Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway
Abstract
:1. Introduction
2. Results
2.1. The Retarding Effect of Antrodan Against the HFD Regarding the Liver- and Body-Weight
2.2. Effect of Antrodan on Plasma Levels of Malondialdehyde, Total Cholesterol, Triglyceride, and Ratio LDL-C/HDL-C
2.3. Effect of Antrodan on the Plasma Levels of Glucose, Insulin, Leptin and Adiponectin
2.4. Effect of Antrodan on the Activities of Plasma Levels of GOT, GPT, and Uric Acid
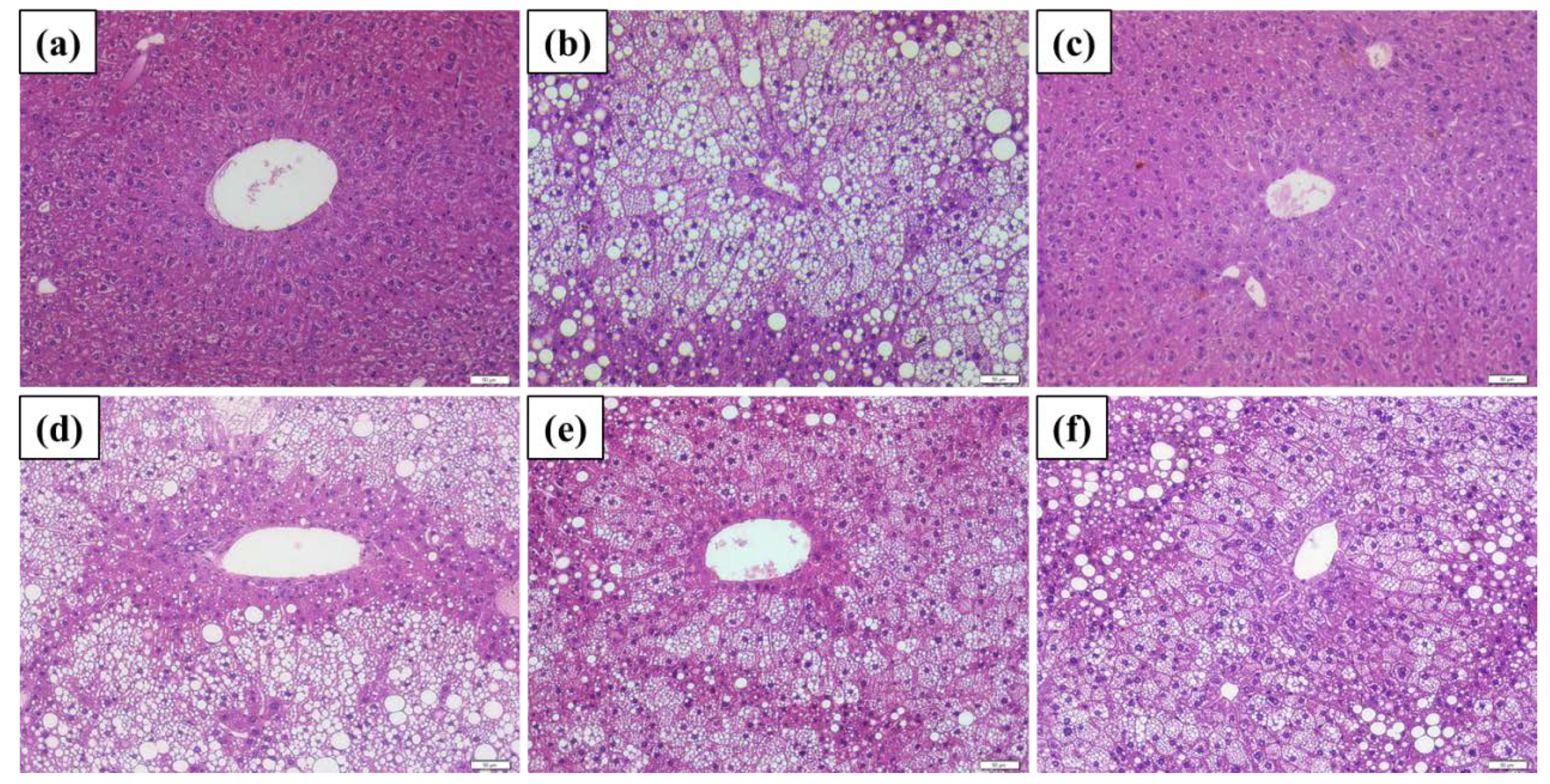
2.5. Histopathological Findings
2.6. Protein Expressions Affected by Antrodan
3. Discussion
3.1. The Adverse Metabolic Role of a High Fat Diet
3.2. Fructose Pays a Higher Energy Cost Regarding the ATP Production
3.3. How Does the High Fructose Diet Affect Llipid Metabolism?
3.4. Why is Antrodan Ineffective at Suppressing the Ratio LDL-C/HDL-C?
3.5. Animal Model Selection Affects the Experimental Outcomes
3.6. Antrodan treatments Appear to be Effective in Regulating Adiponectin but not in Leptin Levels
3.7. Elevated PPARγ and SREBP-1c Increased Lipid Synthesis
3.8. Sirt1 and pAMPK Inhibited PPARγ and SREBP-1c, thereby, Suppressed Lipid Synthesis and Alleviated Insulin Resistance
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Source of Antrodan
4.3. Induction of Fatty Liver Diseases and Treatment with Antrodan
4.4. Assay for the Plasma Biochemical Parameters
4.5. Immunoassay for the Plasma Level of Insulin, Leptin, and Adiponectin
4.6. Western Blotting
4.7. Histological Examination of the Hepatic Tissues
4.8. Statistical Analysis
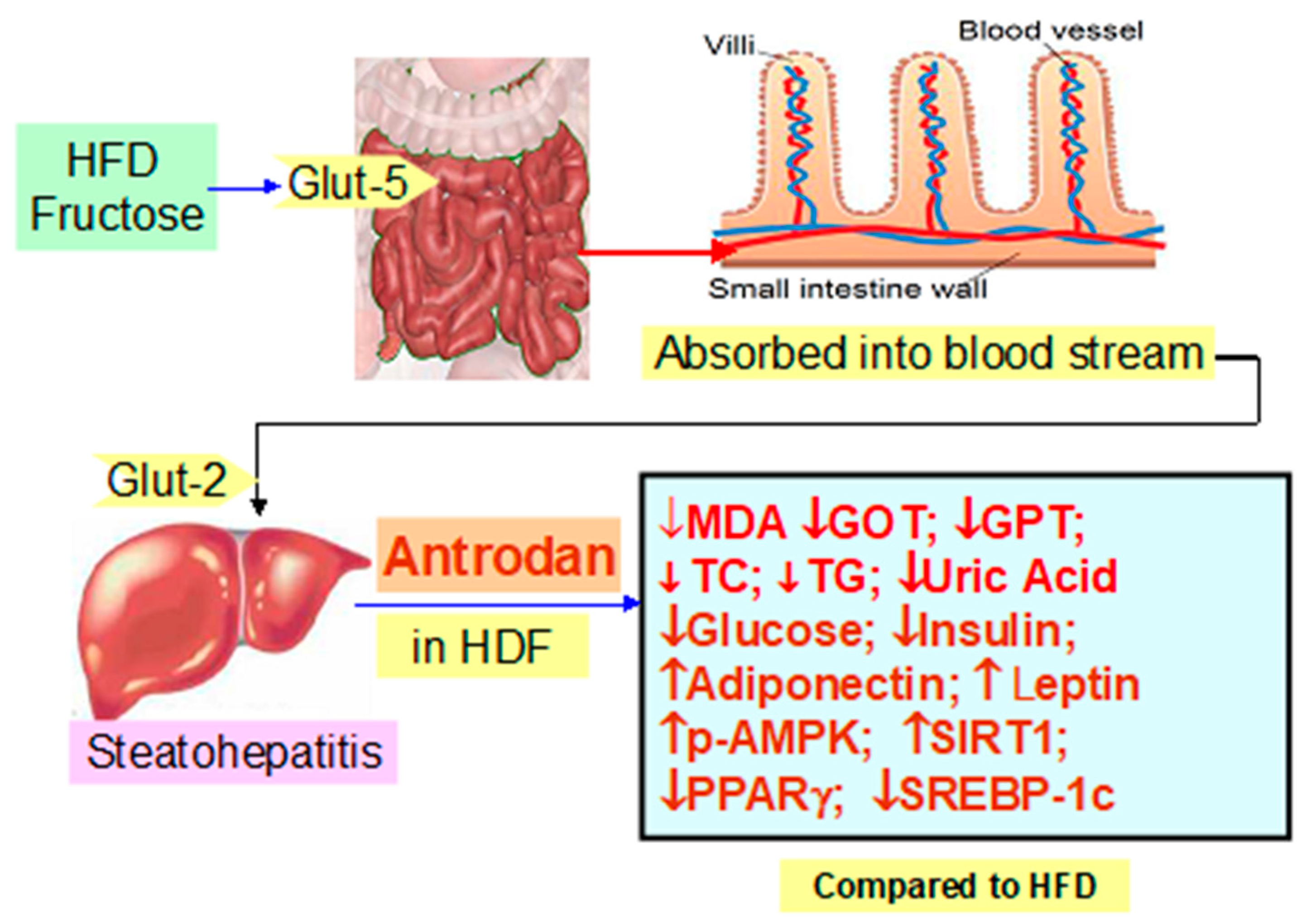
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bril, F.; Cusi, K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care 2017, 40, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig. Dis. 2010, 26, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: A Review. Adv. Nutr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Toop, C.R.; Gentili, S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: A systematic review and meta-analysis. Nutrients 2016, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.M.; Botezelli, J.D.; Da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; Da Silva, A.S.R.; Ropelle, E.R.; De Moura, L.P. Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomed. Pharmacother. 2017, 86, 694–704. [Google Scholar] [CrossRef]
- Parry, S.A.; Hodson, L. Influence of dietary macronutrients on liver fat accumulation and metabolism. J. Investig. Med. 2017, 65, 1102–1115. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chen, K.C.; Peng, R.Y.; Chyau, C.C.; Su, C.H.; Hsieh-Li, H.M. Antrodia camphorata extract induces replicative senescence in superficial TCC and inhibits the absolute migration capability in invasive bladder carcinoma cells. J. Ethnopharm. 2007, 109, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chen, C.C.; Ker, Y.B.; Chang, C.H.; Chyau, C.C.; Hu, M.L. Anti-metastatic effects of antrodan with and without cisplatin on Lewis lung carcinomas in a mouse xenograft model. Int. J. Mol. Sci. 2018, 19, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.H.; Peng, C.C.; Ker, Y.B.; Chen, C.C.; Lee, A.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia. Molecules 2014, 19, 22–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Liu, Y.W.; Ker, Y.B.; Wu, Y.Y.; Lai, E.Y.; Chyau, C.C.; Hseu, T.H.; Peng, R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 2007, 55, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Ker, Y.B.; Peng, C.C.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Hepatoprotective bioactivity of the glycoprotein, Antrodan, isolated from Antrodia cinnamomea mycelia. PLoS ONE 2014, 9, e93191. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Lin, Y.T.; Chen, K.C.; Chyau, C.C.; Peng, R.Y. Antrodan, A β-glucan obtained from Antrodia cinnamomea mycelia, is beneficial to benign prostate hyperplasia. Food Funct. 2015, 6, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Fa, K.N.; Yang, C.M.; Chen, P.C.; Lee, Y.Y.; Chyau, C.C.; Hu, M.L. Anti-metastatic effects of Antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells. Int. J. Biol. Macromol. 2015, 74, 476–482. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metabol. 2017, 26, 545–600. [Google Scholar] [CrossRef]
- Ding, R.B.; Bao, J.L.; Deng, C.X. Emerging roles of Sirt1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Matsusue, K.; Aibara, D.; Hayafuchi, R.; Matsuo, K.; Takiguchi, S.; Gonzalez, F.J.; Yamano, S. Hepatic PPARγ and LXRα independently regulate lipid accumulation in the livers of genetically obese mice. FEBS Lett. 2014, 588, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Lu, K.H.; Ho, C.T.; Sheen, L.Y. Protective effects of Antrodia cinnamomea against liver injury. J. Tradit. Complement. Med. 2012, 2, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Ma, J.; Huang, Q.; Yin, H.; Han, J.; Li, M.; Deng, Y.; Wang, B.; Hassan, W.; Shang, J. Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int. J. Mol. Sci. 2018, 19, 4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, J.E.; Wilson, L.; Brunt, E.M.; Yeh, M.M.; Kleiner, D.E.; Unalp-Arida, A.; Kowdley, K.V. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 2011, 53, 448–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sun, R.Q.; Zeng, X.Y.; Zhou, X.; Li, S.; Jo, E.; Molero, J.C.; Ye, J.M. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology 2015, 156, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappy, L.; Egli, L.; Lecoultre, V.; Schneider, P. Effects of fructose-containing caloric sweeteners on resting energy expenditure and energy efficiency: A review of human trials. Nutr. Metab. 2013, 10, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uldry, M.; Thorens, B. The SLC2 family of facilitated hexose and polyol transporters. Pflug. Arch. 2004, 447, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary sugars intake and cardiovascular health: A scientific statement from the American heart association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Henriques, M.C.; Oliveira, M.C.; Menezes-Garcia, Z.; Marques, P.E.; Souza, D.G.; Menezes, G.B.; Teixeira, M.M.; Ferreira, A.V.M. Acute intake of a high-fructose diet alters the balance of adipokine concentrations and induces neutrophil influx in the liver. J. Nutr. Biochem. 2014, 25, 388–394. [Google Scholar] [CrossRef]
- Thomas, S.; Senthilkumar, G.P.; Sivaraman, K.; Bobby, Z.; Paneerselvam, S.; Harichandrakumar, K.T. Effect of s-methyl-L-cysteine on oxidative stress, inflammation and insulin resistance in male wistar rats fed with high fructose diet. Iran. J. Med. Sci. 2015, 40, 45–50. [Google Scholar]
- Foster, D.W. Malonyl-CoA: The regulator of fatty acid synthesis and oxidation. J. Clin. Investig. 2012, 122, 1958–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, Y.; Yonemitsu, S.; Erion, D.M.; Iwasaki, T.; Stark, R.; Weismann, D.; Dong, J.; Zhang, D.; Jurczak, M.J.; Löffler, M.G.; et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009, 9, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayes, P.A. Intermediary metabolism of fructose. Am. J. Clin. Nutr. 1993, 58, 754S–765S. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Kleiner, D.E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016, 65, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 2004, 6, 120–126. [Google Scholar] [CrossRef]
- Aydin, S.; Aksoy, A.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Kuloglu, T.; Citil, C.; Catak, Z. Today’s and yesterday’s of pathophysiology: Biochemistry of metabolic syndrome and animal models. Nutrition 2014, 30, 1–9. [Google Scholar] [CrossRef]
- Hvizdos, K.M.; Markham, A. Orlistat: A review of its use in the management of obesity. Drugs 1999, 58, 743–760. [Google Scholar] [CrossRef]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef]
- Yin, H.; Hu, M.; Liang, X.; Ajmo, J.M.; Li, X.; Bataller, R.; You, M. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology 2014, 146, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, K.; Hagi, A.; Inoue, Y. The relationship between plasma high density lipoprotein cholesterol levels and cholesteryl ester transfer protein activity in six species of healthy experimental animals. Biol. Pharm. Bull. 2001, 24, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Waterson, M.J.; Horvath, T.L. Neuronal regulation of energy homeostasis: Beyond the hypothalamus and feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedemann, C.; Heneghan, C.; Mahtani, K.; Thompson, M.; Perera, R.; Ward, A.M. Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta-analysis. BMJ 2012, 345, e4759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettinelli, P.; Videla, L.A. Up-regulation of PPAR-γ mRNA expression in the Liver of obese patients: An additional reinforcing lipogenic mechanism to SREBP-1c induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Repa, J.J. The liver X receptor (LXR) and hepatic lipogenesis: The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 2006, 262, 743–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memon, R.A.; Tecott, L.H.; Nonogaki, K.; Beigneux, A.; Moser, A.H.; Grunfeld, C.; Feingold, K.R. Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPAR-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 2000, 141, 4021–4031. [Google Scholar] [PubMed]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and Sirt1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Fu, M.; Pestell, R.; Sauve, A.A. Sirt1 and endocrine signaling. Trends Endocrinol. Metab. 2006, 17, 186–191. [Google Scholar] [CrossRef]
- Potente, M.; Dimmeler, S. Emerging roles of Sirt1 in vascular endothelial homeostasis. Cell Cycle 2008, 7, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Pulla, V.K.; Battu, M.B.; Alvala, M.; Sriram, D.; Yogeeswari, P. Can targeting SIRT-1 to treat type 2 diabetes be a good strategy? A review. Expert Opin. Targets 2012, 16, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschop, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the Sirt1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwa, M.; Nakano, H.; Radak, Z.; Kumagai, S. Endurance exercise increases the Sirt1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism 2008, 57, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Fouhy, F.; Deane, J.; Rea, M.C.; O’Sullivan, Ó.; Ross, R.P.; O’Callaghan, G.; Stanton, C. A remarkable age-related Increase in Sirt1 protein expression against oxidative stress in elderly: Sirt1 gene variants and longevity in human. PLoS ONE 2015, 10, e0117954. [Google Scholar] [CrossRef]
- Lin, S.J.; Defossez, P.A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000, 269, 2126–2128. [Google Scholar] [CrossRef] [Green Version]
- Massudi, H.; Grant, R.; Guillemin, G.J.; Braidy, N. NAD+ metabolism and oxidative stress: The golden nucleotide on a crown of thorns. Redox Rep. 2012, 17, 26–46. [Google Scholar] [CrossRef]
- Kohli, R.; Kirby, M.; Xanthakos, S.A.; Softic, S.; Feldstein, A.E.; Saxena, V.; Tang, P.H.; Miles, L.; Miles, M.V.; Balistreri, W.F.; et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010, 52, 934–944. [Google Scholar] [CrossRef] [Green Version]










| Group | Control | HFD | Ant-H | HFD+Orl | HFD+Ant-L | HDF+Ant-H |
|---|---|---|---|---|---|---|
| Body weight (g) | 25.47 ± 0.58 | 34.20 ± 1.11 ### | 26.50 ± 0.63 | 33.80 ± 0.92 ** | 32.40 ± 0.89 | 31.98 ± 0.56 * |
| Liver weight (g) | 1.15 ± 0.04 | 2.05 ± 0.21 ### | 1.08 ± 0.07 | 1.89 ± 0.14 | 1.87 ± 0.15 | 1.61 ± 0.05 ** |
| Liver weight/Body weight (%) | 4.49 ± 0.14 | 5.93 ± 0.43 # | 4.04 ± 0.19 | 5.58 ± 0.35 | 5.71 ± 0.32 | 5.04 ± 0.09 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chyau, C.-C.; Wang, H.-F.; Zhang, W.-J.; Chen, C.-C.; Huang, S.-H.; Chang, C.-C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int. J. Mol. Sci. 2020, 21, 360. https://doi.org/10.3390/ijms21010360
Chyau C-C, Wang H-F, Zhang W-J, Chen C-C, Huang S-H, Chang C-C, Peng RY. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. International Journal of Molecular Sciences. 2020; 21(1):360. https://doi.org/10.3390/ijms21010360
Chicago/Turabian StyleChyau, Charng-Cherng, Hsueh-Fang Wang, Wen-Juan Zhang, Chin-Chu Chen, Shiau-Huei Huang, Chun-Chao Chang, and Robert Y. Peng. 2020. "Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway" International Journal of Molecular Sciences 21, no. 1: 360. https://doi.org/10.3390/ijms21010360
APA StyleChyau, C.-C., Wang, H.-F., Zhang, W.-J., Chen, C.-C., Huang, S.-H., Chang, C.-C., & Peng, R. Y. (2020). Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. International Journal of Molecular Sciences, 21(1), 360. https://doi.org/10.3390/ijms21010360





