Physical-Chemical Study of Anthracene Selective Oxidation by a Fe(III)-Phenylporhyrin Derivative
Abstract
:1. Introduction
2. Results and Discussion
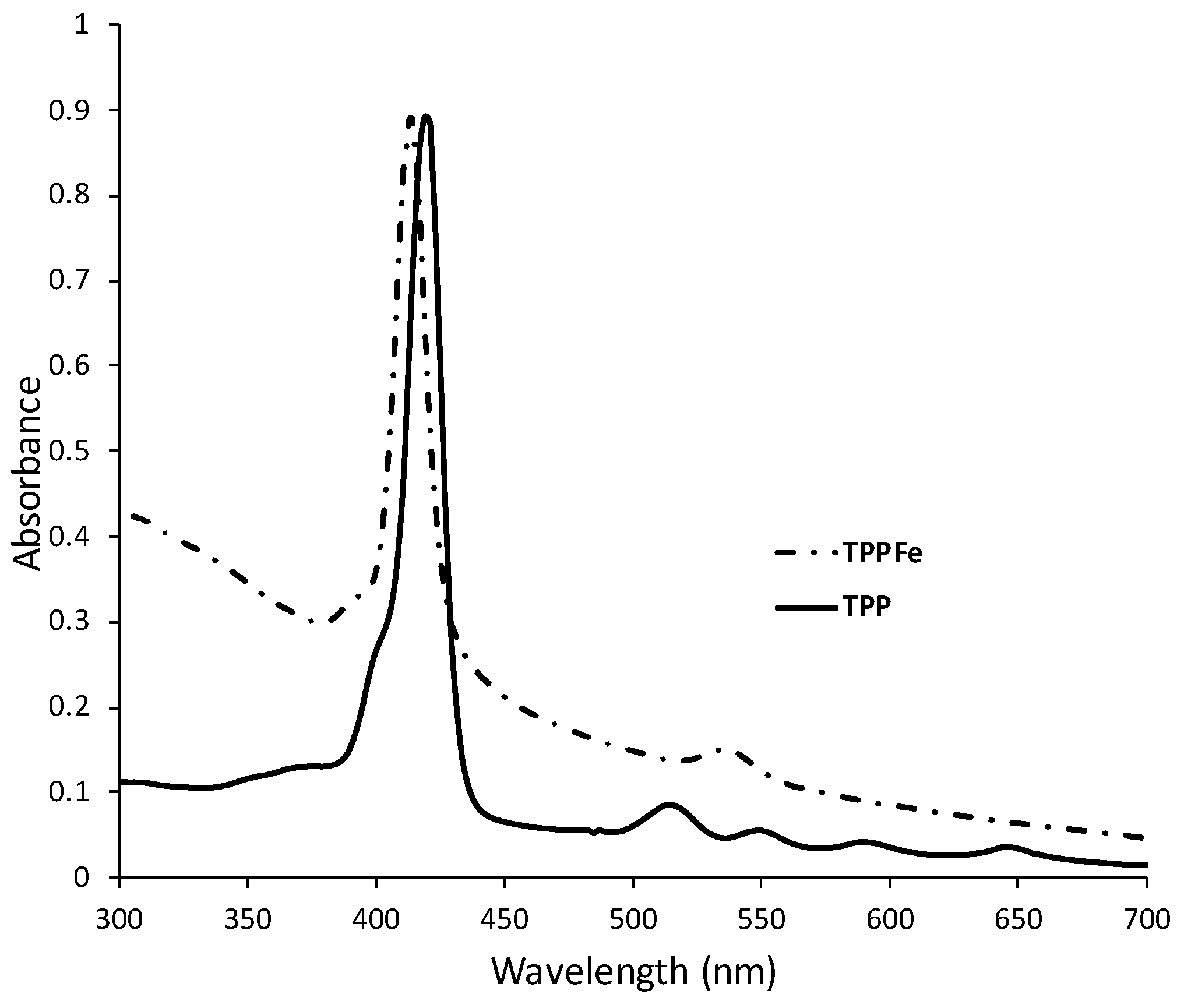
2.1. UV-Vis Assay
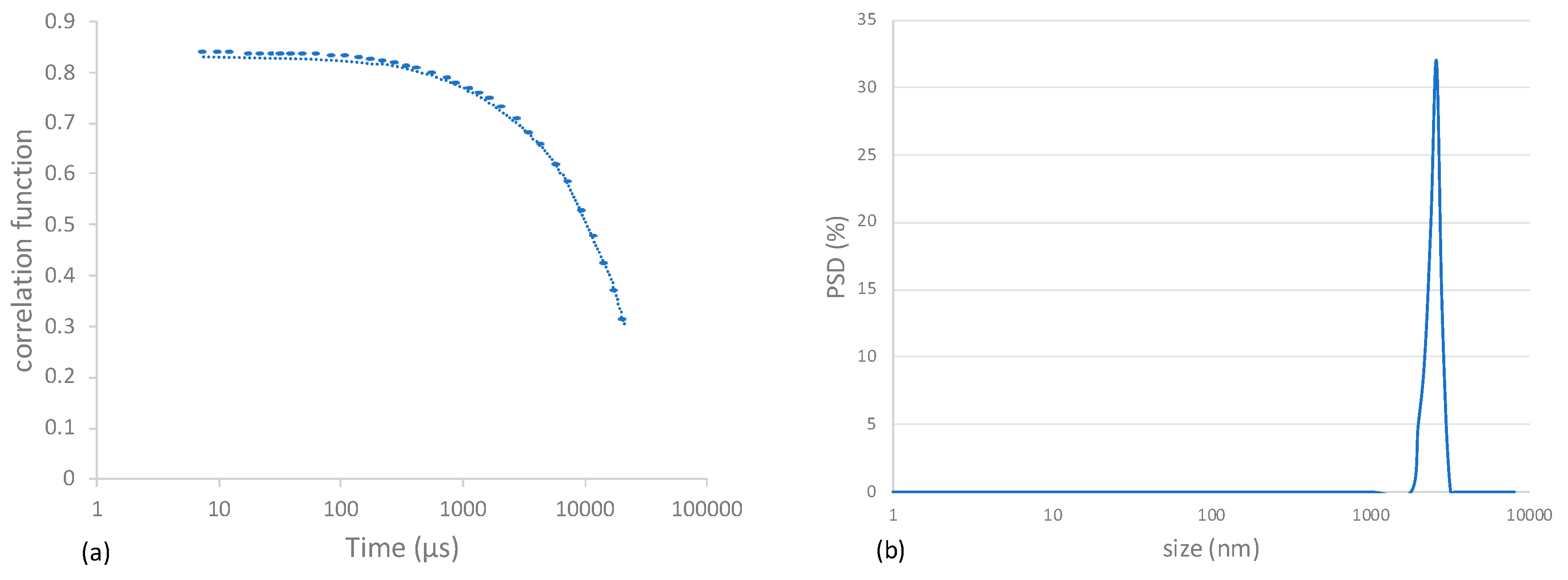
2.2. Dynamic Light Scattering Characterization
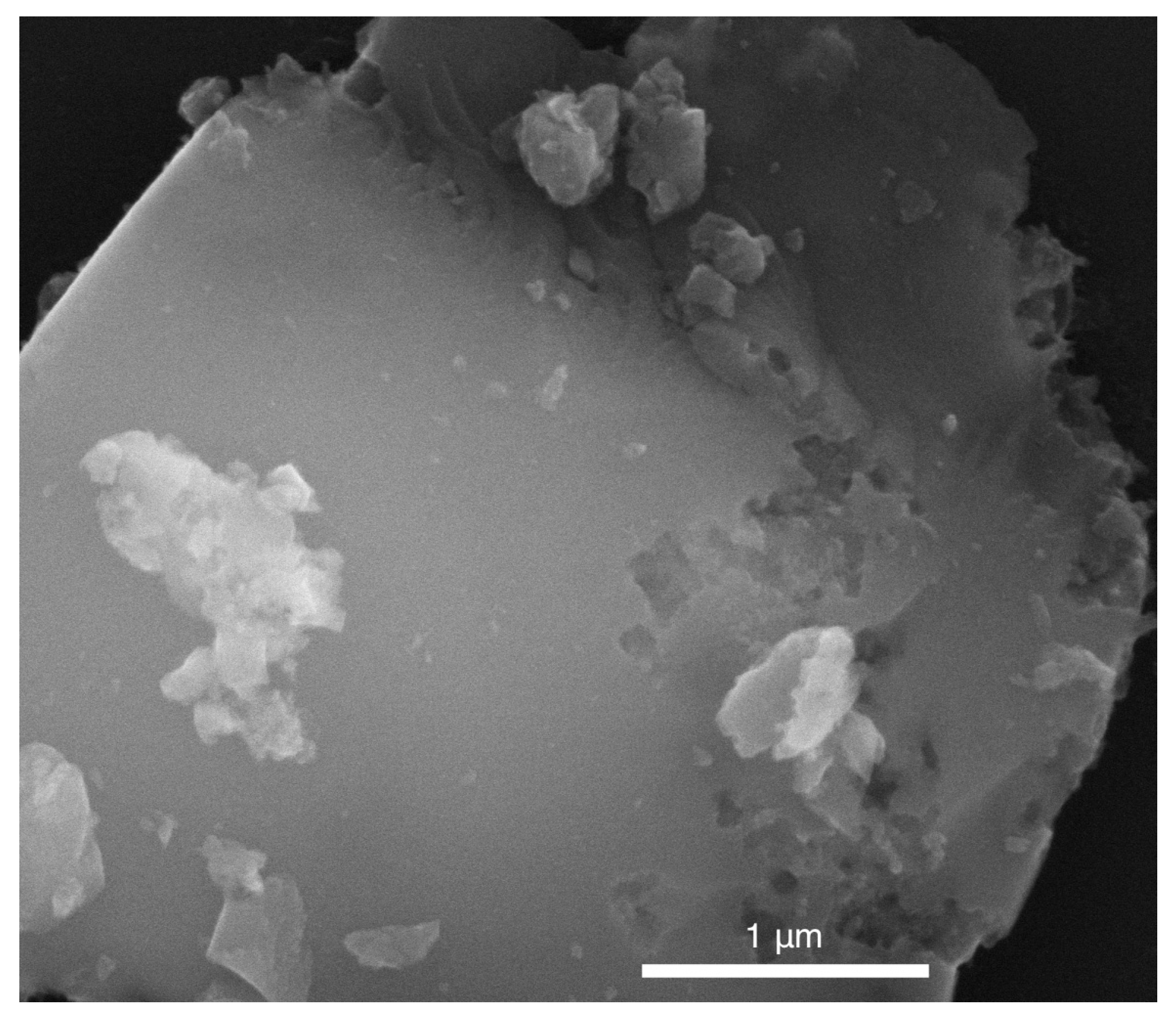
2.3. SEM Characterization
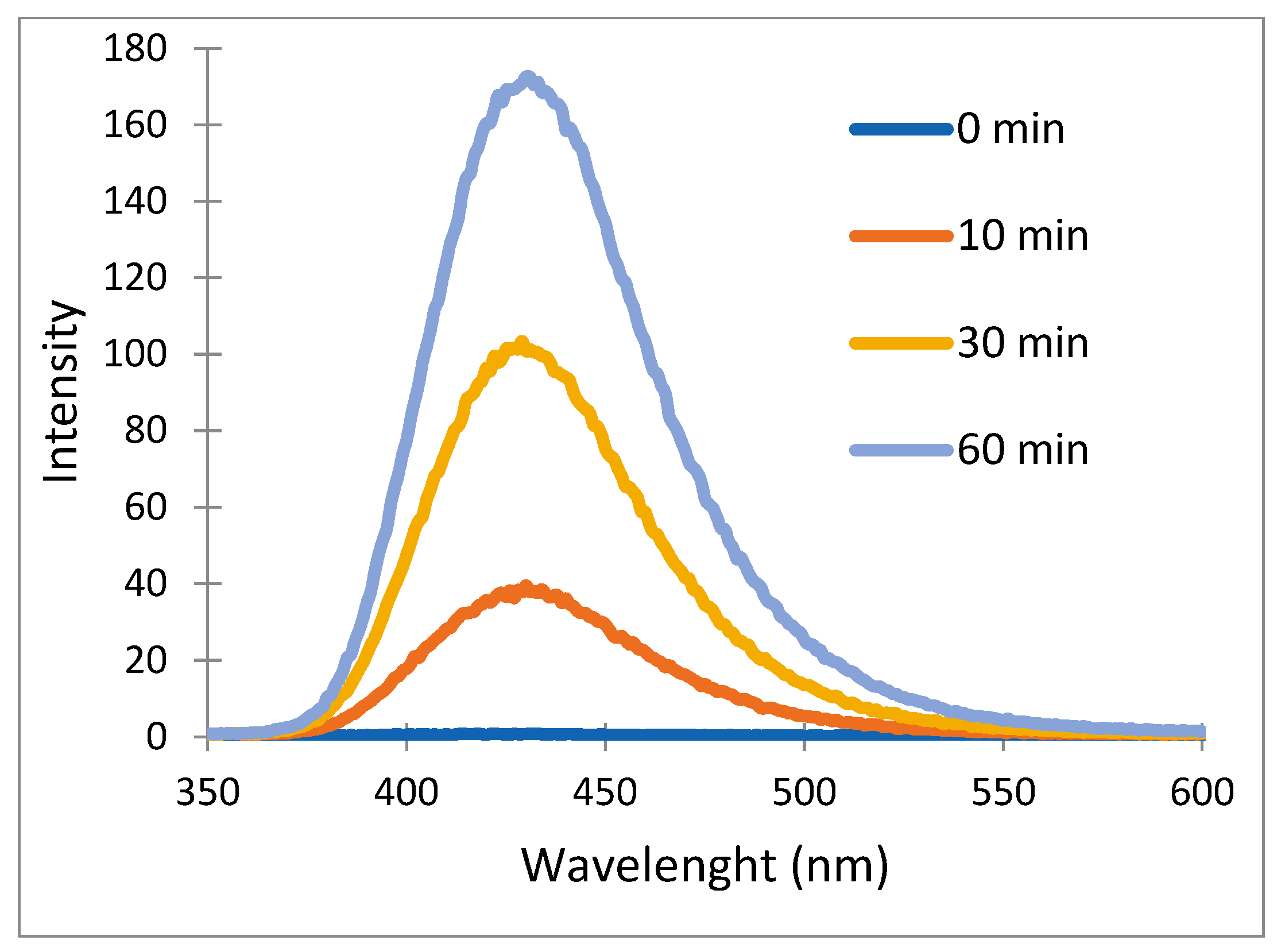
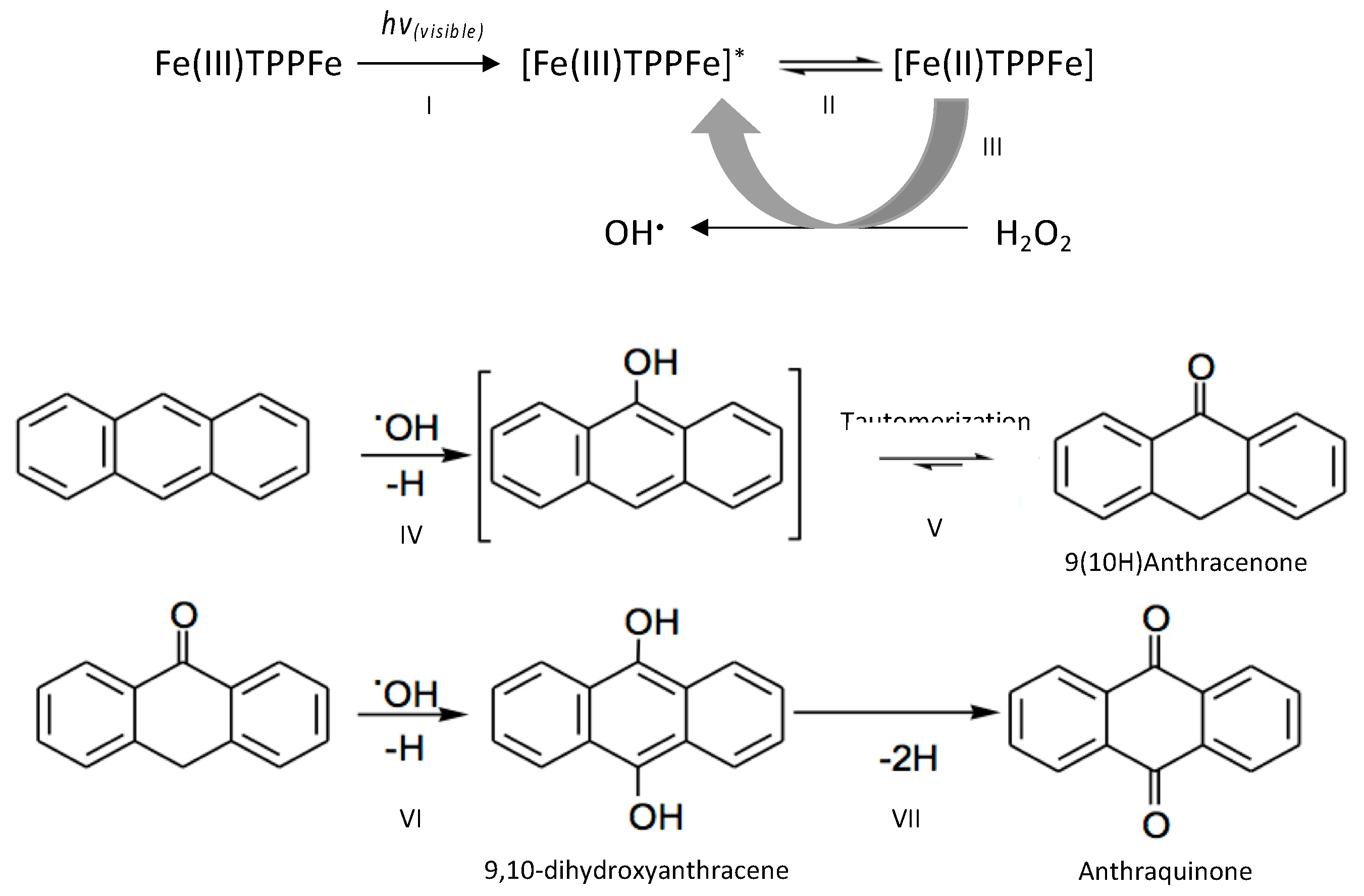
2.4. Identification of Hydroxyl Radical
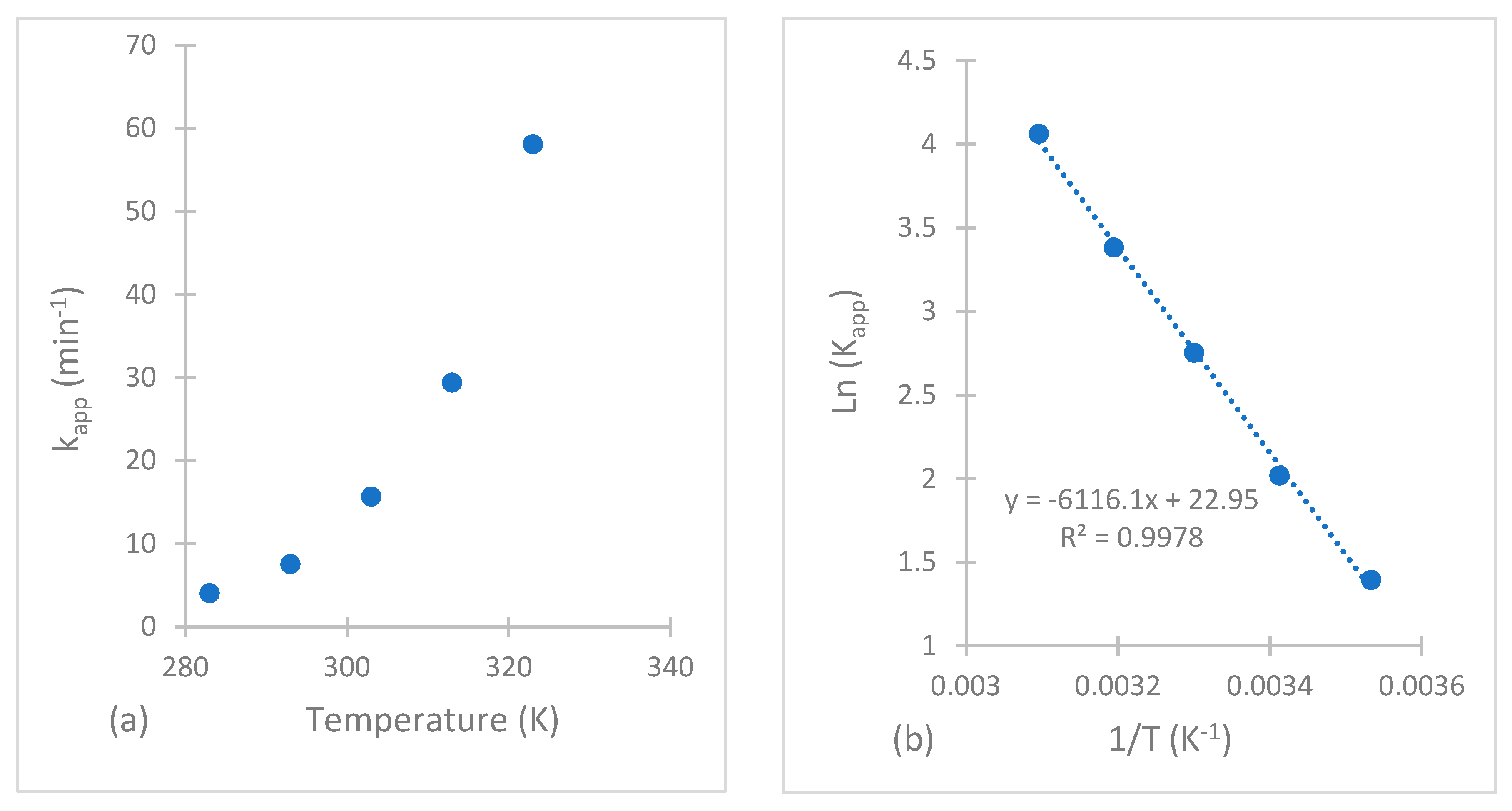
2.5. Kinetic Study
2.6. Analysis of the Products of Oxidation
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Physicochemical Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Liu, Y.; He, H. Degradation kinetics of anthracene by ozone on mineral oxides. Atmos. Environ. 2010, 44, 4446–4453. [Google Scholar] [CrossRef]
- Ukiwe, L.N.; Egereonu, U.U.; Njoku, P.C.; Nwoko, C.I.A.; Allinor, J.I. Polycyclic aromatic hydrocarbons degradation techniques: A review. Int. J. Chem. 2013, 5, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Krumova, K.; Cosa, G. Chapter 1: Overview of reactive oxygen species. In Singlet Oxygen: Applications in Biosciences and Nanosciences, 1st ed.; RSC: London, UK, 2016; pp. 1–21. [Google Scholar]
- Kolarova, H.; Nevrelova, P.; Tomankova, K.; Kolar, P.; Bajgar, R.; Mosinger, J. Production of reactive oxygen species for photodynamic therapy by porphyrin sensitizers. Gen. Physiol. Biophys. 2008, 27, 101–105. [Google Scholar]
- Boyle, R.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Ananthula, R.; Yamada, T.; Taylor, P.H. Kinetics of OH radical reaction with anthracene and anthracene-d10. J. Phys. Chem. A 2006, 110, 3559–3566. [Google Scholar] [CrossRef]
- Silva, M.; Severino, D.; Manso, F.; Oliveira, M.; de Oliveira, M.B.; Baptista, M.; de Medeiros, M.G.; di Mascio, P. Synthesis and characterization of new anthracene derivatives used as singlet molecular oxygen chemical traps. Free Radic. Biol. Med. 2010, 49, S99–S100. [Google Scholar]
- Goulay, F.; Rebrion-Rowe, C.; le Garrec, J.L.; le Picard, S.D.; Canosa, A.; Rowe, B.R. The reaction of anthracene with OH radicals: An experimental studyof the kinetics between 58 and 470 K. J. Chem. Phys. 2005, 122, 104308. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carella, A.; Borbone, F.; Centore, R. Research progress on photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Huang, Z.; Wang, L.; Kan, F. Porphyrin-based nanostructures for photocatalytic applications. Nanomaterials (Basel) 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargari, S.; Rahimi, R.; Yousefi, A. An efficient visible light photocatalyst based on tin porphyrin intercalated between TiO2 graphene nanosheets for inactivation of E. coli and investigation of charge transfer mechanism. RSC Adv. 2016, 6, 24218–24228. [Google Scholar] [CrossRef]
- Bonnett, R.; Roberts, S.; Phillips, D.; O’Brien, P. Chemical Aspects of Photodynamic Therapy, 1st ed.; CRC Press: London, UK, 2000; pp. 1–324. [Google Scholar]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative spices formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Rodríguez, F.; Dolores, M.; Adrados, L.F.; Burillo, J.C.; Tijero, J.F. Selective oxidation of anthracene to anthraquinone in acetic acid with air in presence of nitric acid. Tetrahedron Lett. 1989, 30, 2417–2420. [Google Scholar] [CrossRef]
- Safari, N.; Naghavi, S.; Khavasi, H.R. Homogeneous m-CPBA-oxidation of anthracene by electron-withdrawing metalloporphyrins in different reaction conditions. Appl. Catal. A Gen. 2005, 285, 59–64. [Google Scholar] [CrossRef]
- Maranzana, A.; Ghigo, G.; Tonachini, G. Anthracene and phenanthrene tropospheric oxidation promoted by the nitrate radical in the gas-phase. Theoretical modelistic study. Atmos. Environ. 2017, 167, 181–189. [Google Scholar] [CrossRef]
- Biermann, H.W.; Mac Leod, H.; Atkinson, R.; Winer, A.M.; Pitts, J.N. Kinetics of the gas-phase reactions of the hydroxyl radical with naphthalene, phenanthrene, and anthracene. Environ. Sci. Technol. 1985, 19, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhang, Q.; Wang, W. Atmospheric oxidation of phenanthrene initiated by OH radicals in the presence of O2 and NOx—A theoretical study. Sci. Total Environ. 2016, 563–564, 1008–1015. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, R.; Xu, F.; Zhou, Q.; Jiang, G.; Wang, T.; Chen, J.; Hu, J.; Jiang, W.; Wang, W. Role of water molecule in the gas-phase formation process of nitrated polycyclic aromatic hydrocarbons in the atmosphere: A computational study. Environ. Sci. Technol. 2014, 48, 5051–5057. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of persulfate by quinones: Free radical reactions and implication for the degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Wei, B.; Sun, J.; Mei, Q.; An, Z.; Wang, X.; He, M. Theoretical study on gas-phase reactions of nitrate radicals with methoxyphenols: Mechanism, kinetic and toxicity assessment. Environ. Pollut. 2018, 243, 1772–1780. [Google Scholar] [CrossRef]
- Liu, W.; Lv, G.; Sun, X.; He, L.; Zhang, C.; Li, Z. Theoretical study on the reaction of anthracene with sulfate radical and hydroxyl radical in aqueous solution. Ecotoxicol. Environ. Saf. 2019, 183, 109551. [Google Scholar] [CrossRef]
- Karam, F.F.; Hussein, F.H.; Baqir, S.J.; Halbus, A.F.; Dillert, R.; Bahnemann, D. Photocatalytic degradation of anthracene in closed system reactor. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Kozak, J.; Włodarczyk, M. Photo-oxidation of PAHs with calcium peroxide as a source of the hydroxyl radicals. E3S Web Conf. 2018, 30, 02009. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Wang, J.; Song, Y.; Zheng, X.; Qu, L.L.; Wu, Z.; Wu, X. Remediation of phenanthrene contaminated soil by a solid-state photo-fenton reagent based on mesoporous magnetite/carboxylate-rich carbon composites and its phytotoxicity evaluation. ACS Sustain. Chem. Eng. 2018, 6, 13262–13275. [Google Scholar] [CrossRef]
- Ke, Y.; Ning, X.A.; Liang, J.; Zou, H.; Sun, J.; Cai, H.; Lin, M.; Li, R.; Zhang, Y. Sludge treatment by integrated ultrasound-Fenton process: Characterization of sludge organic matter and its impact on PAHs removal. J. Hazard. Mater. 2018, 343, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bocos, E.; Fernández-Costas, C.; Pazos, M.; Sanromán, M. Ángeles removal of PAHs and pesticides from polluted soils by enhanced electrokinetic-Fenton treatment. Chemosphere 2015, 125, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qin, L.; Gatheru, M.; Cheng, P.; Yang, B.; Wang, J.; Ling, W. Removal of bound PAH residues in contaminated soils by Fenton oxidation. Catalysts 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Boopathy, R.; Gupta, V.K.; Sekaran, G. Preparation, characterizations and its application of heterogeneous Fenton catalyst for the treatment of synthetic phenol solution. J. Mol. Liq. 2013, 177, 402–408. [Google Scholar] [CrossRef]
- Tryba, B.; Morawski, A.W.; Inagaki, M.; Toyoda, M. The kinetics of phenol decomposition under UV irradiation with and without H2O2 on TiO2, Fe-TiO2 and Fe-C-TiO2 photocatalysts. Appl. Catal. B Environ. 2006, 63, 215–221. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Bozic, A.L.; Selanec, I. Photo-assisted Fenton type processes for the degradation of phenol: A kinetic study. J. Hazard. Mater. 2006, 136, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Shan, N.; Yu, L.; Wang, X. UV-visible, fluorescence and EPR properties of porphyrins and metalloporphyrins. Dyes Pigments 2008, 77, 153–157. [Google Scholar] [CrossRef]
- Papkovsky, D.B.; Ponomare, G.V.; Trettnak, W.; O’Leary, P. Phosphorescent complexes of porphyrin ketones: Optical properties and application to oxygen sensing. Anal. Chem. 1995, 67, 4112–4117. [Google Scholar] [CrossRef]
- Díaz, C.; Vallejo, W.; Miranda, J. Photo-Fenton oxidation of phenol with Fe(III)-tetra-4-carboxyphenylporphyrin/SiO2 assisted with visible light. J. Photochem. Photobiol. A Chem. 2014, 294, 75–80. [Google Scholar] [CrossRef]
- Zhdanova, K.A.; Ezhov, A.V.; Bragina, N.A.; Mironov, A.F. Synthesis of new binary porphyrin-cyanine conjugates and their self-aggregation in organic-aqueous media. Mendeleev Commun. 2018, 28, 626–628. [Google Scholar] [CrossRef]
- Andrade, S.M.; Teixeira, R.; Costa, S.M.B.; Sobral, A.J.F.N. Self-aggregation of free base porphyrins in aqueous solution and in DMPC vesicles. Biophys. Chem. 2008, 133, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Malm, A.V.; Corbett, J.C.W. Improved dynamic light scattering using an adaptive and statistically driven time resolved treatment of correlation data. Sci. Rep. 2019, 9, 13519. [Google Scholar] [CrossRef]
- Mark, G.; Tauber, A.; Laupert, R.; Schuchmann, H.; Schulz, D.; Mues, A.; Sonntag, C. OH-radical formation by ultrasound in aqueous solution—Part II: Terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason. Sonochem. 1998, 5, 41–52. [Google Scholar] [CrossRef]
- House, J.E. Principles of Chemical Kinetics, 1st ed.; Elsevier: New York, NY, USA, 2007; pp. 10–31. [Google Scholar]
- Kohtani, S.; Tomohiro, M.; Tokumura, K.; Nakagaki, R. Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts. Appl. Catal. B Environ. 2005, 58, 265–272. [Google Scholar] [CrossRef]
- Paddon, C.A.; Banks, C.E.; Davies, I.G.; Compton, R.G. Oxidation of anthracene on platinum macro- and micro-electrodes: Sonoelectrochemical, cryoelectrochemical and sonocryoelectrochemical studies. Ultrason. Sonochem. 2006, 13, 126–132. [Google Scholar] [CrossRef]
- Cordeiro, D.S.; Corio, P. Electrochemical and photocatalytic reactions of polycyclic aromatic hydrocarbons investigated by raman spectroscopy. J. Braz. Chem. Soc. 2009, 2, 80–87. [Google Scholar] [CrossRef]
- Adler, A.; Longo, F.; Finarelli, J.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Uribe, C.; Vallejo, W.; Quiñones, C. Physical-Chemical Study of Anthracene Selective Oxidation by a Fe(III)-Phenylporhyrin Derivative. Int. J. Mol. Sci. 2020, 21, 353. https://doi.org/10.3390/ijms21010353
Diaz-Uribe C, Vallejo W, Quiñones C. Physical-Chemical Study of Anthracene Selective Oxidation by a Fe(III)-Phenylporhyrin Derivative. International Journal of Molecular Sciences. 2020; 21(1):353. https://doi.org/10.3390/ijms21010353
Chicago/Turabian StyleDiaz-Uribe, Carlos, William Vallejo, and Cesar Quiñones. 2020. "Physical-Chemical Study of Anthracene Selective Oxidation by a Fe(III)-Phenylporhyrin Derivative" International Journal of Molecular Sciences 21, no. 1: 353. https://doi.org/10.3390/ijms21010353
APA StyleDiaz-Uribe, C., Vallejo, W., & Quiñones, C. (2020). Physical-Chemical Study of Anthracene Selective Oxidation by a Fe(III)-Phenylporhyrin Derivative. International Journal of Molecular Sciences, 21(1), 353. https://doi.org/10.3390/ijms21010353




