NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain
Abstract
1. Introduction
2. Results
2.1. Comparison of the Immunoreactive Profile of NDRG2 with a Panel of Astrocyte Markers
2.2. NDRG2 Does Not Correlate with the Expression of Other Astrocyte Markers
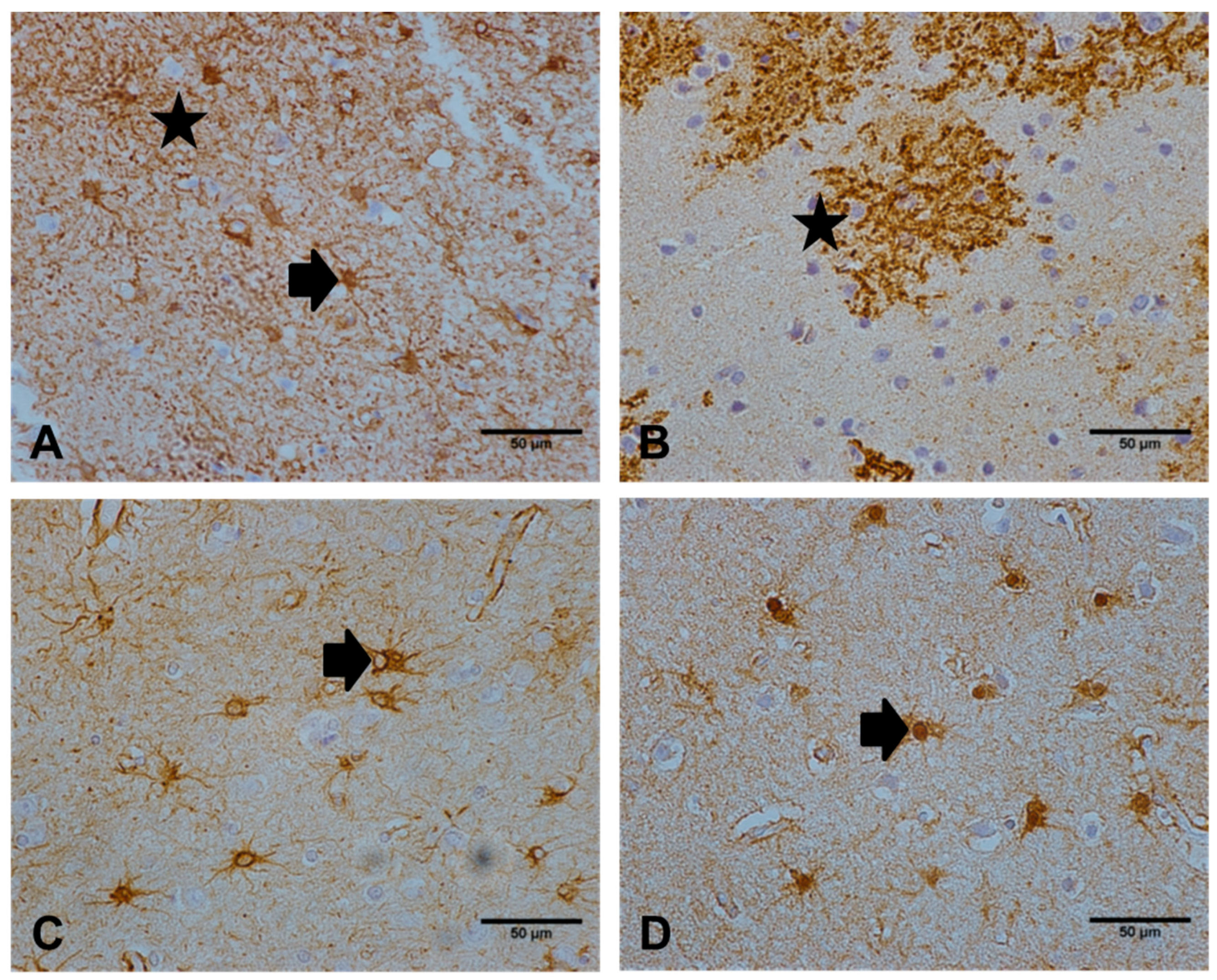
2.3. Not All Astrocytes are NDRG2+
2.4. NDRG2 Expression Correlates with Local Tau Pathology
2.5. NDRG2 Expression Correlates with CD68 but not with Oxidative DNA Damage
3. Discussion
4. Materials and Methods
4.1. Human CNS Cases
4.2. Immunohistochemistry
4.3. Quantitative Analysis of NDRG2
4.4. Previously Assessed Astrocyte Markers and Age-Associated Pathology
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AD | Alzheimer’s disease |
| CFANS | Cognitive Function and Ageing Study |
| CFAS | Cognitive Function and Ageing Neuropathology Study |
| CNS | Central nervous system |
| EAAT2 | Excitatory amino acid transporter 2 |
| GFAP | Glial fibrillary acidic protein |
| GS | Glutamine synthetase |
| NDRG2 | N-myc downstream regulated gene 2 |
| NFT | Neurofibrillary tangle |
References
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar] [CrossRef]
- Masliah, E.; Alford, M.; DeTeresa, R.; Mallory, M.; Hansen, L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Broer, S.; Brookes, N. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 2001, 77, 705–719. [Google Scholar] [CrossRef]
- Newington, J.T.; Harris, R.A.; Cumming, R.C. Reevaluating Metabolism in Alzheimer’s Disease from the Perspective of the Astrocyte-Neuron Lactate Shuttle Model. J. Neurodegener. Dis. 2013, 2013, 234572. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging 2010, 31, 578–590. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Olabarria, M.; Noristani, H.N.; Yeh, C.Y.; Rodriguez, J.J. Astrocytes in Alzheimer’s disease. Neurotherapeutics 2010, 7, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhai, Y.; Wei, H.; Zhang, C.; Xing, G.; Yu, Y.; He, F. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol. Cell Biochem. 2002, 229, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Flugge, G.; Araya-Callis, C.; Garea-Rodriguez, E.; Stadelmann-Nessler, C.; Fuchs, E. NDRG2 as a marker protein for brain astrocytes. Cell Tissue Res. 2014, 357, 31–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okuda, T.; Kokame, K.; Miyata, T. Differential expression patterns of NDRG family proteins in the central nervous system. J. Histochem. Cytochem. 2008, 56, 175–182. [Google Scholar] [CrossRef]
- Mitchelmore, C.; Buchmann-Moller, S.; Rask, L.; West, M.J.; Troncoso, J.C.; Jensen, N.A. NDRG2: A novel Alzheimer’s disease associated protein. Neurobiol. Dis 2004, 16, 48–58. [Google Scholar] [CrossRef]
- Rong, X.F.; Sun, Y.N.; Liu, D.M.; Yin, H.J.; Peng, Y.; Xu, S.F.; Wang, L.; Wang, X.L. The pathological roles of NDRG2 in Alzheimer’s disease, a study using animal models and APPwt-overexpressed cells. CNS Neurosci. 2017, 23, 667–679. [Google Scholar] [CrossRef]
- Herskowitz, J.H.; Seyfried, N.T.; Duong, D.M.; Xia, Q.; Rees, H.D.; Gearing, M.; Peng, J.; Lah, J.J.; Levey, A.I. Phosphoproteomic analysis reveals site-specific changes in GFAP and NDRG2 phosphorylation in frontotemporal lobar degeneration. J. Proteome. Res. 2010, 9, 6368–6379. [Google Scholar] [CrossRef]
- Ma, Y.L.; Zhang, L.X.; Liu, G.L.; Fan, Y.; Peng, Y.; Hou, W.G. N-Myc Downstream-Regulated Gene 2 (Ndrg2) Is Involved in Ischemia-Hypoxia-Induced Astrocyte Apoptosis: A Novel Target for Stroke Therapy. Mol. Neurobiol. 2017, 54, 3286–3299. [Google Scholar] [CrossRef]
- Deng, Y.; Yao, L.; Chau, L.; Ng, S.S.; Peng, Y.; Liu, X.; Au, W.S.; Wang, J.; Li, F.; Ji, S.; et al. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int. J. Cancer 2003, 106, 342–347. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.; Kang, H.B.; Jaroszewski, L.; Deacon, A.M.; Lee, H.; Choi, W.C.; Kim, K.J.; Kim, C.H.; Kang, B.S.; et al. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J. Biol. Chem. 2011, 286, 12450–12460. [Google Scholar] [CrossRef]
- Takarada-Iemata, M.; Yoshikawa, A.; Ta, H.M.; Okitani, N.; Nishiuchi, T.; Aida, Y.; Kamide, T.; Hattori, T.; Ishii, H.; Tamatani, T.; et al. N-myc downstream-regulated gene 2 protects blood–brain barrier integrity following cerebral ischemia. Glia 2018, 66, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Guo, H.; Tao, L.; Cai, G.; Wang, Y.; Yao, L.; Xiong, L.; Zhang, J.; Li, Y. NDRG2 Protects the Brain from Excitotoxicity by Facilitating Interstitial Glutamate Uptake. Transl. Stroke Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.B.; Brayne, C.; Savva, G.M.; Matthews, F.E.; Forster, G.; Simpson, J.; Lace, G.; Ince, P.G. Epidemiological neuropathology: The MRC Cognitive Function and Aging Study experience. J. Alzheimers Dis. 2011, 25, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yin, A.; Yao, L.; Li, Y. N-myc downstream-regulated gene 2 in the nervous system: From expression pattern to function. Acta Biochim. Biophys. Sin. 2015, 47, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.C.; Yao, L.B.; Liu, X.P.; Nie, X.Y.; Wang, X.G.; Su, C.Z. Exploring a new gene containing ACP like domain in human brain and expression in E. coli. Prog. Biochem. Biophys. 2001, 28, 72–76. [Google Scholar]
- Zhang, Z.; Ma, Z.; Zou, W.; Guo, H.; Liu, M.; Ma, Y.; Zhang, L. The Appropriate Marker for Astrocytes: Comparing the Distribution and Expression of Three Astrocytic Markers in Different Mouse Cerebral Regions. Biomed. Res. Int. 2019, 2019, 9605265. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, H.; Li, X.; Peng, Y.; Kinden, R.; Liang, W.; Li, X.; Shi, M.; Liu, L.; Wang, Q.; et al. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 50, 305–313. [Google Scholar] [CrossRef]
- Tao, L.; Zhu, Y.; Wang, R.; Han, J.; Ma, Y.; Guo, H.; Tang, W.; Zhuo, L.; Fan, Z.; Yin, A.; et al. N-myc downstream-regulated gene 2 deficiency aggravates memory impairment in Alzheimer’s disease. Behav. Brain Res. 2019, 112384. [Google Scholar] [CrossRef]
- Takarada-Iemata, M.; Kezuka, D.; Takeichi, T.; Ikawa, M.; Hattori, T.; Kitao, Y.; Hori, O. Deletion of N-myc downstream-regulated gene 2 attenuates reactive astrogliosis and inflammatory response in a mouse model of cortical stab injury. J. Neurochem. 2014, 130, 374–387. [Google Scholar] [CrossRef]
- Bouvier, D.S.; Murai, K.K. Synergistic actions of microglia and astrocytes in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2015, 45, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.; Boche, D. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflammation 2016, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhao, Z.Y.; Wang, Y.Z.; Ji, S.P.; Liu, X.P.; Liu, X.W.; Che, H.L.; Lin, W.; Li, X.; Zhang, J.; et al. Immunohistochemical detection of Ndrg2 in the mouse nervous system. Neuroreport 2008, 19, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, T.; Takarada-Iemata, M.; Hashida, K.; Sudo, H.; Okuda, T.; Kokame, K.; Hatano, T.; Takanashi, M.; Funabe, S.; Hattori, N.; et al. The effect of Ndrg2 expression on astroglial activation. Neurochem. Int. 2011, 59, 21–27. [Google Scholar] [CrossRef]
- Li, Y.; Xu, N.; Cai, L.; Gao, Z.; Shen, L.; Zhang, Q.; Hou, W.; Zhong, H.; Wang, Q.; Xiong, L. NDRG2 is a novel p53-associated regulator of apoptosis in C6-originated astrocytes exposed to oxygen-glucose deprivation. PLoS ONE 2013, 8, e57130. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Zou, W.; Zhang, L.; Li, Y.; Zhang, J.; Liu, M.; Hou, W.; Ma, Y. N-myc downstream-regulated gene 2 controls astrocyte morphology via Rho-GTPase signaling. J. Cell Physiol. 2019, 234, 20847–20858. [Google Scholar] [CrossRef]
- Iida, T.; Furuta, A.; Nishioka, K.; Nakabeppu, Y.; Iwaki, T. Expression of 8-oxoguanine DNA glycosylase is reduced and associated with neurofibrillary tangles in Alzheimer’s disease brain. Acta Neuropathol. 2002, 103, 20–25. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Haynes, L.J.; Theaker, R.; Gelsthorpe, C.; Baxter, L.; Forster, G.; Lace, G.L.; Shaw, P.J.; Matthews, F.E.; et al. Population variation in oxidative stress and astrocyte DNA damage in relation to Alzheimer-type pathology in the ageing brain. Neuropathol. Appl Neurobiol. 2010, 36, 25–40. [Google Scholar] [CrossRef]
- Cognitive function and dementia in six areas of England and Wales: The distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Psychol. Med. 1998, 28, 319–335.
- Mirra, S.S. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: A commentary. Neurobiol Aging 1997, 18, S91–S94. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Fluteau, A.; Ince, P.G.; Minett, T.; Matthews, F.E.; Brayne, C.; Garwood, C.J.; Ratcliffe, L.E.; Morgan, S.; Heath, P.R.; Shaw, P.J.; et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci. Lett. 2015, 609, 11–17. [Google Scholar] [CrossRef] [PubMed]



| Astrocyte Marker | NDRG2 | GFAP | GS | EAAT2 |
|---|---|---|---|---|
| Mean (SD) | 16.46 (11.75) | 8.49 (8.98) | 0.76 (0.84) | 29.94 (26.87) |
| Median (IQR) | 14.43 (7.25–22.1) | 5.79 (2.12–11.55) | 0.51 (0.23–0.99) | 23.11 (8.42–43.61) |
| Specificity | Isotype | Dilution | Antigen Retrieval | Supplier |
|---|---|---|---|---|
| AT8 (tau) | mouse IgG1 | 1:400 | PC, AS pH9.5 | Endogen, UK |
| CD68 | mouse IgG3κ | 1:100 | MW 10 min, TSC | DakoCytomation, UK |
| EAAT2 (Glt-1) | mouse IgG2A | 1:20 | PC, AS pH9.5 | Novocastra, UK |
| GFAP | rabbit IgG | 1:1000 | MW 10 min, TSC | DakoCytomation, UK |
| GS | goat IgG | 1:500 | PC, AR pH6.4 | Millipore, UK |
| MHC II | mouse IgG1κ | 1:20 | MW 10 min, TSC | DakoCytomation, UK |
| NDRG2 | goat IgG | 1:75 | PC, AS pH9.5 | Santa Cruz, USA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadul, M.M.; Garwood, C.J.; Waller, R.; Garrett, N.; Heath, P.R.; Matthews, F.E.; Brayne, C.; Wharton, S.B.; Simpson, J.E. NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain. Int. J. Mol. Sci. 2020, 21, 340. https://doi.org/10.3390/ijms21010340
Fadul MM, Garwood CJ, Waller R, Garrett N, Heath PR, Matthews FE, Brayne C, Wharton SB, Simpson JE. NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain. International Journal of Molecular Sciences. 2020; 21(1):340. https://doi.org/10.3390/ijms21010340
Chicago/Turabian StyleFadul, Motaz M., Claire J. Garwood, Rachel Waller, Navonna Garrett, Paul R. Heath, Fiona E Matthews, Carol Brayne, Stephen B. Wharton, and Julie E. Simpson. 2020. "NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain" International Journal of Molecular Sciences 21, no. 1: 340. https://doi.org/10.3390/ijms21010340
APA StyleFadul, M. M., Garwood, C. J., Waller, R., Garrett, N., Heath, P. R., Matthews, F. E., Brayne, C., Wharton, S. B., & Simpson, J. E. (2020). NDRG2 Expression Correlates with Neurofibrillary Tangles and Microglial Pathology in the Ageing Brain. International Journal of Molecular Sciences, 21(1), 340. https://doi.org/10.3390/ijms21010340





