Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model
Abstract
1. Introduction
2. Results
2.1. Changes in Antioxidant Parameters in Sow Plasma and Piglet Plasma and Liver
2.2. Changes in Inflammatory Factors in Sow and Piglet Plasma
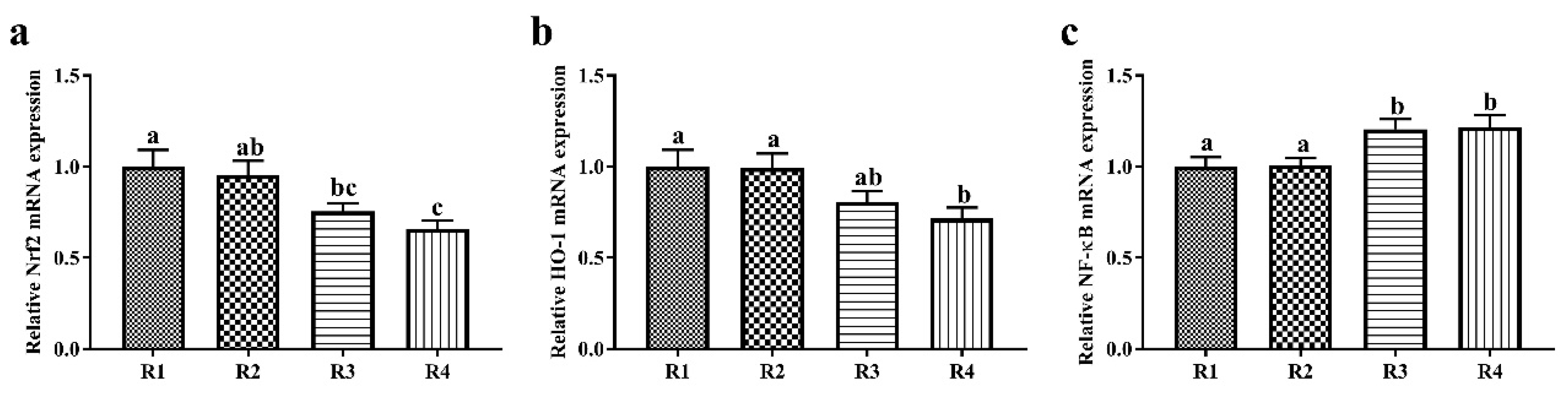
2.3. Changes in Relative mRNA Expression in Piglet Liver
2.4. Changes in Microbial Metabolites SCFAs in Sow Feces and Piglet Colonic Contents
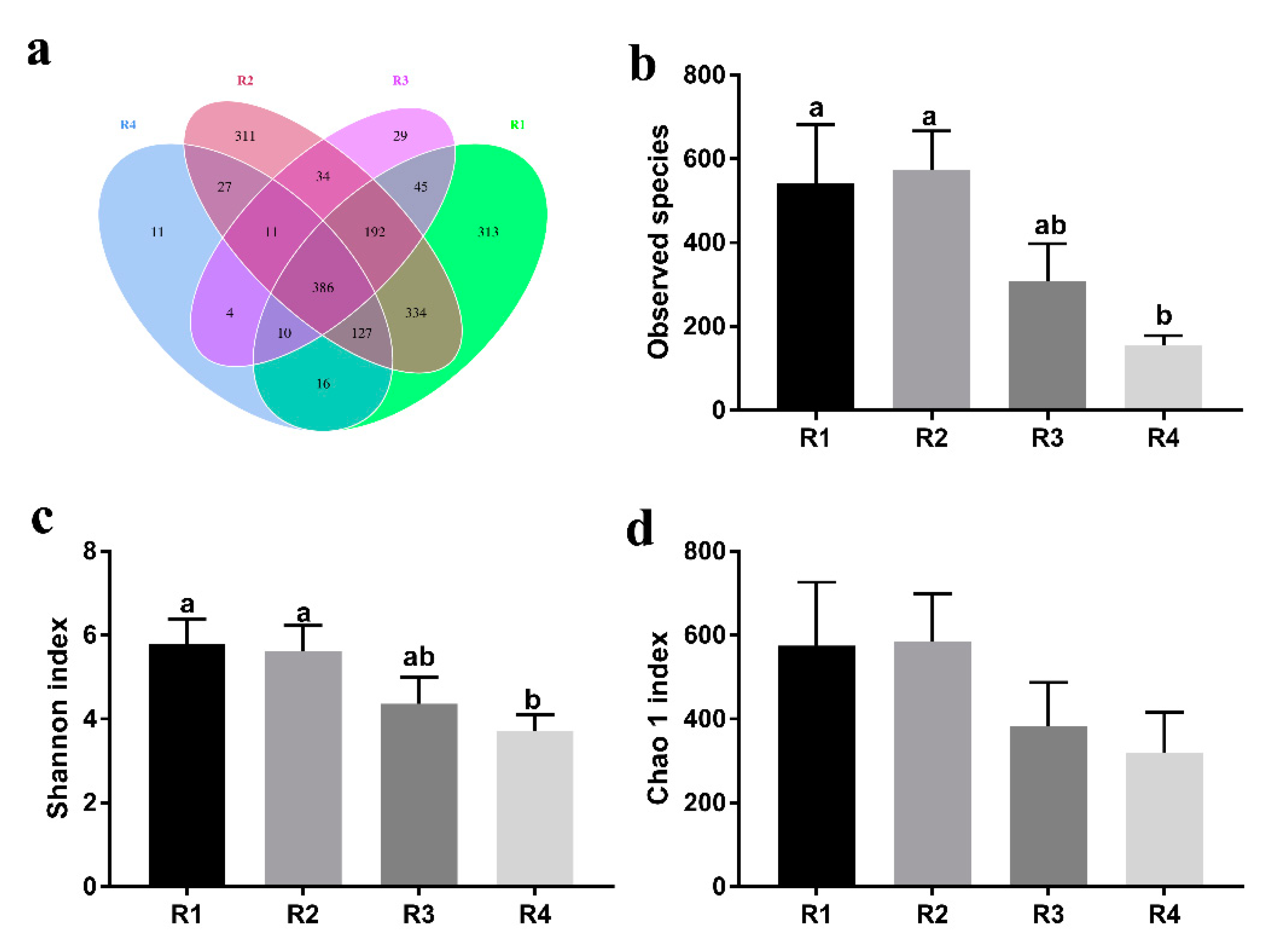
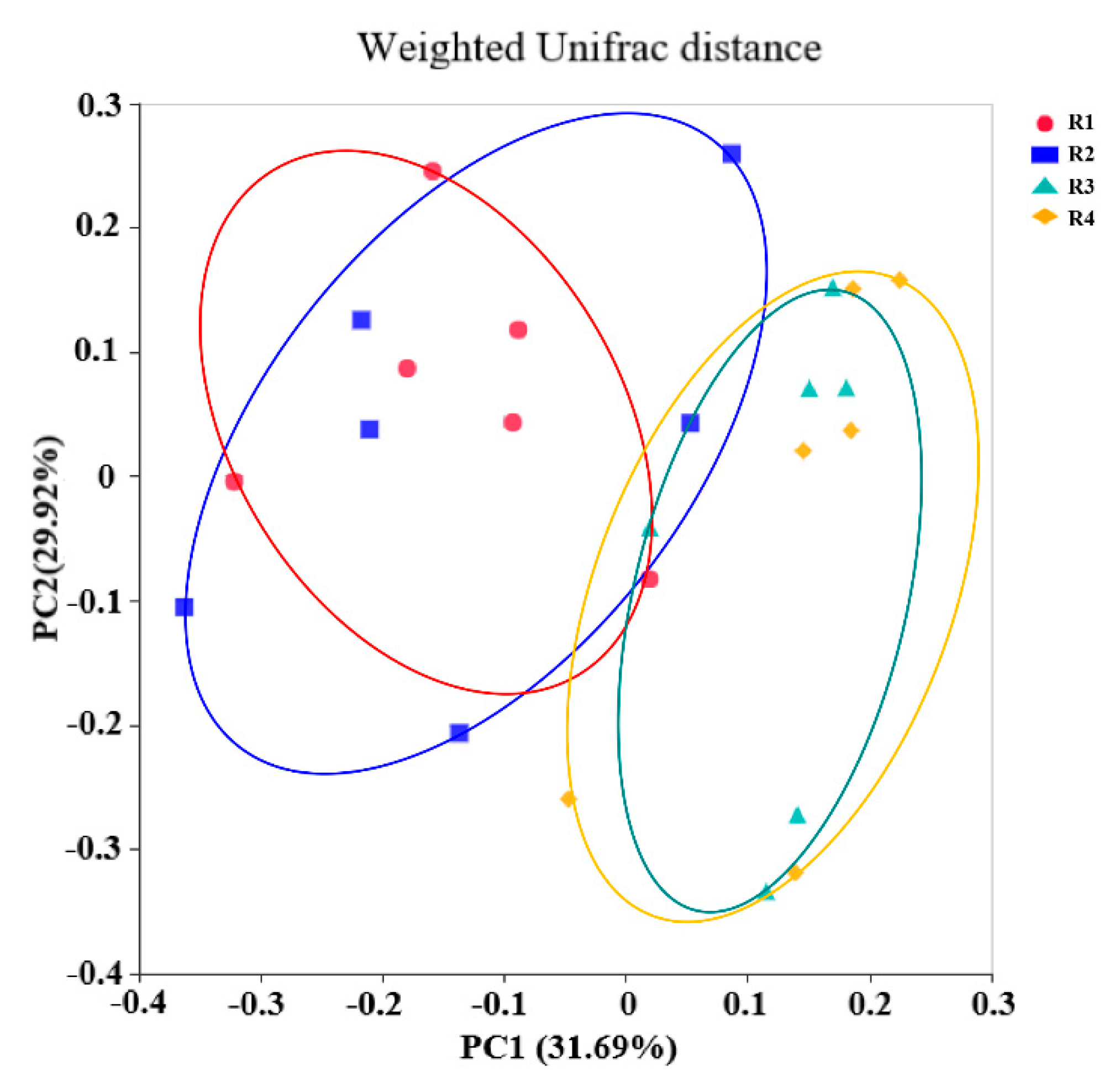
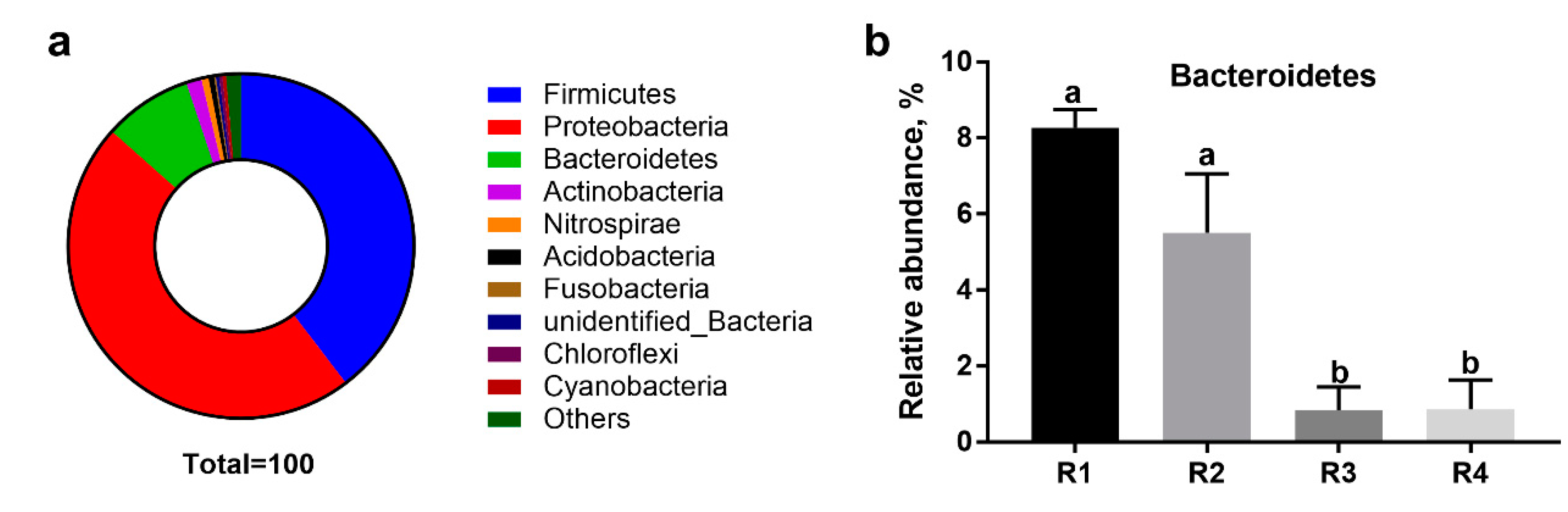
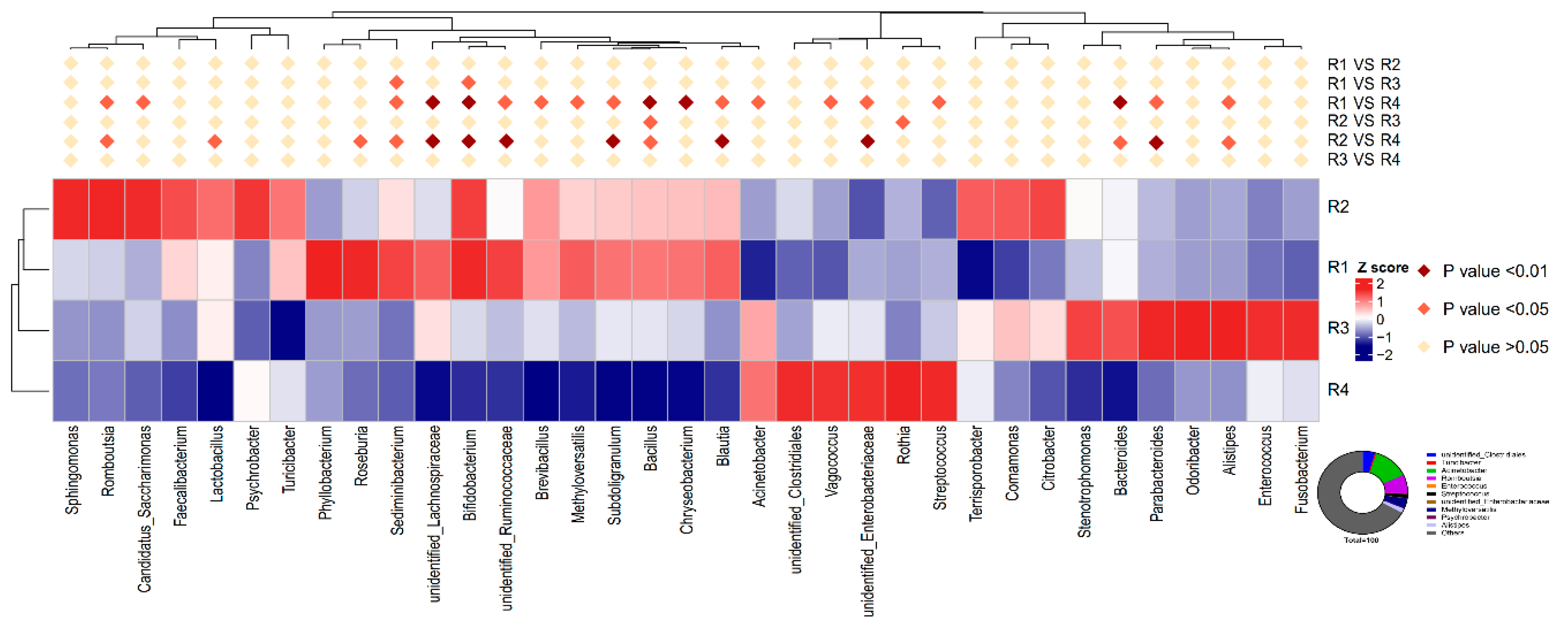
2.5. Changes in Microbial Composition and Diversity in Sow Feces
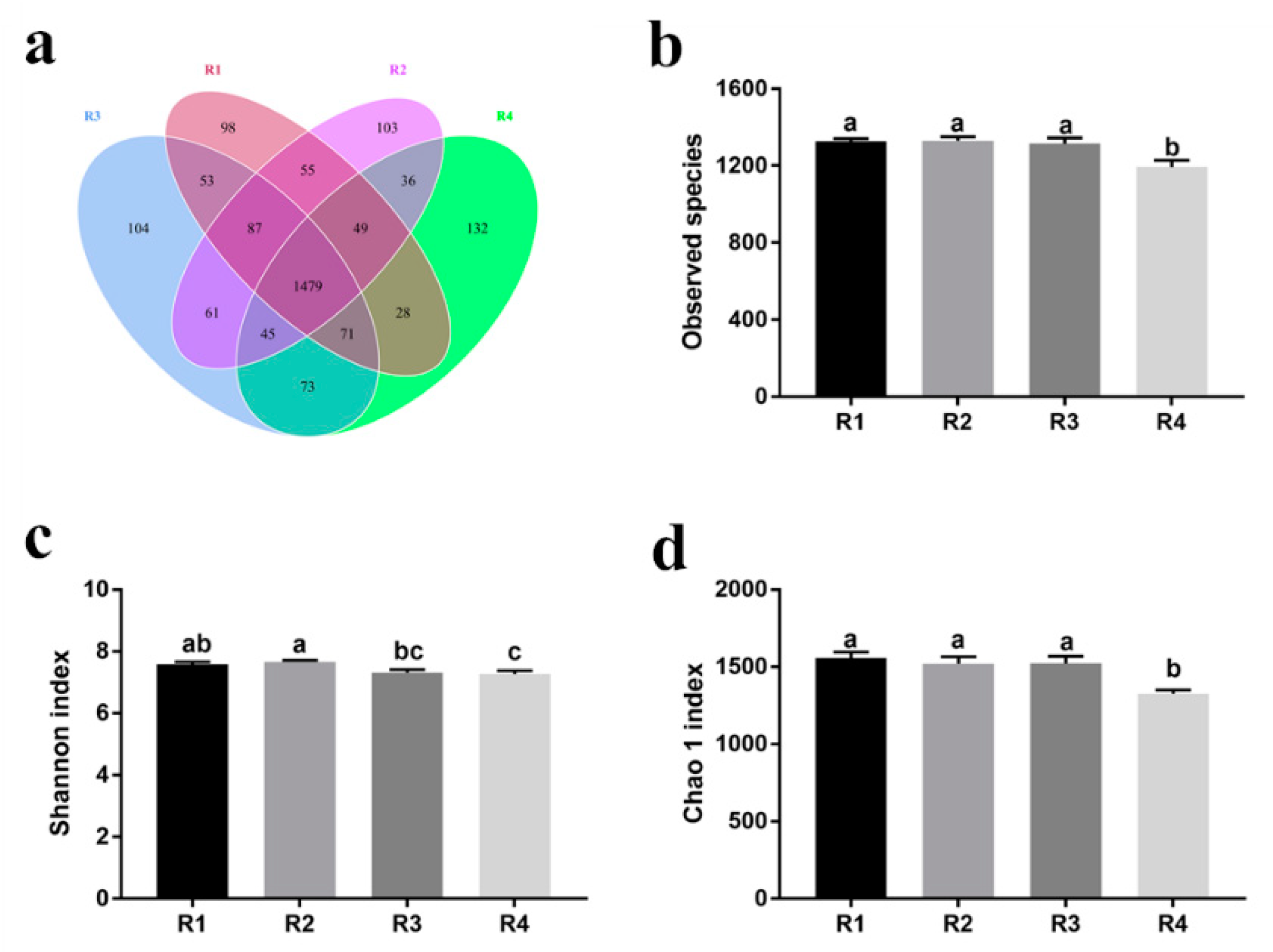
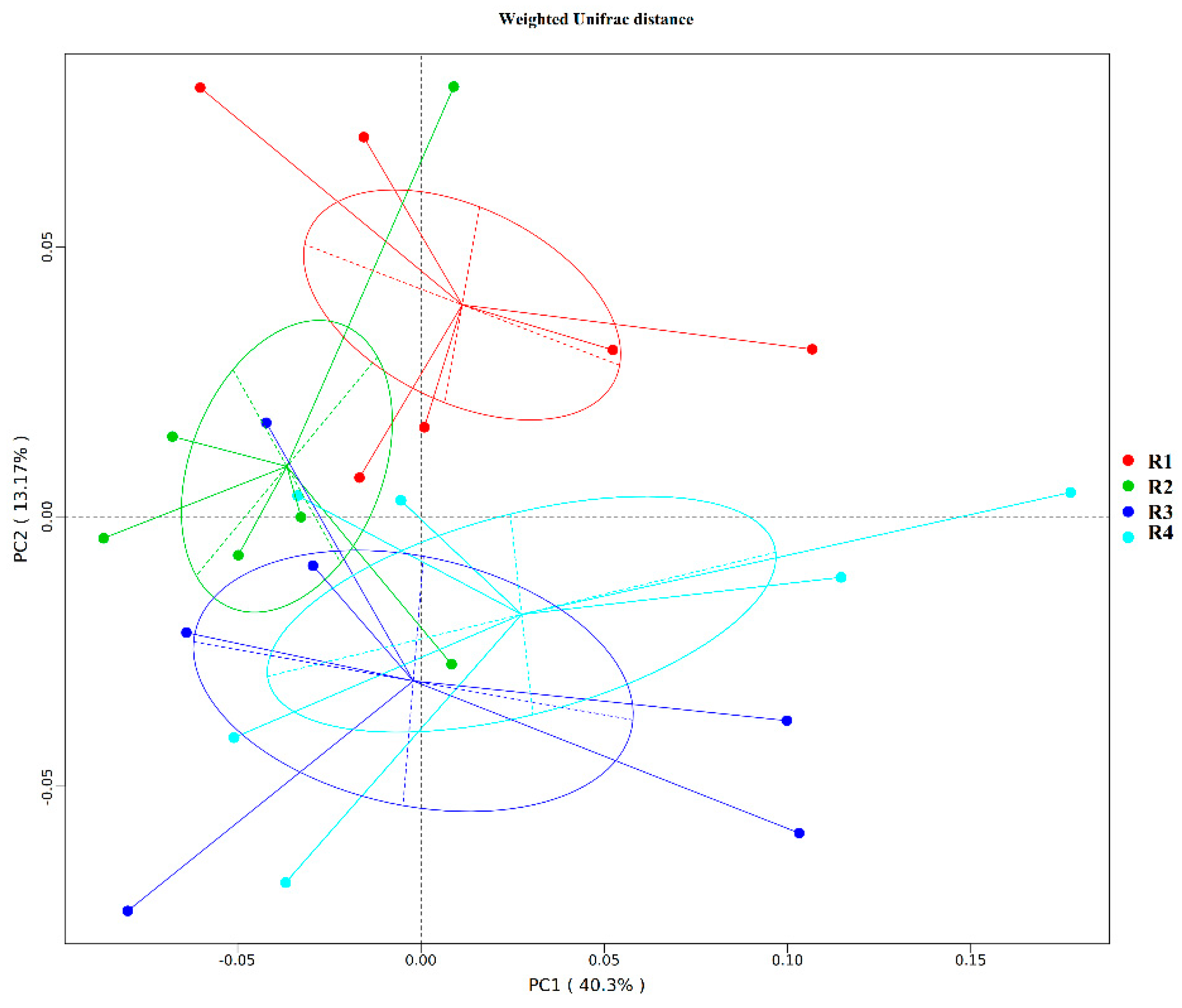
2.5.1. Changes in Fecal Microbial Diversity
2.5.2. Changes in Relative Abundance at the Phylum Level
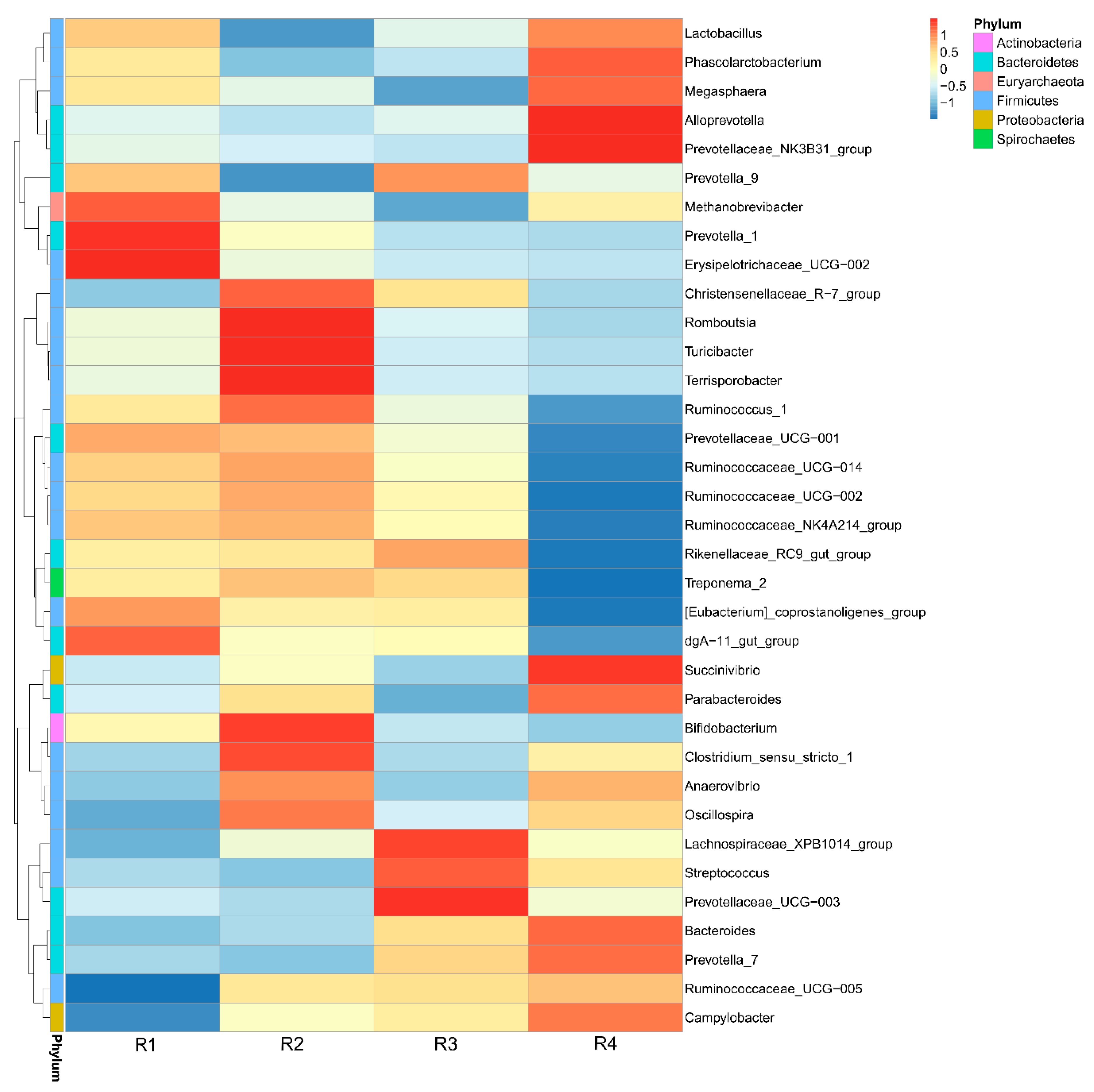
2.5.3. Changes in Relative Abundance at the Genus Level
2.6. Changes in Microbial Composition and Diversity in Piglet Colonic Contents
2.6.1. Changes in Colonic Microbial Diversity
2.6.2. Changes in Relative Abundance at the Phylum Level
2.6.3. Changes in Relative Abundance at the Genus Level
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals and Diets
4.3. Sampling Procedure
4.4. Analysis of Oxidative and Antioxidative Parameters
4.5. Analysis of Inflammatory Factors
4.6. Measurement of Gene Expression
4.7. Detection of Fecal SCFAs
4.8. Microbial Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| T-AOC | Total antioxidant capacity |
| CAT | Catalase |
| T-SOD | Total peroxide dismutase |
| GSH-Px | Glutathione peroxidase |
| MDA | Malondialdehyde |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor-α |
| SCFAs | Short-chain fatty acids |
| OTUs | Operational taxonomic units |
References
- Nappi, A.J.; Vass, E. Hydroxyl radical formation via iron-mediated Fenton chemistry is inhibited by methylated catechols. Biochim. Biophys. Acta General Subj. 1998, 1425, 159–167. [Google Scholar] [CrossRef]
- Lin, Y.; Han, X.F.; Fang, Z.F.; Che, L.Q.; Wu, D.; Wu, C.M. The beneficial effect of fiber supplementation in high- or low-fat diets on fetal development and antioxidant defense capacity in the rat. Eur. J. Nutr. 2012, 51, 19–27. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease, an epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Mutinati, M.; Pantaleo, M.; Roncetti, M.; Piccinno, M.; Rizzo, A.; Sciorsci, R. Oxidative stress in neonatology. A review. Reprod. Domest. Anim. 2014, 49, 7–16. [Google Scholar] [CrossRef]
- Barker, D. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Gohir, W.; Kennedy, K.M.; Wallace, J.G.; Saoi, M.; Britz-McKibbin, P.; Petrik, J.J.; Surette, M.G.; Sloboda, D.M. High-fat diet intake modulates maternal intestinal adaptations to pregnancy, and results in placental hypoxia and impaired fetal gut development. bioRxiv 2018, 436816. [Google Scholar] [CrossRef]
- Vuillermin, P.J.; Macia, L.; Nanan, R.; Tang, M.L.; Collier, F.; Brix, S. The maternal microbiome during pregnancy and allergic disease in the offspring. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 39, pp. 669–675. [Google Scholar]
- Wang, Y.S.; Zhou, P.; Liu, H.; Li, S.; Zhao, Y.; Deng, K.; Cao, D.D.; Che, L.Q.; Fang, Z.F.; Xu, S.Y.; et al. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 2016, 51, 492–500. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- de Agüero, M.G.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Paßlack, N.; Vahjen, W.; Zentek, J. Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet. Res. 2015, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- de Vega, J.M.A.; Díaz, J.; Serrano, E.; Carbonell, L.F. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit. Care Med. 2002, 30, 1782–1786. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1697. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Sheikh, S.Z.; Maharshak, N.; Otterbein, L.E.; Plevy, S.E. Heme oxygenase-1 and carbon monoxide regulate intestinal homeostasis and mucosal immune responses to the enteric microbiota. Gut Microbes 2014, 5, 220–224. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Li, H.; Smidt, H.; Wright, A.D.G.; Zhang, K.; Ding, X.; Zeng, Q.; Bai, S.; Wang, J.; et al. Different types of dietary fibers trigger specific alterations in composition and predicted functions of colonic bacterial communities in BALB/c mice. Front. Microbiol. 2017, 8, 966. [Google Scholar] [CrossRef]
- Jonathan, M.C.; Borne, J.J.G.C.v.d.; Wiechen, P.V.; Silva, C.S.D.; Scholsa, H.A. In vitro fermentation of 12 dietary fibres by faecal inoculum from pigs and humans. Food Chem. 2012, 133, 889–897. [Google Scholar] [CrossRef]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O. Mechanisms of tissue injury by oxygen radicals, implications for neonatal disease. Acta Paediatr. 1996, 85, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Oxidative stress in the newborn—A 30-year perspective. Neonatology 2005, 88, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Kendall, M.J.; Dunne, F. Oxidative stress and normal pregnancy. Clin. Endocrinol. 2010, 57, 609–613. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clin. Lipidol. 2010, 5, 899–911. [Google Scholar] [CrossRef]
- Mou, D.; Wang, J.; Liu, H.; Chen, Y.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Feng, B.; Li, J.; et al. Maternal methyl donor supplementation during gestation counteracts bisphenol A—Induced oxidative stress in sows and offspring. Nutrition 2018, 45, 76–84. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging, A role for prostate cancer? Biochim. Biophys. Acta 2009, 1795, 83–91. [Google Scholar] [CrossRef]
- David, M.; Munaswamy, V.; Halappa, R.; Marigoudar, S.R. Impact of sodium cyanide on catalase activity in the freshwater exotic carp, Cyprinus carpio (Linnaeus). Pestic. Biochem. Physiol. 2008, 92, 15–18. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, F.; Duan, L.-R.; Sheng, J.-J.; Xie, Y.-H.; Yang, Q.; Chen, Y.; Dong, Q.Q.; Zhang, B.L.; Wang, S.W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress, Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. UK 2016, 6, 23693. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Hou, Y.; Wang, L.; Ding, B.; Yang, Z.; Li, J.; Long, M.; Liu, Y.; Wu, G. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Brit. J. Nutr. 2014, 111, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury, role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420. [Google Scholar]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.-S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology, from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Maes, M.; Scharpé, S.; Meltzer, H.Y.; Bosmans, E.; Suy, E.; Calabrese, J.; Cosyns, P. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993, 49, 11–27. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6, regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I. Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 2004, 39, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Van Antwerp, D.J.; Martin, S.J.; Kafri, T.; Green, D.R.; Verma, I.M. Suppression of TNF-α-induced apoptosis by NF-κB. Science 1996, 274, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Xu, C.; Xie, X.; Jiang, S.; Peng, J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microbiol. 2018, 84, e01047-18. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- King, D.E. Dietary fiber, inflammation, and cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 594–600. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. BioMed Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer, how are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, H.; Yang, Y.; He, J.; Cao, M.; Yang, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; et al. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals 2019, 9, 422. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027. [Google Scholar] [CrossRef] [PubMed]
- Tsatsaronis, J.A.; Walker, M.J.; Sanderson-Smith, M.L. Host responses to group a streptococcus, cell death and inflammation. PLoS Pathog. 2014, 10, e1004266. [Google Scholar] [CrossRef] [PubMed]
- Heuvelin, E.; Lebreton, C.; Grangette, C.; Pot, B.; Cerf-Bensussan, N.; Heyman, M. Mechanisms involved in alleviation of intestinal inflammation by Bifidobacterium breve soluble factors. PLoS ONE 2009, 4, e5184. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kamiya, S.; Hanawa, T.; Fukuda, M.; Kawakami, H.; Takahashi, H.; Yokota, H. Effect of probiotic bacterial strains of Lactobacillus, Bifidobacterium, and Enterococcus on enteroaggregative Escherichia coli. J. Infect. Chemother. 2010, 16, 10–18. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, E.J.; Lee, J.C.; Kim, W.K.; Kim, H.S. Anti-inflammatory effects of short chain fatty acids in IFN-γ-stimulated RAW 264.7 murine macrophage cells, Involvement of NF-κB and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease, the multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.L.; Deng, X.; Zhu, T.T.; Xiong, J.F.; Xu, Y.H.; Xu, Y. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Yaku, K.; Enami, Y.; Kurajyo, C.; Matsui-Yuasa, I.; Konishi, Y.; Kojima-Yuasa, A. The enhancement of phase 2 enzyme activities by sodium butyrate in normal intestinal epithelial cells is associated with Nrf2 and p53. Mol. Cell. Biochem. 2012, 370, 7–14. [Google Scholar] [CrossRef]
- Place, R.F.; Noonan, E.J.; Giardina, C. HDAC inhibition prevents NF-κB activation by suppressing proteasome activity, down-regulation of proteasome subunit expression stabilizes IκBα. Biochem. Pharmacol. 2005, 70, 394–406. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Leblois, J.; Massart, S.; Li, B.; Wavreille, J.; Bindelle, J.; Everaert, N. Modulation of piglets’ microbiota, differential effects by a high wheat bran maternal diet during gestation and lactation. Sci. Rep. UK 2017, 7, 7426. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. UK 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Gohir, W.; Whelan, F.J.; Surette, M.G.; Moore, C.; Schertzer, J.D.; Sloboda, D.M. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes 2015, 6, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Harris, R.A.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Rolhion, N.; Darfeuille-Michaud, A. Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 1277–1283. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, G.; Yao, Y.; Guo, S.; Lu, K.; Sheng, Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J. Trauma 2006, 61, 650–657. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter infection. New Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef]
- Joly-Guillou, M.L. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 2005, 11, 868–873. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii, emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhao, Y.; Zhang, P.; Li, Y.; Gui, T.; Wang, J.; Jin, C.; Che, L.; Li, J.; Lin, Y.; et al. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front. Microbiol. 2017, 8, 2242. [Google Scholar] [CrossRef] [PubMed]
| Parameters 2 | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||
| Sow plasma | |||||
| T-AOC, U/mL | 1.16 ± 0.21 | 1.70 ± 0.14 | 1.90 ± 0.31 | 1.44 ± 0.30 | 0.202 |
| CAT, U/mL | 5.95 ± 0.37 a | 5.70 ± 0.24 a | 3.05 ± 0.19 b | 2.22 ± 0.22 c | <0.001 |
| T-SOD, U/mL | 0.94 ± 0.02 | 0.95 ± 0.05 | 0.83 ± 0.03 | 0.86 ± 0.02 | 0.052 |
| GSH-Px, U/mL | 1012.42 ± 51.68 | 941.36 ± 80.10 | 946.68 ± 47.26 | 966.66 ± 31.76 | 0.860 |
| MDA, mmol/mL | 3.19 ± 0.30 bc | 2.57 ± 0.29 c | 3.54 ± 0.25 ab | 4.19 ± 0.24 a | 0.004 |
| Piglet plasma | |||||
| T-AOC, U/mL | 3.70 ± 0.15 a | 4.06 ± 0.53 a | 3.14 ± 0.52 ab | 2.22 ± 0.49 b | 0.035 |
| CAT, U/mL | 4.47 ± 0.35 | 5.47 ± 0.71 | 4.45 ± 0.12 | 3.55 ± 0.44 | 0.054 |
| T-SOD, U/mL | 0.27 ± 0.04 | 0.27 ± 0.05 | 0.25 ± 0.04 | 0.25 ± 0.04 | 0.977 |
| GSH-Px, U/mL | 266.16 ± 31.82 a | 223.66 ± 21.67 a | 112.25 ± 20.80 b | 104.49 ± 22.64 b | <0.001 |
| MDA, mmol/mL | 4.72 ± 0.23 | 5.16 ± 0.60 | 5.41 ± 0.41 | 6.08 ± 0.96 | 0.440 |
| Parameters 2 | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||
| T-AOC, U/mL | 2.38 ± 0.58 | 2.59 ± 0.52 | 2.16 ± 0.56 | 2.22 ± 0.30 | 0.924 |
| CAT, U/mL | 11.75 ± 0.94 a | 11.00 ± 0.90 a | 7.47 ± 1.03 ab | 3.55 ± 0.69 b | 0.041 |
| T-SOD, U/mL | 16.65 ± 2.18 | 17.64 ± 2.46 | 13.26 ± 1.64 | 10.35 ± 1.20 | 0.059 |
| GSH-Px, U/mL | 68.99 ± 12.39 a | 50.68 ± 7.20 ab | 14.21 ± 1.71 c | 32.71 ± 5.56 bc | 0.001 |
| MDA, mmol/mL | 1.22 ± 0.46 | 1.03 ± 0.19 | 1.76 ± 0.46 | 0.97 ± 0.22 | 0.404 |
| Items 2 | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||
| Sow plasma | |||||
| IL-2, pg/mL | 479.67 ± 39.03 | 527.00 ± 47.89 | 544.80 ± 34.18 | 458.03 ± 29.60 | 0.423 |
| IL-6, ng/L | 942.65 ± 51.78 b | 919.20 ± 52.88 b | 1152.62 ± 62.17 a | 1128.28 ± 52.20 a | 0.012 |
| IL-10, ng/L | 177.30 ± 10.13 | 191.15 ± 14.68 | 170.81 ± 9.18 | 198.84 ± 14.94 | 0.458 |
| TNF-α, pg/mL | 389.76 ± 34.64 | 435.95 ± 31.29 | 405.95 ± 40.61 | 350.33 ± 20.91 | 0.303 |
| Piglet plasma | |||||
| IL-2, pg/mL | 542.91 ± 38.96 | 581.53 ± 34.17 | 493.44 ± 23.85 | 464.82 ± 18.47 | 0.068 |
| IL-6, ng/L | 968.27 ± 109.26 | 1013.16 ± 96.44 | 1087.09 ± 68.82 | 1030.72 ± 95.86 | 0.827 |
| IL-10, ng/L | 177.49 ± 11.23 | 185.24 ± 13.79 | 190.35 ± 14.62 | 195.09 ± 14.42 | 0.830 |
| TNF-α, pg/mL | 310.51 ± 21.47 a | 392.80 ± 35.92 ab | 485.21 ± 10.60 c | 448.17 ± 32.04 bc | 0.002 |
| Parameters 2 | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||
| Sow feces | |||||
| Acetate | 47.17 ± 2.73 a | 42.56 ± 1.05 ab | 36.12 ± 2.54 bc | 31.97 ± 3.75 c | 0.005 |
| Propionate | 18.34 ± 1.45 a | 18.50 ± 1.22 a | 17.09 ± 1.60 ab | 12.87 ± 1.13 b | 0.048 |
| Butyrate | 9.01 ± 0.65 | 7.52 ± 0.48 | 8.75 ± 2.19 | 6.09 ± 1.35 | 0.425 |
| Total SCFAs 2 | 74.52 ± 4.66 a | 68.59 ± 2.04 a | 61.96 ± 2.10 ab | 50.93 ± 6.59 b | 0.008 |
| Piglet colonic contents | |||||
| Acetate | 14.72 ± 1.62 a | 12.78 ± 0.97 a | 6.59 ± 1.09 b | 4.14 ± 1.23 b | 0.001 |
| Propionate | 0.62 ± 0.24 | 0.78 ± 0.20 | 0.30 ± 0.10 | 0.20 ± 0.02 | 0.082 |
| Butyrate | 1.76 ± 0.46 a | 1.60 ± 0.32 a | 0.59 ± 0.18 b | 0.64 ± 0.26 b | 0.031 |
| Total SCFAs 2 | 17.10 ± 2.21 a | 15.15 ± 0.87 a | 7.47 ± 1.32 b | 4.99 ± 1.51 b | 0.001 |
| Taxonomy, % | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | ||
| Firmicutes | 52.57 ± 2.07 | 55.87 ± 1.77 | 55.48 ± 2.21 | 54.83 ± 2.79 | 0.663 |
| Bacteroidetes | 33.06 ± 1.79 | 28.94 ± 1.69 | 32.42 ± 2.53 | 32.89 ± 2.32 | 0.368 |
| Spirochaetes | 4.50 ± 0.89 ab | 5.14 ± 0.65 a | 4.93 ± 0.92 a | 2.20 ± 0.63 b | 0.020 |
| Proteobacteria | 2.63 ± 0.39 b | 3.40 ± 0.46 ab | 2.40 ± 0.26 b | 4.48 ± 0.72 a | 0.009 |
| Tenericutes | 2.17 ± 0.17 a | 1.61 ± 0.13 ab | 1.42 ± 0.25 ab | 1.06 ± 0.23 b | 0.013 |
| Euryarchaeota | 2.37 ± 0.33 | 1.45 ± 0.34 | 0.98 ± 0.38 | 1.70 ± 0.33 | 0.095 |
| Actinobacteria | 1.30 ± 0.23 ab | 2.03 ± 0.66 a | 0.97 ± 0.09 b | 0.92 ± 0.08 b | 0.018 |
| Verrucomicrobia | 0.96 ± 0.23 | 0.75 ± 0.11 | 0.72 ± 0.06 | 0.54 ± 0.09 | 0.133 |
| Cyanobacteria | 0.35 ± 0.12 | 0.19 ± 0.03 | 0.21 ± 0.04 | 0.47 ± 0.23 | 0.106 |
| Planctomycetes | 0.13 ± 0.03 | 0.23 ± 0.08 | 0.19 ± 0.12 | 0.27 ± 0.14 | 0.567 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Feng, B.; Xu, S.; et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. Int. J. Mol. Sci. 2020, 21, 31. https://doi.org/10.3390/ijms21010031
Li Y, Liu H, Zhang L, Yang Y, Lin Y, Zhuo Y, Fang Z, Che L, Feng B, Xu S, et al. Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. International Journal of Molecular Sciences. 2020; 21(1):31. https://doi.org/10.3390/ijms21010031
Chicago/Turabian StyleLi, Yang, Haoyu Liu, Lijia Zhang, Yi Yang, Yan Lin, Yong Zhuo, Zhengfeng Fang, Lianqiang Che, Bin Feng, Shengyu Xu, and et al. 2020. "Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model" International Journal of Molecular Sciences 21, no. 1: 31. https://doi.org/10.3390/ijms21010031
APA StyleLi, Y., Liu, H., Zhang, L., Yang, Y., Lin, Y., Zhuo, Y., Fang, Z., Che, L., Feng, B., Xu, S., Li, J., & Wu, D. (2020). Maternal Dietary Fiber Composition during Gestation Induces Changes in Offspring Antioxidative Capacity, Inflammatory Response, and Gut Microbiota in a Sow Model. International Journal of Molecular Sciences, 21(1), 31. https://doi.org/10.3390/ijms21010031






