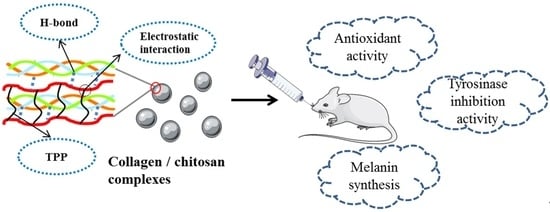
Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis
Abstract
:1. Introduction
2. Results and Discussion
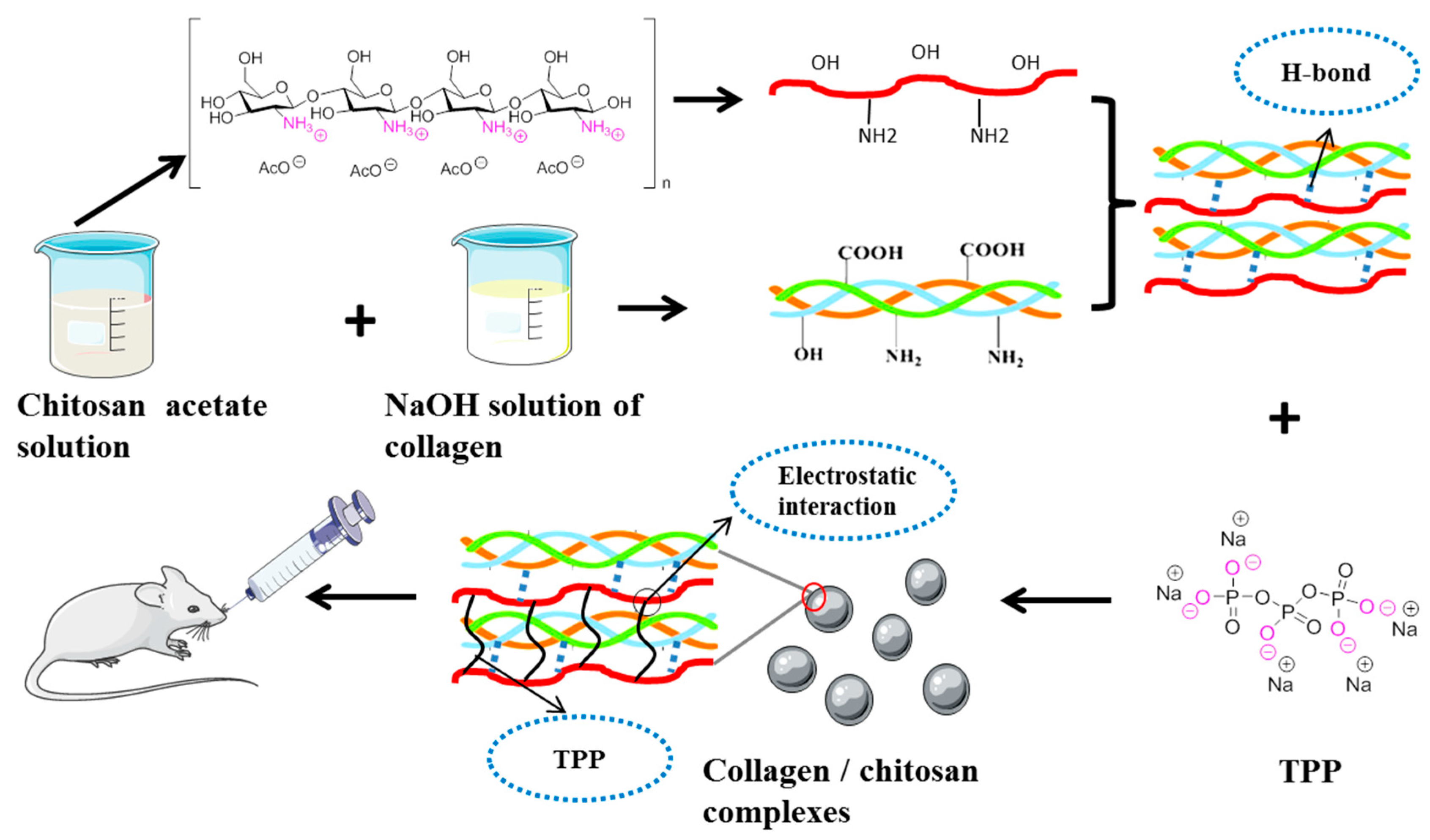
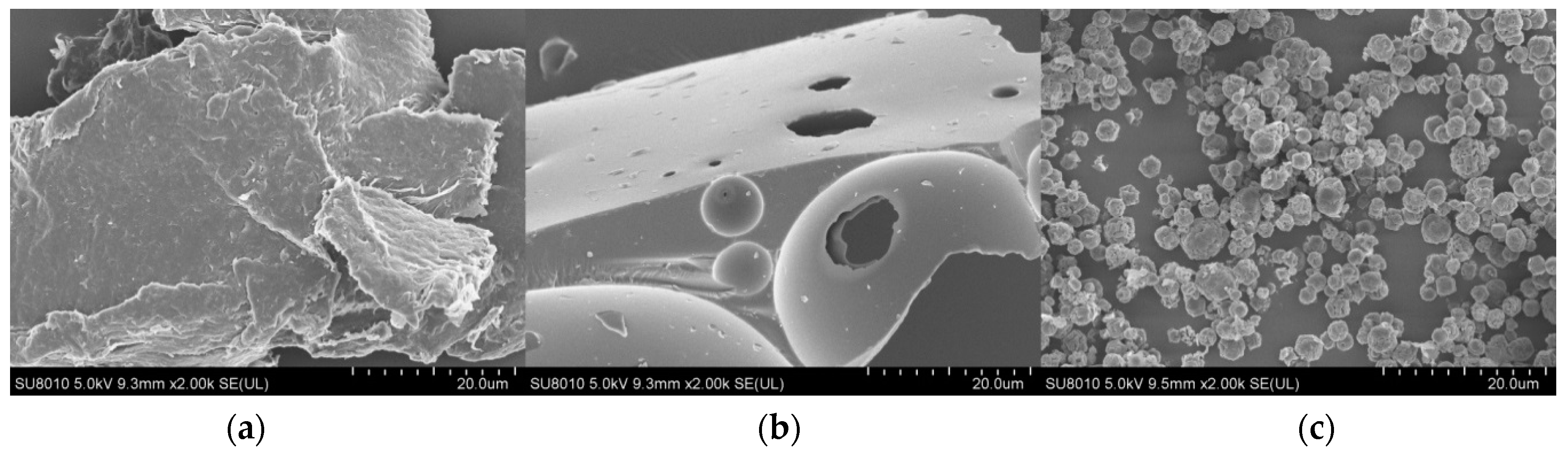
2.1. Preparation of the Collagen/Chitosan Complexes and Surface Morphology
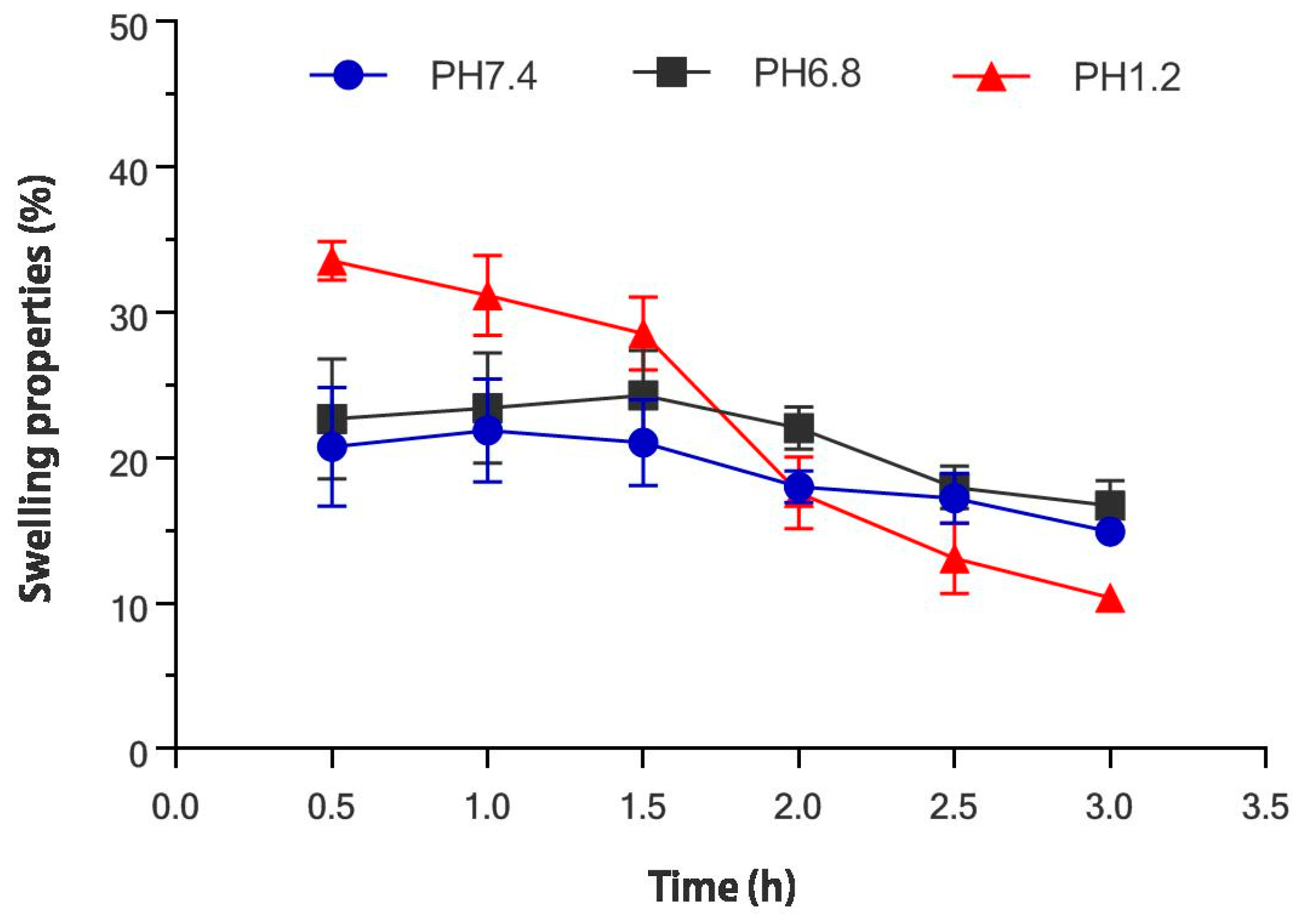
2.2. Swelling Properties
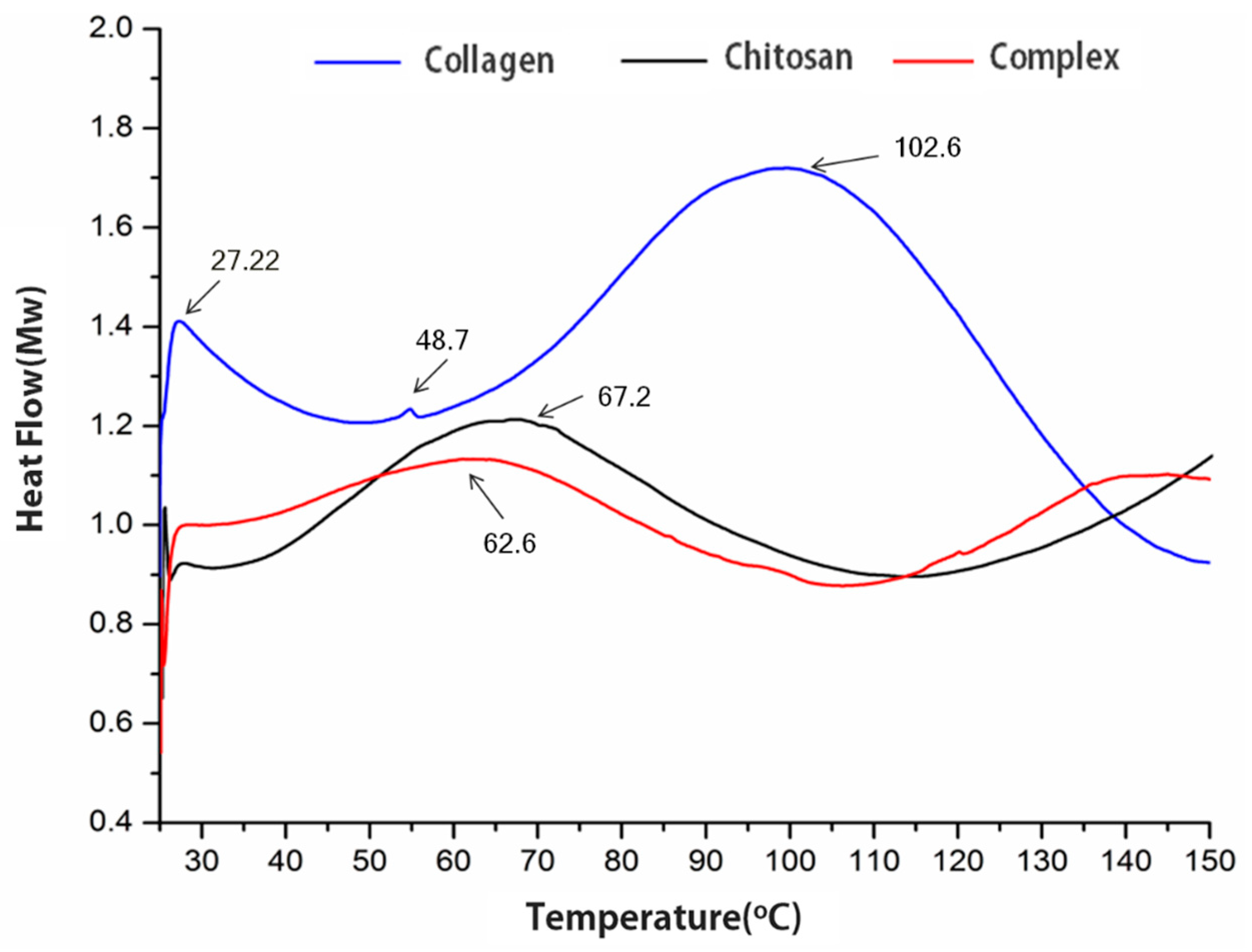
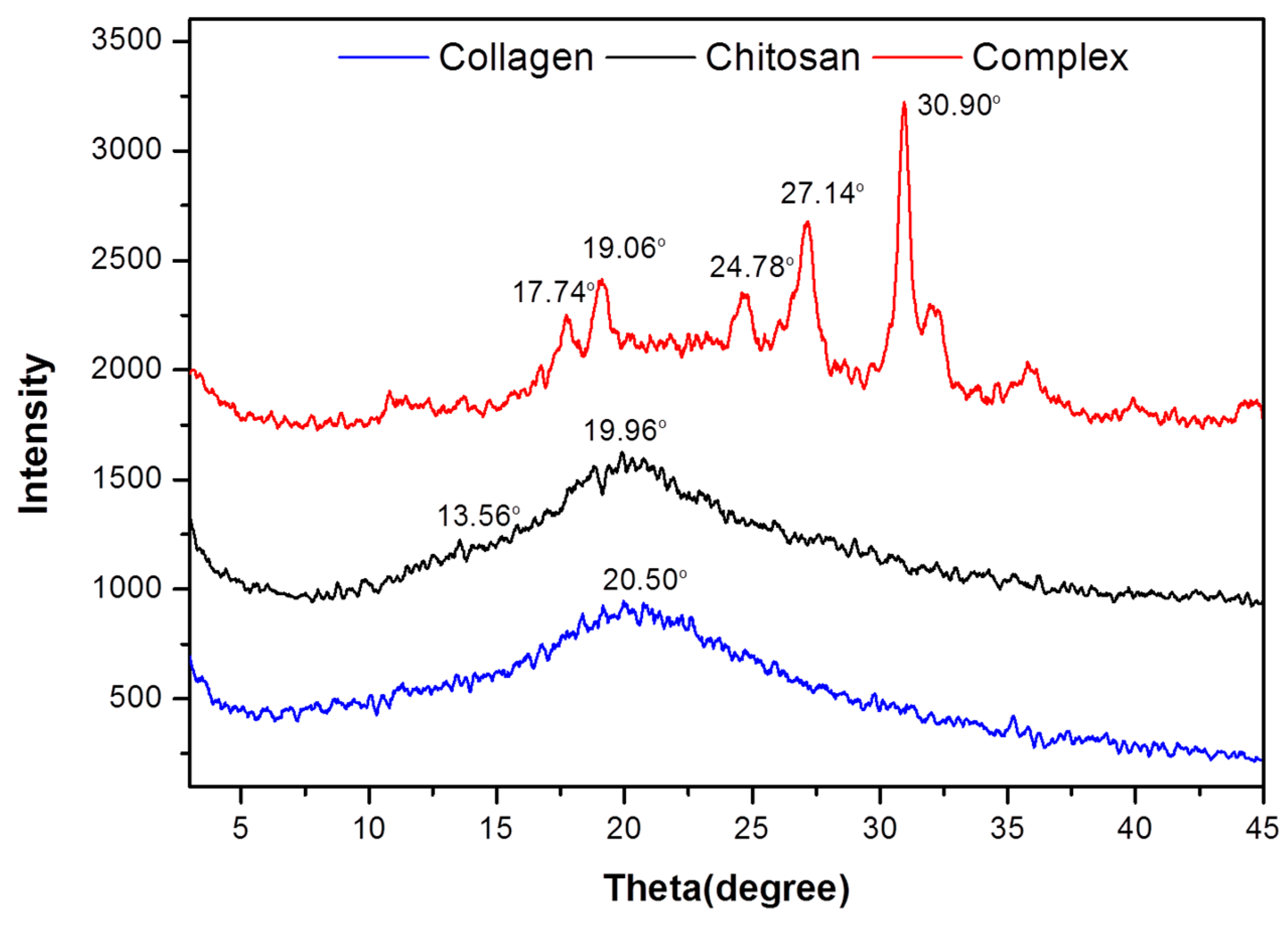
2.3. DSC and XRD Analysis
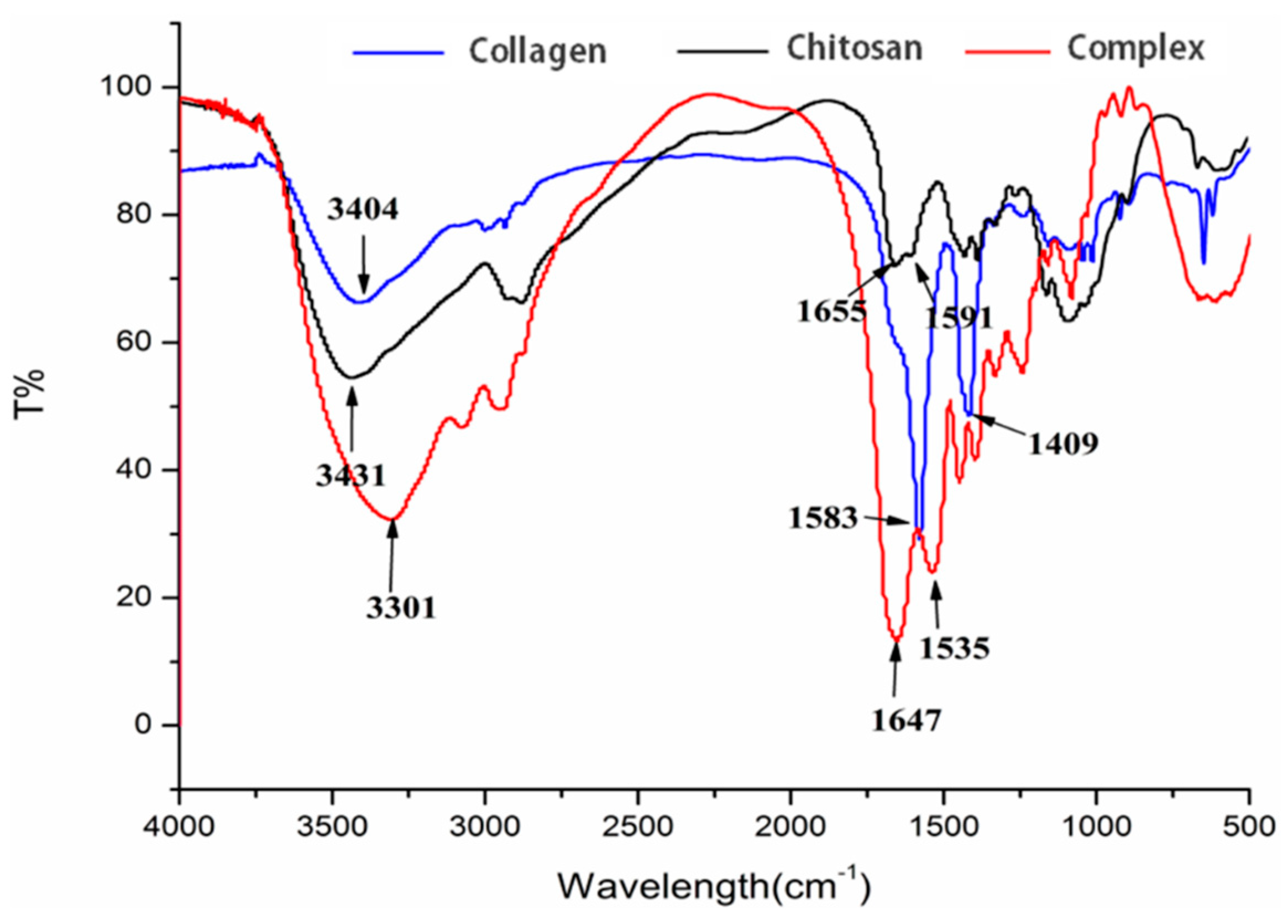
2.4. Fourier Transform Infrared Spectrum (FTIR) Identification
2.5. Antioxidation Activity In Vivo
2.6. Effect on Melanin Synthesis
2.6.1. Determination of Melanin Content in B16 Melanoma Cells
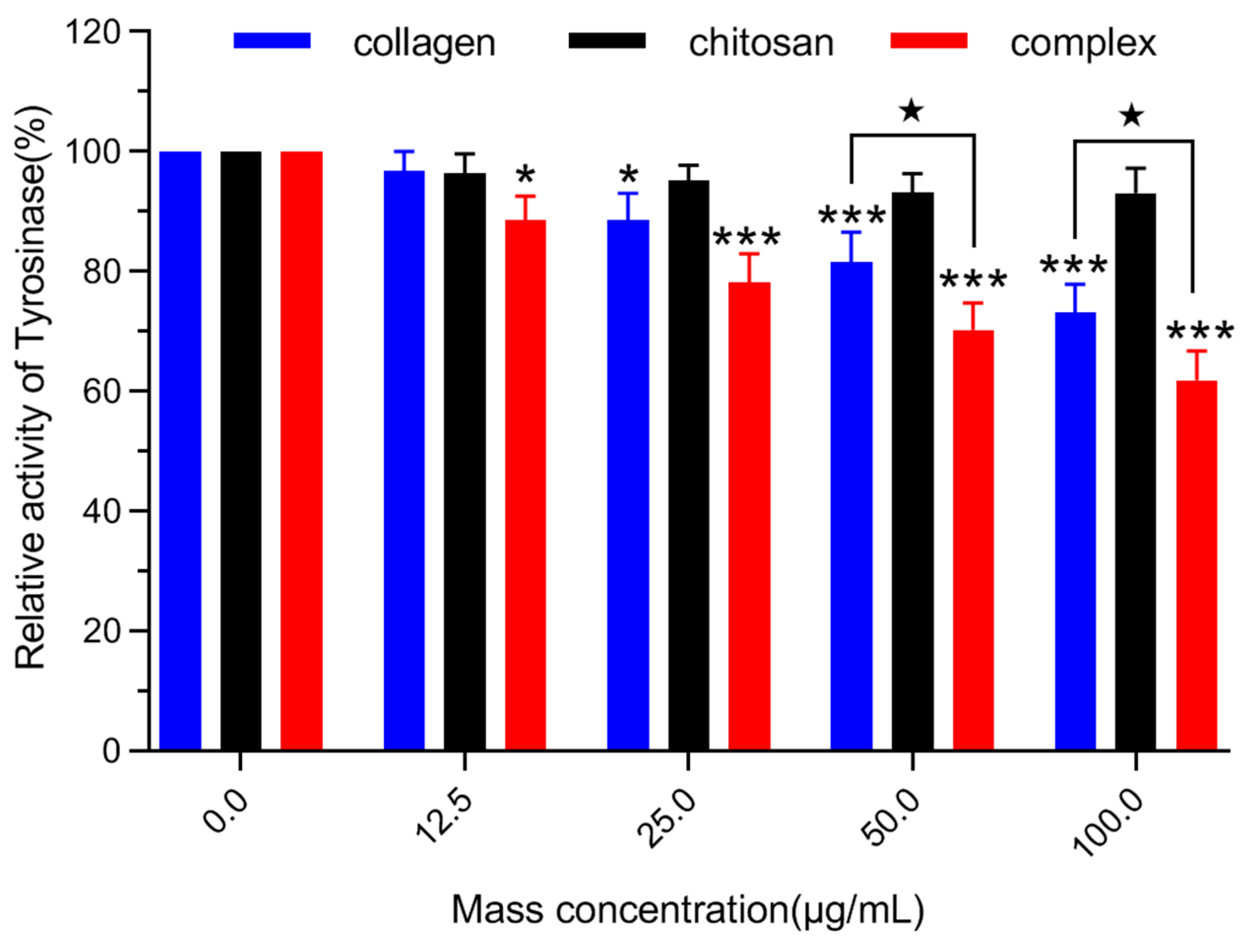
2.6.2. Detection of Tyrosinase Activity in B16 Melanoma Cells
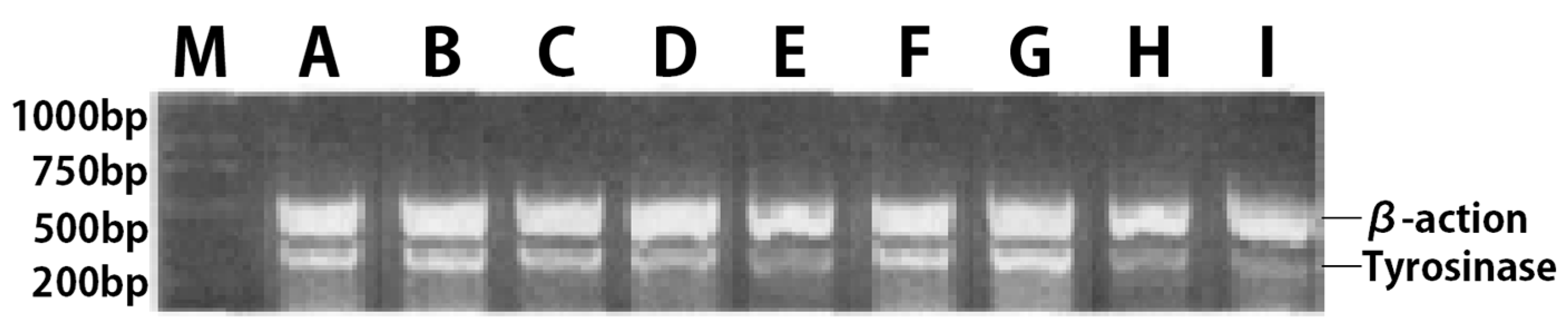
2.6.3. Detection of Tyrosinase mRNA Expression in B16 Melanoma Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan Acetic Acid Solution
3.3. Complex Preparation
3.4. Physicochemical Characterization of Complex
3.4.1. Scanning Electron Microscopy
3.4.2. Swelling Properties
3.4.3. Differential Scanning Calorimetry
3.4.4. X-ray Diffraction Analysis
3.4.5. Fourier Transform Infrared Spectrum Identification
3.5. Antioxidant Activity In Vivo
3.6. Effect of Melanin Synthesis
3.6.1. Determination of Melanin Content
3.6.2. Detection of Tyrosinase Activity
3.6.3. Detection of Tyrosinase mRNA Expression
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.I.; Sotelo, C.G. Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Mar. Drugs 2018, 16, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Li, J.; Guan, H. The antioxidant effects of complexes of tilapia fish skin collagen and different marine oligosaccharides. J. Ocean Univ. China 2010, 9, 399–407. [Google Scholar] [CrossRef]
- Kumar, B.; Rani, S. Technical note on the isolation and characterization of collagen from fish waste material. Int. J. Food Sci. Technol. 2017, 54, 276–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saidi, G.; Rahman, M.S.; Al-Alawi, A.; Guizani, N. Thermal characteristics of gelatin extracted from shaari fish skin. J. Therm. Anal. Calorim. 2011, 104, 593–603. [Google Scholar] [CrossRef]
- Blanco, M.; Vazquez, J.A.; Perez-Martin, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Zhaoming, W.U.; Jingjing, L.I.; Yan, Z.; Zhao, H.; Jianen, H.U.; Long, W.U. Advances in Biological Activity and Application of Fish Skin Collagen-Derived Oligopeptides. Food Technol. 2018, 29, 304–310. [Google Scholar]
- Gauza, M.; Kubisz, L. Effect of water content on electrical conductivity of fish skin collagen. J. Non Cryst. Solids 2011, 357, 686–690. [Google Scholar] [CrossRef]
- Heu, M.S.; Lee, J.H.; Kim, H.J.; Jee, S.J.; Lee, J.S.; Jeon, Y.J.; Shahidi, F.; Kim, J. Characterization of acid- and pepsin-soluble collagens from flatfish skin. Food Sci. Biotechnol. 2010, 19, 27–33. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella). J. Food Sci. 2010, 72, E450–E455. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singaravelu, S.; Raja, M.D.; Sobhana, S.S.L.; Sivagnanam, U.T. Extraction and Characterization of Collagen from the Skin of Arothron stellatus Fish—A Novel Source of Collagen for Tissue Engineering. J. Biomater. Tissue Eng. 2014, 4, 203–209. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.C.; Oh, G.W.; Heo, S.Y.; Nguyen, V.T.; Jeon, Y.J.; Lee, B.; Jang, C.H.; Kim, G.H.; Park, W.S. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int. J. Biol. Macromol. 2015, 81, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, S.; Velswamy, P.; Uma, T.S. Isolation and Characterization of Biocompatible Collagen from the Skin of Puffer Fish (Lagocephalus inermis). J. Polym. Environ. 2017, 26, 2086–2095. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2017, 109, 273–286. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Rostamizadeh, K.; Rezaei, S.; Abdouss, M.; Sadighian, S.; Arish, S. A hybrid modeling approach for optimization of PMAA–chitosan–PEG nanoparticles for oral insulin delivery. RSC Adv. 2015, 5, 69152–69160. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, X.; Zhang, H.; Lin, D.; Yao, S.; Ling, J. Determination of Apparent Drug Permeability Coefficients through Chitosan-Sodium Cellulose Sulfate Polyelectrolyte Complex Films. Acta Phys. Chim. Sin. 2014, 30, 365–370. [Google Scholar]
- Sun, B.; Ma, W.; Su, F.; Wang, Y.; Liu, J.; Wang, D.; Liu, H. The osteogenic differentiation of dog bone marrow mesenchymal stem cells in a thermo-sensitive injectable chitosan/collagen/β-glycerophosphate hydrogel: In vitro and in vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Woods, B.; Marques, A.L.P.; Moreira-Silva, J.; Silva, T.H.; Cryan, S.A.; Rui, L.R.; O’Brien, F.J. Multifunctional Biomaterials from the Sea: Assessing the effects of Chitosan incorporation into Collagen Scaffolds on Mechanical and Biological Functionality. Acta Biomater. 2016, 43, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive-A review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tan, F.J.; Marra, K.G.; Jan, S.S.; Liu, D.C. Synthesis and characterization of collagen/hyaluronan/chitosan composite sponges for potential biomedical applications. Acta Biomater. 2009, 5, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, C.; Nandhini, D.P.; Pandimadevi, M. Preparation and Characterization of Biomaterial from Collagen, Chitosan and Hibiscus rosa-sinensis Nanoparticles. Int. J. Pharm. Sci. Rev. Res. 2016, 38, 190–197. [Google Scholar]
- Tan, W.; Krishnaraj, R.; Desai, T. Evaluation of nanostructured composite collagen--chitosan matrices for tissue engineering. Tissue Eng. 2001, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Cuy, J.L.; Beckstead, B.L.; Brown, C.D.; Hoffman, A.S.; Giachelli, C.M. Adhesive protein interactions with chitosan: Consequences for valve endothelial cell growth on tissue-engineering materials. J. Biomed. Mater. Res. Part A 2003, 67, 538. [Google Scholar] [CrossRef]
- Kishen, A.; Shrestha, S.; Shrestha, A.; Cheng, C.; Goh, C. Characterizing the collagen stabilizing effect of crosslinked chitosan nanoparticles against collagenase degradation. Dent. Mater. 2016, 32, 968–977. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Krishnamoorthy, G.; Ramkumar, K.M.; Raichur, A.M. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: An effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 378–385. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Mu, C. Preparation and biological characteristics of collagen-chitosan composite scaffold:Proportion of chitosan and collagen. J. Clin. Rehabil. Tissue Eng. Res. 2010, 14, 5367–5370. [Google Scholar]
- Rubina, M.S.; Kamitov, E.E.; Zubavichus, Y.V.; Peters, G.S.; Naumkin, A.V.; Suzer, S.; Vasil’Kov, A.Y. Collagen-chitosan scaffold modified with Au and Ag nanoparticles: Synthesis and structure. Appl. Surf. Sci. 2016, 366, 365–371. [Google Scholar] [CrossRef]
- Cho, M.H.; Kim, K.S.; Ahn, H.H.; Kim, M.S.; Kim, S.H.; Khang, G.; Lee, B.; Lee, H.B. Chitosan gel as an in situ-forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng. Part A 2008, 14, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Hemant, K.S.Y.; Shivakumar, H.G. Development of Chitosan Acetate Films for Transdermal Delivery of Propranolol Hydrochloride. Trop. J. Pharm. Res. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Ren, B.; Wang, F.; Zhang, Q. Preparation and characterization of collagen-hydroxyapatite composite used for bone tissue engineering scaffold. Artif. Cells Blood Substit. Immobil. Biotechnol. 2003, 31, 435–448. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Synthesis and characterization of a novel amphiphilic chitosan?polylactide graft copolymer. Carbohyd. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Jin, W.; Fei, J.; Xu, Q.; Chen, D.; He, J. Alginic acid oligosaccharide accelerates weaned pig growth through regulating antioxidant capacity, immunity and intestinal development. RSC Adv. 2016, 6, 87026–87035. [Google Scholar]
- Dmitriev, L.F.; Titov, V.N. Lipid peroxidation in relation to ageing and the role of endogenous aldehydes in diabetes and other age-related diseases. Ageing Res. Rev. 2010, 9, 200–210. [Google Scholar] [CrossRef]
- Antonyan, I.S. Role of Dopaminergic Mechanisms in the Formation of Stress-Induced Ulcer Damages to the Duodenum and Stomach in Rats. Neirofiziologiia 2012, 44, 79–82. [Google Scholar] [CrossRef]
- Wen, Z.S.; Liu, L.J.; Ouyang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef]
- Bafakih, F.F.; Gyure, K.A. Melanocytic Lesions of the Central Nervous System. AJSP Rev. Rep. 2013, 18, 262–265. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Pigment Cell. Res. 2010, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Menter, J.M.; Townsel, M.E.; Moore, C.L.; Williamson, G.D.; Soteres, B.J.; Fisher, M.S.; Willis, I. Melanin accelerates the tyrosinase-catalyzed oxygenation of p-hydroxyanisole (MMEH). Pigment Cell. Res. 2010, 3, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Tamara, F.R.; Lin, C.; Mi, F.L.; Ho, Y.C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. J. Nanomater. 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.J.; Park, M.; Choi, H.R.; Kang, H.S. P047 Role of IL-18 in the melanin synthesis of B16F10 melanoma cells. Cytokine 2012, 59, 533–534. [Google Scholar] [CrossRef]
- Shen, T.; Heo, S.I.; Wang, M.H. Involvement of the p38 MAPK and ERK signaling pathway in the anti-melanogenic effect of methyl 3,5-dicaffeoyl quinate in B16F10 mouse melanoma cells. Chem. Biol. Interact. 2012, 199, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Inhibitory Effect of Saponin Consituents from Xanthoceras Nutshell on Tyrosinase Activity. J. Chin. Cereals Oils Assoc. 2013, 28, 53–56. [Google Scholar]
- Wan, X.; Chai, B.; Liao, Y.; Su, Y.; Ye, T.; Shen, P.; Chen, X. Molecular and biochemical characterization of a distinct tyrosinase involved in melanin production from Aeromonas media. Appl. Microbiol. Biotechnol. 2009, 82, 261–269. [Google Scholar] [CrossRef]
- Satooka, H.; Cerda, P.; Kim, H.J.; Wood, W.F.; Kubo, I. Effects of matsutake mushroom scent compounds on tyrosinase and murine B16-F10 melanoma cells. Biochem. Biophys. Res. Commun. 2017, 487, 840–846. [Google Scholar] [CrossRef]

| Group | Dose (mg/(kg·day)) | Animal (n) | MDA nmol/mL | SOD × 103 Nu/mL |
|---|---|---|---|---|
| Young control | - | 10 | 5.68 ± 1.23 ** | 7.64 ± 0.87 ** |
| Aged control | - | 10 | 10.24 ± 1.15 | 5.57 ± 0.38 |
| Chitosan | 200 | 10 | 8.81 ± 1.06 * | 6.17 ± 0.12 * |
| Collagen | 200 | 10 | 8.03 ± 1.46 ** | 6.54 ± 0.57 ** |
| Complex | 400 (contain same amount of collagen and chitosan) | 10 | 7.61 ± 1.43 ** | 6.77 ± 0.33 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. https://doi.org/10.3390/ijms21010313
Hua Y, Ma C, Wei T, Zhang L, Shen J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. International Journal of Molecular Sciences. 2020; 21(1):313. https://doi.org/10.3390/ijms21010313
Chicago/Turabian StyleHua, Yingying, Chenjun Ma, Tiantian Wei, Liefeng Zhang, and Jian Shen. 2020. "Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis" International Journal of Molecular Sciences 21, no. 1: 313. https://doi.org/10.3390/ijms21010313
APA StyleHua, Y., Ma, C., Wei, T., Zhang, L., & Shen, J. (2020). Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. International Journal of Molecular Sciences, 21(1), 313. https://doi.org/10.3390/ijms21010313