further to go. Lancet Glob. Health 2015, 3, e10–e12. [Google Scholar] [CrossRef] [Green Version]
- Bellamy, R.; Beyers, N.; McAdam, K.P.W.J.; Ruwende, C.; Gie, R.; Samaai, P.; Bester, D.; Meyer, M.; Corrah, T.; Collin, M. Genetic susceptibility to tuberculosis in Africans: A genome-wide scan. Proc. Natl. Acad. Sci. USA 2000, 97, 8005–8009. [Google Scholar] [CrossRef] [Green Version]
- Bernin, H.; Lotter, H. Sex bias in the outcome of human tropical infectious diseases: Influence of steroid hormones. J. Infect. Dis. 2014, 209, S107–S113. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Rege, M.; Gogtay, N.J.; Aigal, U.; Sharma, S.K.; Valecha, N.; Bhanot, G.; Kshirsagar, N.A.; Sharma, S. Age-dependent sex bias in clinical malarial disease in hypoendemic regions. PLoS ONE 2012, 7, e35592. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; Doudi, M.; Setorki, M. Assessing epidemiology of cutaneous leishmaniasis in Isfahan, Iran. J. Vector Borne Dis. 2013, 50, 30. [Google Scholar] [PubMed]
- Marlow, M.A.; da Silva Mattos, M.; Makowiecky, M.E.; Eger, I.; Rossetto, A.L.; Grisard, E.C.; Steindel, M. Divergent profile of emerging cutaneous leishmaniasis in subtropical Brazil: New endemic areas in the southern frontier. PLoS ONE 2013, 8, e56177. [Google Scholar] [CrossRef] [PubMed]
- Murback, N.D.N.; Hans Filho, G.; Nascimento, R.A.F.d.; Nakazato, K.R.d.O.; Dorval, M.E.M.C. American cutaneous leishmaniasis: Clinical, epidemiological and laboratory studies conducted at a university teaching hospital in Campo Grande, Mato Grosso do Sul, Brazil. An. Bras. Dermatol. 2011, 86, 55–63. [Google Scholar] [CrossRef]
- Sarkari, B.; Hatam, G.; Ghatee, M.A. Epidemiological features of visceral leishmaniasis in Fars province, southern Iran. Iran. J. Public Health 2012, 41, 94. [Google Scholar]
- McClelland, E.E.; Hobbs, L.M.; Rivera, J.; Casadevall, A.; Potts, W.K.; Smith, J.M.; Ory, J.J. The role of host gender in the pathogenesis of Cryptococcus neoformans infections. PLoS ONE 2013, 8, e63632. [Google Scholar] [CrossRef] [Green Version]
- Aaby, P.; Samb, B.; Simondon, F.; Seck, A.M.C.; Knudsen, K.; Whittle, H. Non-specific beneficial effect of measles immunisation: Analysis of mortality studies from developing countries. BMJ 1995, 311, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Ingersoll, M.A. Sex differences shape the response to infectious diseases. PLoS Pathog. 2017, 13, e1006688. [Google Scholar] [CrossRef]
- Harper, M.; Fowlis, G. 3. Management of urinary tract infections in men. Trends Urol. Gynaecol. Sex. Health 2007, 12, 30–35. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Dance, A. Why the sexes don’t feel pain the same way. Nature 2019, 567, 448–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnall, B.D.; Stacey, B.R.; Chou, R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012, 13, 1181–1211. [Google Scholar] [CrossRef] [PubMed]
- Christov-Moore, L.; Simpson, E.A.; Coude, G.; Grigaityte, K.; Iacoboni, M.; Ferrari, P.F. Empathy: Gender effects in brain and behavior. Neurosci. Biobehav. Rev. 2014, 46, 604–627. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why is depression more prevalent in women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef]
- Packiasabapathy, S.; Sadhasivam, S. Gender, genetics, and analgesia: Understanding the differences in response to pain relief. J. Pain Res. 2018, 11, 2729–2739. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.K.; Samntaray, S.; Banik, N.L. Future directions for using estrogen receptor agonists in the treatment of acute and chronic spinal cord injury. Neural Regen. Res. 2016, 11, 1418–1419. [Google Scholar] [CrossRef]
- Bi, R.; Foy, M.R.; Thompson, R.F.; Baudry, M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol. Aging 2003, 24, 977–983. [Google Scholar] [CrossRef]
- Tang, B.; Ji, Y.; Traub, R.J. Estrogen alters spinal NMDA receptor activity via a PKA signalling pathway in a visceral pain model in the rat. Pain 2008, 137, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Gu, Y.J.; Zhang, H.; Zhang, J. Estrogen affects neuropathic pain through upregulating N-methyl-D-aspartate acid receptor 1 expression in the dorsal root ganglion of rats. Neural Regen. Res. 2017, 12, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Renteria, M.; Juarez-Contreras, R.; Gonzalez-Ramirez, R.; Islas, L.D.; Sierra-Ramirez, F.; Llorente, I.; Simon, S.A.; Hiriart, M.; Rosenbaum, T.; Morales-Lazaro, S.L. TRPV1 channels and the progesterone receptor Sig-1R interact to regulate pain. Proc. Natl. Acad. Sci. USA 2018, 115, E1657–E1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, J.D. Preclinical Pain Research: Can We Do Better? Anesthesiology 2016, 125, 846–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travers, A. Gender and pain is it an issue? SAJAA 2009, 15, 7–10. [Google Scholar] [CrossRef]
- Mazure, C.M. Our evolving science: Studying the influence of sex in preclinical research. Biol. Sex Differ. 2016, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Mazure, C.M.; Jones, D.P. Twenty years and still counting: Including women as participants and studying sex and gender in biomedical research. BMC Womens Health 2015, 15, 94. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Yamamotova, A.; Hrabak, P.; Hribek, P.; Rokyta, R. Do multiple body modifications alter pain threshold? Physiol. Res. 2017, 66, S493–S500. [Google Scholar]
- Belfer, I. Pain in women. Agri 2017, 29, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Aloisi, A.M.; Bachiocco, V.; Costantino, A.; Stefani, R.; Ceccarelli, I.; Bertaccini, A.; Meriggiola, M.C. Cross-sex hormone administration changes pain in transsexual women and men. Pain 2007, 132, S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Ronald, L.M. Sex, gender, and pharmaceutical politics: From drug development to marketing. Gend. Med. 2010, 7, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Doyle, H.H.; Eidson, L.N.; Sinkiewicz, D.M.; Murphy, A.Z. Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J. Neurosci. 2017, 37, 3202–3214. [Google Scholar] [CrossRef] [PubMed]
- Averitt, D.L.; Eidson, L.N.; Doyle, H.H.; Murphy, A.Z. Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 2019, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Aubrun, F.; Salvi, N.; Coriat, P.; Riou, B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology 2005, 103, 156–160. [Google Scholar] [CrossRef]
- Els, C.; Jackson, T.D.; Kunyk, D.; Lappi, V.G.; Sonnenberg, B.; Hagtvedt, R.; Sharma, S.; Kolahdooz, F.; Straube, S. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 10, CD012509. [Google Scholar] [CrossRef]
- Legato, M.; Glezerman, M. The International Society for Gender Medicine, 1st ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Gianazza, E.; Miller, I.; Guerrini, U.; Palazzolo, L.; Parravicini, C.; Eberini, I. Gender proteomics I. Which proteins in non-sexual organs. J. Proteom. 2018, 178, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Gianazza, E.; Miller, I.; Guerrini, U.; Palazzolo, L.; Parravicini, C.; Eberini, I. Gender proteomics II. Which proteins in sexual organs. J. Proteom. 2018, 178, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Lusis, A.J. Understanding the sexome: Measuring and reporting sex differences in gene systems. Endocrinology 2012, 153, 2551–2555. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P. Promoting the understanding of sex differences to enhance equity and excellence in biomedical science. Biol. Sex Differ. 2010, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.E.; Coffman, K.E.; Miller, V.M. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health 2015, 11, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addressed issue. Front. Genet. 2012, 3, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://ec.europa.eu/research/health/pdf/2013-10_personalised_medicine_en.pdf (accessed on 13 December 2019).
- Johnson, P.A.; Fitzgerald, T.; Glynn, A.; Salganicoff, A.; Wood, S.F.; Goldstein, J.M. Precision Medicine: How Sex and Gender Drive Innovation. 2016. Available online: http://www.tbf.org/~/media/TBFOrg/Files/Reports/ConnorsCenterBrochure-2016.pdf2016 (accessed on 13 December 2016).
- Panagiotou, G.; Nielsen, J. Nutritional systems biology: Definitions and approaches. Annu. Rev. Nutr. 2009, 29, 329–339. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Ranjdoost, F.; Asgharian, P.; Mahmoodpoor, A.; Sanaie, S. Vitamins, Are They Safe? Adv. Pharm. Bull. 2016, 6, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K.; Tinz, J. Multivitamin/mineral supplements: Rationale and safety—A systematic review. Nutrition 2017, 33, 76–82. [Google Scholar] [CrossRef]
- Tisato, V.; Muggeo, P.; Lupiano, T.; Longo, G.; Serino, M.L.; Grassi, M.; Arcamone, E.; Secchiero, P.; Zauli, G.; Santoro, N.; et al. Maternal Haplotypes in DHFR Promoter and MTHFR Gene in Tuning Childhood Acute Lymphoblastic Leukemia Onset-Latency: Genetic/Epigenetic Mother/Child Dyad Study (GEMCDS). Genes 2019, 10, 634. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2020, 21, 296. https://doi.org/10.3390/ijms21010296
Gemmati D, Varani K, Bramanti B, Piva R, Bonaccorsi G, Trentini A, Manfrinato MC, Tisato V, Carè A, Bellini T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. International Journal of Molecular Sciences. 2020; 21(1):296. https://doi.org/10.3390/ijms21010296
Chicago/Turabian StyleGemmati, Donato, Katia Varani, Barbara Bramanti, Roberta Piva, Gloria Bonaccorsi, Alessandro Trentini, Maria Cristina Manfrinato, Veronica Tisato, Alessandra Carè, and Tiziana Bellini. 2020. "“Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era" International Journal of Molecular Sciences 21, no. 1: 296. https://doi.org/10.3390/ijms21010296
APA StyleGemmati, D., Varani, K., Bramanti, B., Piva, R., Bonaccorsi, G., Trentini, A., Manfrinato, M. C., Tisato, V., Carè, A., & Bellini, T. (2020). “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. International Journal of Molecular Sciences, 21(1), 296. https://doi.org/10.3390/ijms21010296








